Abstract
Ti-6Al-4V is widely used in the aerospace industry, however, Ti-6Al-4V repairs are challenging to perform via cold spray due to the material’s limited capacity for plastic deformation. To promote particle deformation, this study investigated the influence of Ti-6Al-4V powder heat treatment on the powder microstructure, cold spray deposition behavior, and, ultimately, on the coating microstructure and properties. Plasma-atomized Ti-6Al-4V powder was subjected to three different heat treatments (i.e., 540 °C for 5 h, 750 °C for 2 h, and 950 °C for 2.5 h) and subsequently deposited on Ti-6Al-4V substrates using a high-pressure cold spray system. Scanning electron microscopy revealed that the as-received microstructure was primarily characterized by a martensitic alpha microstructure. After low-temperature heat treatment (540 °C), the microstructure retained the original martensitic alpha microstructure. Intermediate heat treatment (750 °C) resulted in the formation of needle-like alpha and acicular alpha with fine beta precipitates. High-temperature treatment (950 °C) resulted in the formation of needle-like alpha, acicular alpha, and plate-like alpha with coarse and fine beta precipitates. The hardness of the powder increased after heat treating at 550 °C and 750 °C but decreased when treated at 950 °C. After cold spray deposition, particle interiors retained the as-processed powder microstructure while regions near particle boundaries exhibited either a featureless microstructure (as-received powder and low-temperature heat treatment) or elongated β (high-temperature heat treatment). With the exception of 750 °C powder heat treatment, all coatings were nearly dense (< 1% porosity). Ultimately the results showed that 950 °C heat treatments have the potential to improve powder deformation behavior and coating properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ti-6Al-4V is a α-β alloy widely used in the aerospace industry because of the material’s high specific strength/corrosion resistance. One of the main disadvantages of the alloy, however, is its high cost when compared to competing materials like aluminum (Ref 1). Thus, maintenance repair and overhaul of titanium and titanium alloy components potentially represent a significant cost avoidance. Among the various repair technologies, cold spray (CS) has gained considerable visibility as a cost-effective technique. In this regard, the CS process has been utilized to repair damaged aircraft and navy components successfully in the recent past (Ref 2,3,4). CS is a solid-state deposition process, where micron scale (i.e., 20–70 μm) powder particles are accelerated to supersonic velocities (~up to 1200 m/s) towards a substrate. On impact the particles experience severe plastic deformation and bond to one another and the substrate via metallurgical bonding and mechanical interlocking. Since the CS process relies primarily on severe plastic deformation, low yield strength FCC metals like aluminum and copper alloys are readily deposited with good properties (Ref 5,6,7,8,9,10). Accordingly, CS of Ti-6Al-4V is challenging on account of its high yield strength and limited capacity for plastic strain.
Various single splat (Ref 11, 12) and full deposition (Ref 13,14,15,16,17,18,19,20) studies have been conducted to ascertain the feasibility of producing Ti-6Al-4V depositions with good mechanical properties using the CS process. Goldbaum et al.(Ref 11) showed that powder particles with high particle velocities (with high gas temperature and pressure) and substrate pre-heating resulted in CS Ti-6Al-4V splats with high adhesion strengths (i.e., ~250–320 MPa). In addition, it was reported that small powder particles possess high adhesion strength due to their high in-flight velocity in the gas stream (Ref 11). Vidaller et al. (Ref 12) employed a single splat study to demonstrate that Ti-6Al-4V powders adhered more strongly to a titanium substrate than to aluminum (7075 Al) or magnesium (AZ 31) substrates when sprayed at 1000 °C and 5 MPa gas temperature and pressure, respectively. This study highlights the importance of the powder-to-substrate hardness ratio on achieving satisfactory adhesion. In addition, substrate preparation and surface roughness can also affect the adhesion strength of Ti-6Al-4V coatings, with ground substrates registering adhesion strengths of 122 MPa versus 82 MPa for grit blasted and 112 MPa for the as-machined substrates (Ref 19). Along with substrate material type and surface condition, cold spray parameters influence the resulting coating quality and mechanical properties. In this regard, Vo et al. (Ref 15) showed that coatings produced by helium gas exhibited lower porosity (< 1% versus 7%) and higher tensile strength (460 MPa versus ~180 MPa) when compared to coatings deposited with nitrogen. This effect is associated with the low molecular weight and thermal properties (i.e., high specific gas constant) of helium, which results in higher gas and particle velocities, and consequently, increased particle deformation at impact. Recently, Bhattiprolu et al. (Ref 20) showed that utilization of a long nozzle in addition to employing helium gas improved deposition quality (porosity ~ 0.3% and adhesion strength > 68 MPa), due to higher particle velocities. Finally, post-deposition heat treatment was also shown to improve the mechanical properties of Ti-6Al-4V depositions with tensile strength reaching 85% of the bulk value (~ 765 MPa) (Ref 15). Regarding this, heat treatment is generally known to reduce coating porosity (Ref 16, 21) and improve the metallurgical bonding between particles (Ref 15), although at times it can result in a slight increase in porosity due to coalescence of submicron pores (Ref 16).
Ti-6Al-4V feedstock powder microstructure and properties are known to have a significant influence on cold spray deposition microstructure and properties (Ref 20, 22,23,24,25). As such, initial powder microstructure affects the level of powder deformation and the resulting CS particle microstructure within the coating (Ref 25). Munagala et al. (Ref 25) showed that particles with irregular morphology and equiaxed alpha (α) grains resulted in a greater degree of particle deformation owing to their lower hardness compared to martensitic α grains. Moreover, their resulting depositions were nearly dense (~ 0.3% porosity) comprising particles characterized by a larger proportion of nanograins. Regarding this, nanograin formation is often reported by various investigators close to the particle–particle interfaces and is attributed to dynamic recrystallization (Ref 20, 24,25,26). Alternately, particle interiors are known to exhibit the as-received powder microstructure and are characterized by either broken martensitic α (Ref 24) or martensitic α grains (Ref 20) when depositions are carried out with plasma-atomized powder. This heterogeneity within the CS particle microstructure results in variation in the local mechanical properties with regions near the particle interfaces exhibiting high hardness due to the presence of nanograins and high dislocation density (Ref 24, 27, 28). Additionally, bulk mechanical properties of the coating are reported to increase by adopting particles with a dual-phase microstructure of coarse α and β grains (Ref 20). Specifically, the presence of β phase precipitates promotes strain localization and adiabatic shear instability (ASI), thereby improving inter-particle bonding during deposition and in turn resulting in coatings with high adhesion strengths (Ref 20).
Recently, improved deposition efficiency and mechanical properties have been reported for aluminum alloy CS depositions performed using heat treated powders (Ref 29,30,31). Solution treatment of the aluminum alloy powders displaced the intercellular dendritic network of solute elements in the as-received powder into the solid solution of the aluminum matrix resulting in a homogeneous microstructure (Ref 29, 31). Consequently, the hardness of AA7075 powders was reduced by 25% (Ref 29) and a thicker coating (when compared to as-sprayed coating) was shown to develop when the other CS parameters (viz gas temperature, gas pressure, traverse speed, number of layers, and standoff distance) were held constant (Ref 29). In a separate study, a ~ 70% increase in the deposition efficiency was reported for CS coatings deposited using solution-treated AA7075 powder (versus as-sprayed coatings) (Ref 31). On the other hand, further aging of the powder was reported to reduce the deposition efficiency due to precipitate strengthening of AA7075 (Ref 30). The above studies show the important influence of powder heat treatment on cold sprayed aluminum alloys.
From the above-reported studies on CS coatings, it is evident that the powder microstructure influences deposition efficiency, porosity, and mechanical properties of the resulting CS coatings. Additionally, powder heat treatment can be successfully employed to manipulate the powder microstructure and in turn the resultant CS coating properties. To the authors knowledge, however, limited (if any) studies have reported on the influence of Ti-6Al-4V powder heat treatment on the microstructure and mechanical properties of CS coatings. The aim of this study is to provide a preliminary understanding of the relationships between powder heat treatment and powder microstructure and in turn their influence on CS coating microstructure and properties. Plasma-atomized Ti-6Al-4V powder, which is widely employed in CS deposition of Ti-6Al-4V alloys, was heat treated to three different temperatures. The goal of the heat treatment was to disrupt the atomized powder microstructure and convert it into a duplex microstructure comprising α and β grains of various sizes. Consequently, relationships between powder microstructure, powder properties, coating microstructure, and coating properties were explored by employing scanning electron microscopy, optical microscopy, microhardness testing, and nanoindentation.
Ti-6Al-4V bulk material is produced and commonly used in the mill-annealed heat treatment condition (Ref 32). Repair of those structures may require CS coatings produced with a similar chemistry and microstructure for some applications. A study of this nature can benefit efforts towards this direction by demonstrating the feasibility of microstructural control in the CS coatings via powder heat treatment. Additionally, powder heat treatment can potentially improve the deposition behavior of powder particles which may enable cold spray additive manufacturing or facilitate the production of cost-effective coatings (i.e., via nitrogen process gas utilization). Finally, this study can also eliminate the requirement for post-deposition heat treatment, especially on large manufactured parts where secondary processing may be impractical.
Materials and Methods
Powder Processing
Commercially available Ti-6Al-4V plasma-atomized powder (AP&C, Boisbriand, Quebec, Canada) was employed in this study. Hereafter, this material will be referred as as-received (AR) powder. Additionally, AR powder was subjected to annealing heat treatment at 540 °C, 750 °C, and 950 °C, for 5 h, 2 h, and 2.5 h, respectively. Hereafter, 540 °C, 750 °C, and 950 °C heat treated powders will be referred as HT-1, HT-2, and HT-3 powders, respectively. For all the heat treatments, the powder was allowed to cool in the furnace after their respective soak times. Heat treatment of the HT-1 powder was conducted in argon atmosphere using a retort furnace (Vulcan 3-1750, ESP Chemicals Inc., Tucson, AZ) situated in a glove box (Inert Pure LabHE, Inert Technology, Amesbury, MA) with oxygen level less than 50 ppm. However, for heat treating HT-2 and HT-3 powders, a different heat treatment setup was designed to prevent oxygen diffusion in the powders. In this regard, Bhattiprolu et al. (Ref 33) have demonstrated that heat treatment in an inert atmosphere (argon) with close oxygen control is not effective in preventing oxygen diffusion in the powder. As such, this heat treatment procedure comprised (i) a high volumetric flow of argon gas through a tube furnace, (ii) employment of additional degassing procedures (at 200 °C for 0.5 h) prior to heat treatment, and (iii) utilization of titanium turnings as ‘oxygen getter.’ Using this method, the detrimental effects of oxygen were reduced significantly. For both heat treatments, a rotating tube furnace (ST-1200 °C, SentroTech, Strongsville, OH) under the argon environment was utilized to reduce the amount of powder sintering. A stainless steel cylindrical chamber (Fig. 1a) fitted with air-tight end seals, with provisions for argon gas flow in the chamber, was filled with roughly 500–750 g of Ti-6Al-4V plasma-atomized powder. This was employed to reduce the risk of contamination during repeated heat treatments. In the case of HT-3 powder heat treatment, fins were added to the cylindrical chamber to further limit powder sintering (Fig. 1b). This addition prevented particles from simply sliding in the vessel while remaining at the bottom throughout the heat treatment. As such, the particles were carried along the sidewall to result in a tumbling action of powder throughout the heat treatment. Post-heat treatment sintering was observed; however, the degree of sintering was lower for the HT-3 powder (Fig. 1). Subsequently, the sintered powder was broken using sieving, and a mortar and pestle, for 10–15 min. Due to lower sintering, HT-3 powder broke easily compared to HT-2 powder.
In general, the heat treatment temperature and time combinations for HT-1 and HT-2 powder were selected to represent stress relieving and mill annealing heat treatment of Ti-6Al-4V. Alternately, a temperature close to β transus (~ 980 °C) and time of 2.5 h was selected for HT-3 powder to allow coarsening of α grains, while limiting the level of oxygen diffusion and powder sintering.
Cold Spray Processing
Cold spray deposition was performed using AR, HT-1, HT-2, and HT-3 powders on grit-blasted Ti-6Al-4V substrates purchased in mill-annealed condition (McMaster-Carr), using a VRC Gen III Max high-pressure cold spray system (VRC Metal Systems, Rapid City, SD). Here, grit blasting was performed to encourage the adhesion of the initial cold spray layer to the substrate via mechanical interlocking. All depositions were carried out using helium carrier gas with a gas pressure of 3.79 MPa and gas temperature of 400 °C. A polybenzimidazole (PBI) nozzle with an expansion ratio 8 and a length of 170 mm was utilized for all the depositions. Additionally, a standoff distance of 25 mm, travel speed of 200 mm/s, powder feed rate of 5.7 g/min, and a deposition angle of 90° were utilized during the deposition process.
Microstructure Characterization
Scanning electron microscopy (SEM) (Supra 40VP, Zeiss, Oberkochen, Germany) was used to characterize the morphology and microstructure of AR, HT-1, HT-2, and HT-3 powder cross-sections as well as the cross-sectional microstructure of the corresponding cold sprayed depositions. The depositions were also characterized using optical microscopy. In addition to secondary electron imaging, SEM analysis was performed using backscattered electron (BSE) imaging mode to characterize the powder and coating cross-sections in the unetched condition. SEM powder morphology analysis was carried out by adhering the as-received powders on carbon tape. Powder and cold spray coating cross-sectional analysis was performed by mounting the samples (cold spray coating in the raster direction) in Bakelite and subsequently subjecting the specimens to metallographic grinding/polishing using a SiC paper, diamond, and alumina suspensions. Final chemical/mechanical polishing was conducted using a mixture of colloidal silica (0.08 μm) and 30% concentrated H2O2. The etching was performed on the polished samples using 2% Kroll’s reagent. For secondary electron and BSE imaging, an electron energy of 10 keV and 15 keV was employed, respectively.
Finally, the crystal structure of the AR, HT-1, HT-2, and HT-3 powders was characterized by X-Ray diffraction (XRD) using a powder diffractometer (Ultima Plus, Rigaku, The Woodlands, TX) equipped with a CuKα X-Ray source. 2θ values were varied between 20° and 85° with a step size of 0.02° and a counting time of 1 s per step.
Nanoindentation
Nanohardness testing was conducted on AR, heat treated powders, and their corresponding coatings using a nanoindenter (MTS Nanoindenter XP, Keysight Technologies Inc, Santa Rosa, CA, USA) under load control with maximum loads of 5 mN and 10 mN for powder and coatings, respectively. These loads were selected to limit the influence of substrate compliance and resulted in a maximum penetration depth of ≤ 500 nm. Moreover, elastic modulus and hardness were recorded as a function of indention depth. For each powder type, nanohardness measurements were performed on at least eight powder particles of various sizes. For coatings, an array of 100 indents were conducted over an area of 10,000 µm2. The Oliver–Pharr method was used for evaluating nanohardness as well as elastic modulus (Ref 34). From the obtained nanohardness and elastic modulus values, only moduli between 90 and 120 GPa for powders were utilized for calculation of average and standard deviations. The above moduli were selected based on three factors: (1) the elastic modulus of Ti-6Al-4V (~ 110 GPa), (2) to avoid the influence of underlying mounting material, and (3) to avoid the influence of surface roughness at low depths.
Porosity
The porosity of the cold sprayed coatings was evaluated using ASTM standard E2109 (Ref 35). 20 optical micrographs were collected using a magnification of 500X. To obtain representative porosity measurements, micrographs were obtained throughout the length and thickness of the coating. Subsequently, porosity measurements were obtained using image analysis (Image J, National Institutes of Health (NIH)) and were reported as the percent porosity by area.
Microhardness Testing
Microhardness measurements were conducted using a Vickers microhardness testing system (MicroMet 4, Buehler ltd., Lake Bluff, IL, USA). All measurements were performed using a load of 100 gf and dwell time of 15 s. 18–20 measurements were performed along the longitudinal direction of the coating (along the raster direction) and also through the coating thickness. Indentation diagonals were measured using an optical microscope at a magnification of 500X.
Results
Powder Characteristics
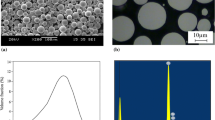
Figure 2 shows SEM micrographs and laser diffraction analysis revealing the morphology and particle sizes of the powders before and after various heat treatments. From (Fig. 2i–l), the powders have a wide distribution of particle sizes ranging between < 5 μm and over 100 μm. The average powder particle sizes for AR, HT-1, HT-2, and HT-3 powders were 33 ± 12 μm, 29 ± 9 μm, 51 ± 30 μm, and 42 ± 13 μm, respectively. Interestingly, the HT-2 powder exhibited a bimodal size distribution with both small (~ 40 μm)- and large (~ 100 μm)-sized particles present. Roughly, 25% of the HT-2 powder particles had sizes > 60 μm with ~ 3% of particles exceeding 115 μm. The large particle size resulted due to the utilization of broken sintered powder for analysis. Here, the degree of sintering was the highest post HT-2 powder heat treatment due to heat treatment being conducted in a vessel without fins (see Section Powder Processing). With these observations in mind, the fraction of particles within the size range of 20–45 μm (typical cold spray deposition range) in AR, HT-1, HT-2, and HT-3 powders were 70%, 80%, 55%, and 28%, respectively. It is important to note that the HT-3 powder had the lowest proportion of small/medium-sized powder particles. The observed changes in the size distribution and average particle size of the powders were the outcome of the (a) degree of sintering and (b) extent of mechanical processing after powder heat treatment.
(a–d) Low magnification SEM images, (e–h) high magnification SEM images, and (i–l) powder particle size analysis of (a, e, i) AR, (b, f, j) HT-1, (c, g, k) HT-2, and (d, h, l) HT-3 powders. The black arrow in Fig. (e) represents cellular surface grain structure and represented more clearly as an inset in the figure. The yellow arrow and white arrow in Fig. (f) represent featureless and martensitic α surface grain structures. The martensitic α surface grain is represented more clearly as an inset in Fig (f) (Color figure online)
From inspection of (Fig. 2a–h), all powders exhibit a spherical morphology. The AR and HT-1 powders (Fig. 2(e, f)) also showed a variety of structures on the powder surface: (i) fine need-like surface features associated with martensitic α titanium (white arrow in (Fig. 2f) and more clearly as an inset in the figure), (ii) cellular structures (black arrow in Fig. 2e and more clearly as an inset in the figure), and (iii) featureless regions (yellow arrow in Fig. 2f). These structures are the result of variations in undercooling, solidification rate, and cooling rate that occurred during the plasma atomization process (Ref 20). Alternately, the HT-2 and HT-3 powders (Fig. 2g, h) exhibited a rough surface morphology. Here, the fine surface grain features present on the as-received powder were removed due to particle–particle collision and mechanical abrasion associated with heat treating in the rotating tube furnace. In addition, craters of various sizes were also observed on the surface of the HT-2 and HT-3 powders (Fig. 2g, h). Crater formation was likely due to weak bonding of the particles (sintering) and subsequent detachment during sieving or mechanical processing after heat treatment. Finally, some satellite particles in AR and HT-1 powder (Fig. 2e, f) and small sintered particles of size ~ 10–15 μm in HT-2 and HT-3 powder (Fig. 2g, h) can be observed clearly.
Powder Microstructure
Characterization Using XRD Analysis
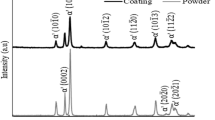
Figure 3(a, b) shows the XRD patterns for the AR and heat treated powders. The patterns showed peaks for HCP titanium (α’ phase) but no distinct peaks for BCC titanium (β phase) owing to its low volume fraction (Fig. 3a). However, the small peaks occurring at 2-θ angles 40.18° and 40.20° in HT-3 and HT-2 powder may be attributed to the BCC plane (110) (pointed out by arrows in Fig. 3b) (Ref 36). From the same figure, similar peaks and shoulders were observed in HT-3 and HT-2 powders at 2-θ angles 38.20°, 35.14°, and 38.24°, 35.12°, respectively. As such, it is likely that the powders may comprise a mixed microstructure representing both α and martensitic α microstructure. Regarding this, peak splitting at temperatures > 500 °C had been associated with α’\(\to\) α + β phase transformation (Ref 36). Alternately, the above characteristics were absent in AR and HT-1 powders. Additionally, peak broadening was reduced (qualitatively) in heat treated powders due to recovery of dislocations and associated increase in crystal size post heat treatment.
Characterization Using SEM Imaging
Figure 4 shows low and high magnification SEM micrographs of AR and heat treated powders. The AR powder was characterized by a largely martensitic α (α’) microstructure throughout the particle (Fig. 4a and more clearly from Fig. 4b). This figure also portrays twins within a single α’ grain (pointed out by a white arrow in Fig. 4b). Similar to the AR powder, the HT-1 powder (Fig. 4c, d) was also characterized by a microstructure with α’ grain morphology. From inspection of Fig. 4(e, f), the HT-2 powder is characterized by acicular α grains, that are coarse compared to α’, with fine β phase precipitates interspersed in the powder particle. Alternately, HT-3 powder (see Fig. 4g, h) was characterized by coarse β phase precipitates and large α grains (much coarser than HT-2 powder). In addition, from Fig. 4(h), β phase precipitates are positioned along the α phase grain boundaries and at times reach sizes greater than (qualitatively) the α grains observed in HT-2 powder.
Low magnification SEM cross-sectional micrographs of (a) AR, (c) HT-1, (e) HT-2, and (g) HT-3 powders. High magnifications of the same are represented in (b) AR, (d) HT-1, (f) HT-2, and (h) HT-3. The white arrow in Fig (b) represents twins in AR powder. The black and yellow arrows in Fig (f) and Fig (h) represent α and β phases. All the specimens were etched using 2% Kroll’s reagent (Color figure online)
Figure 5 shows high magnification SEM micrographs of HT-2 powders. It is interesting to note two different microstructures that resulted for the same heat treatment. From Fig. 5, the HT-2 powders comprised the following microstructures: (i) needle-like α (NA) grains similar to α’ grain morphology (represented by a yellow arrow in Fig. 5(a)) and (ii) acicular α (AA) grains (black arrow in Fig. 5b) with fine β precipitates (white arrow in Fig. 5(b)).
High magnification SEM micrographs of HT-3 powders are presented in Fig. 6. Again, a wide variety of microstructures are observed in the heat treated powder: (i) NA grains (yellow arrow in Fig. 6(a)), (ii) AA grains (black arrow in Fig. 6(b)) with fine β precipitates (white arrow in Fig. 6(b)), and (iii) plate-like α (PA) grains (red arrow in Fig. 6(c)) with coarse β precipitates (double-sided white arrow in Fig. 6(c)). Note, powder particles with NA grains were not only observed in HT-3 powder but also in HT-2 and HT-1 powders. Also, when comparing Fig. 5(b) and Fig. 6(b) it can be observed that the microstructure characteristics of the HT-3 powder particle (Fig. 6(b)) overlapped those depicted for the HT-2 powder (Fig. 5(b)).
(a–c) High magnification SEM cross-sectional micrographs of HT-3 powders. The yellow arrow, black arrow, and red arrow represent needle-like α, acicular α, and plate-like α, respectively, in (a–c). The white arrow in (b) and double-sided white arrow in (c) depict fine and coarse β phase precipitates (Color figure online)
Powder Mechanical Properties
The nanohardness of the AR and heat treated powders as a function of powder processing condition is provided in Fig. 7(a). Low temperature heat treatment (HT-1 and HT-2) resulted in an increase in hardness compared to the AR powder, however, higher temperature heat treatment (HT-3) resulted in similar hardness to AR powder. The average nanohardness values for AR, HT-1, HT-2, and HT-3, powders were measured to be 4.4 ± 0.9 GPa, 5.6 ± 1.1 GPa, 5.4 ± 0.6 GPa, and 4.4 ± 0.9 GPa, respectively.
Figure 7(b, c) shows backscatter electron (BSE) micrographs of two particles that were subjected to the HT-3 treatment. These particles clearly exhibited a different microstructure and corresponding variation in hardness (reported in upper right corner). In addition, vanadium-rich beta phase regions are clearly observed in Fig. 7(b, c) and exhibit a bright contrast due to atomic number BSE contrast. Moreover, when the dual-phase (α + β) microstructure is coarse (Fig. 7b), the hardness is much lower than when fine α + β microstructure is present (due to Hall–Petch strengthening). Thus, it is expected that a coarse α + β microstructure would be preferred to improve particle deformation during cold spray processing and reduce the required critical velocity (Ref 37).
CS coating Microstructure
Characterization Using Optical Microscopy
Low magnification optical micrographs of CS coatings produced using AR, HT-1, HT-2, and HT-3 powders are presented in Fig. 8. Hereafter, these coatings will be referred as AR, HT-1, HT-2, and HT-3 coatings. All depositions demonstrated some bond line defects although metallurgical bonding to the substrate was observed in several regions. Also, the AR, HT-1, and HT-3 coatings exhibited limited porosity characteristic of high-quality cold spray depositions. Further, coatings produced by AR and HT-3 powder show similar levels of porosity (Fig. 8a and d). The CS coating produced by HT-2 powder, on the other hand, exhibited comparatively higher porosity and will be discussed in Section Porosity.
Characterization Using SEM Imaging
Figure 9 shows low and high magnification SEM cross-sectional micrographs of AR, HT-1, HT-2, and HT-3 coatings. As seen from Fig. 9(a, c, e, and g), all coatings are dense with some triple junction (yellow arrow) and inter-particle (black arrow) porosity. These defects are typical for the cold spray process and result due to insufficient localized plastic deformation for complete consolidation (Ref 20). In addition, all coatings exhibit residual abrasive media from the surface preparation grit blasting procedure, located at the coating–substrate interface (white arrow). Although not ideal, this is a frequent occurrence for specimens prepared using grit blasting.
Low magnification SEM cross-sectional micrographs of (a) AR, (c) HT-1, (e) HT-2, and (g) HT-3 coatings. High magnifications of the same are represented in (b) AR, (d) HT-1, (f) HT-2, and (h) HT-3. All the specimens were etched using 2% Kroll’s reagent. The yellow arrow, black arrow, and white arrow in Fig. (a, c, and e), represent triple junction porosity, particle–particle porosity, and embedded grit, respectively. The double-sided black arrows in Fig. (b and d) represent featureless microstructure (Color figure online)
Figure 9(b, d, f, and h) portray individual CS particles within the CS coating. AR and HT-1 CS particles are characterized by α’ in regions exhibiting low plastic strain and featureless microstructure in severely deformed regions (double-sided black arrows) as seen from Fig. 9(b and d). Note, AR and HT-1 powders were characterized by α’ microstructure throughout the particle. Thus, the AR powder microstructure was largely preserved in the CS coatings. From inspection of Fig. 9(f), the HT-2 CS particle is characterized by AA grains with fine β phase precipitates interspersed throughout the CS particle. Fig. 9(h) demonstrates that the HT-3 CS particle comprises PA grains with coarse intergranular β phase precipitates.
High magnification SEM cross-sectional micrographs of HT-2 CS particles from regions subjected to limited plastic strain and from a region near particle–particle boundary are presented in Fig. 10. The particles were characterized by the following two microstructures away from the particle boundary: (i) NA grains (yellow arrow in Fig. 10(a)) and (ii) AA grains (black arrow in Fig. 10b) with fine β precipitates (white arrow in Fig. 10(b)). Note, the above microstructural characteristics are also observed for the HT-2 powder as shown in Fig. 5(a, b). Again, this demonstrates that the as-processed powder microstructure was retained in the CS coating. This is especially important because the as-processed powder was characterized by multiple microstructures suggesting that effective CS coating microstructure control is possible. Further, near the particle boundary (represented by dashed black line in Fig. 10(c)) β precipitate elongation was clearly observed (red arrow) along with the presence of an AA grain (pointed out by the white arrow).
High magnification SEM cross-sectional micrographs of HT-2 CS particles from regions subjected to (a, b) limited plastic strain and (c) from a region near particle–particle interface. The yellow arrow and black arrow represent needle-like α and acicular α grains in (a, b). The white arrow in (b) depicts a fine β phase precipitate. The red arrow in (c) represent elongated β phase precipitates. The white arrow in (c) points to an acicular α grain. The particle boundary is represented by a dashed black line (Color figure online)
Figure 11 depicts the high magnification SEM cross-sectional micrographs of HT-3 CS particles collected from regions subjected to limited plastic strain. HT-3 CS particles were characterized by three microstructures: (i) NA grains (yellow arrow in Fig. 11a), (ii) AA grains (black arrow in Fig. 11(b)) with fine β phase precipitates (white arrow in Fig. 11b), and (iii) PA grains (red arrow in Fig. 11(c)) with coarse β precipitates (double-sided white arrow in Fig. 11c). These microstructures are similar to the HT-3 powder microstructures (Fig. 6a–c) confirming as-processed powder microstructure is conserved in the coatings.
(a–c) High magnification SEM cross-sectional micrographs of HT-3 CS particles from regions subjected to limited plastic strain. The yellow arrow, black arrow, and red arrow represent needle-like α, acicular α, and plate-like α, respectively, in (a–c). The white and double-sided white arrows in (b–c) depict fine and coarse β phase precipitates (Color figure online)
Figure 12 shows high magnification SEM cross-sectional micrographs of HT-3 CS particles collected from regions near particle–particle boundaries (represented by dashed yellow lines). From inspection, two microstructural features can be observed: (i) elongated β phase precipitates (yellow arrows in Fig. 12a, b) and (ii) elongated α grains (black arrow in Fig. 12b).
(a, b) High magnification SEM cross-sectional micrographs of HT-3 CS particles from regions near particle–particle interface. The yellow arrows in (a, b) represent elongated β phase precipitates. The black arrow in Fig. 12b represents elongated α grain (Color figure online)
Properties of CS Coatings Deposited by AR and Heat Treated Powders
Fig. 13(a, b) provides the porosity and microhardness of the AR, HT-1, HT-2, and HT-3 coatings. Average porosity of the AR, HT-1, HT-2, and HT-3 coatings were 0.25%, 0.54%, 3.27%, and 0.43%, respectively. With the exception of the HT-2 coating, all coatings had similar porosity, ≤ 0.5% (Fig. 13a). The low porosity of the AR coating and the slight changes in the porosity between AR and heat treated powder coatings (i.e., HT-1 and HT-3) arise due to the high powder velocities employed in the deposition (Fig. 13a). In this regard, the velocity of plasma-atomized Ti-6Al-4V was measured to be ~ 900 m/s from a previous study conducted by the authors (Ref 20). This value is in line with the critical velocity (velocity required for adhesion of powder particles on the substrate) typically reported for Ti-6Al-4V powders during CS processing ( 960 ± 200 m/s (Ref 24)). Therefore, it is not surprising that AR, HT-1, and HT-3 coatings result in a dense deposit. Incidentally, the microhardness of the AR, HT-1, HT-2, and HT-3 coatings was 415 ± 15 HV0.1, 390 ± 22 HV0.1, 368 ± 43 HV0.1, and 385 ± 15 HV0.1, respectively (Fig. 13b). The HT-2 coating exhibited the lowest microhardness and largest hardness variation (Fig. 13b). HT-1 and HT-3 coatings had similar hardness values. On the other hand, HT-3 coating had slightly lower hardness when compared to the AR coating. Overall, the hardness of coatings produced using heat treated powders was lower than the AR coating.
Nanoindentation heat maps overlaid on BSE micrographs of the CS coatings produced using AR and heat treated powders are shown in Fig. 14. From Fig. 14(a), it can be observed that the hardness for the AR coating varies throughout the indented region and some areas near particle boundaries possess high nanohardness (~ 6–8 GPa). These regions typically experience severe plastic strain. Accordingly, for regions that experienced limited plastic strain (portions of the particle that exhibit less flattening) in the AR coating, low nanohardness (~ 0–3 GPa) was observed (Fig. 14a). This low nanohardness was also seen for regions near pores (Fig. 14a). Finally, the AR coating nanohardness values varied between 3.5 and 5 GPa for a major portion of the indented region (Fig. 14a).
From inspection of Fig. 14(b), the HT-1 coating exhibits the same general behavior observed in the AR coating. In addition, individual powder particles within the coating were characterized by higher overall hardness compared to AR coating (Fig. 14a). On the other hand, the average microhardness (Fig. 13b) of HT-1 coating was lower due to the strong influence of porosity.
The important influence of the powder microstructure modified by the heat treatment on the mechanical properties was evident in HT-2 (Fig. 14(c)) and HT-3 (Fig. 14d) coatings. Here, the majority of the HT-2 coating exhibited nanohardness values higher than HT-1 and AR coatings. Moreover, low hardness near porous regions can be observed clearly. Correspondingly, this influence of porosity was also observed for HT-3 coating (Fig. 14d). Interestingly, some regions that experienced significant particle flattening, or deformation (black arrows in Fig. 14(d)), exhibited a relatively low hardness (~ 2–3 GPa). These regions were characterized by PA grains. In general, the majority of the coating (~ 60%) had hardness values between 3.5 and 5 GPa, similar to AR coating. A small area (Fig. 14d) exhibited high nanohardness and a dark BSE contrast. The lower elevation of the particle resulted in a dark BSE contrast and a variation in the projected area calculation (Ref 34) during nanoindentation.
Discussion
AR Powder Morphology and Microstructure
AR powder was spherical in morphology comprising α’ grains within the particle and various structures on the surface. The spherical morphology results due to rapid solidification (cooling rate of 100–1000 °C/s (Ref 38)) of liquid Ti-6Al-4V droplets dispersed by a plasma jet during the atomization process. Again, due to the high cooling rates, diffusion-less transformation of α from beyond the β transus of Ti-6Al-4V (~ 980 °C (Ref 39)) occurs resulting in α’ grains within the particle and at times on the surface. Cooling rates greater than 410 °C/s from the β phase region (≥ 980 °C) are known to result in α’ grains (Ref 39). Further, due to the high cooling rates, dislocation generation and migration occur in the crystal lattice to accommodate the invariant strain associated with such a transformation. A crystal lattice with enhanced dislocation density, owing to its non-uniform stress state, can partly contribute to XRD peak broadening as shown in Fig. 3. While the defects (enhanced dislocation density) are typically imperceptible via SEM imaging, in some cases they are visible within the microstructure as twins (see Fig. 4b). Similar microstructures (α’ grains comprising twins) have been reported after rapid cooling, both in bulk material (Ref 39) and in powder form (Ref 25). Along with enhanced dislocation density, the high cooling rates also result in a super saturated solid solution of Ti-6Al-4V resulting in a peak shift of the AR XRD pattern (Fig. 3a, b).
Heat Treated Powder Morphology and Microstructure
Microstructure characterization revealed that effective microstructural control is possible with powder heat treatment. In addition, for high temperature heat treatments (HT-2 and HT-3), considerable particle-to-particle microstructure variability was observed after heat treatment.
Sintering was observed in HT-2 and HT-3 powders (See Fig. 1). Regarding this, sintering of titanium is known to occur at temperatures > 700 °C and hold times of 1 h (Ref 40). Further, this can take place readily in small spherical powder particles due to the high surface energy associated with the morphology. Here, the initial bond is created where the curvature gradient is maximum (Ref 40). This can result in necking features between sintered particles as seen in Fig. 2(h). Finally, sintering partly contributed to the increase in average particle size of HT-2 powder (heat treated at 750 °C) (see Fig. 2(k)).
In this study, the AR powder was heat treated to replace the α’ microstructure with a two-phase microstructure comprising coarse α and β grains. When heat treating at temperatures greater than 400 °C, α’ dissociates to α and β with the α phase nucleating along α’ grains. The β precipitates that do form are typically 100–200 nm in size at these low temperatures (\(\le\) 550 °C) (Ref 41). Thus, although β precipitates may be present in the HT-1 powder (Fig. 4(d)), they are nevertheless imperceptible using SEM imaging. Alternately, heat treating at moderate or high temperatures (700–950 °C) results in perceptible α and β grains (Fig. 4(f and h)). Specifically, significant β phase precipitation occurs at temperatures ≥ 700 °C, resulting in a microstructure comprising fine α and β phases (Ref 42). The precipitates coarsen when heated between 750 °C and 950 °C, owing to the low equilibrium volume fraction of β phase at these temperatures (30–35% at 800 °C (Ref 43)). Once the temperature reaches 950 °C, the low equilibrium volume fraction of α (~ 23% (Ref 43)) and increased atomic mobility promote significant α phase coarsening (Ref 42) during the 2.5 h soaking time. Due to the above reasons, the highest coarsening was observed in HT-3 powder (Fig. 4(h) and 6(c)) resulting in PA grains and coarse β precipitates. Finally, during furnace cooling, β transforms to α (often referred to as transformed β) and β. It is unclear whether the AA grains observed in Figs. 4(f), 5, and 6 for HT-2 and HT-3 powders are primary α or transformed β.
HT-2 and HT-3 powders exhibited microstructural characteristics that are typical for heat treatment temperatures of 750 °C and 950 °C. However, the microstructure was not uniform for all the powder particles subjected to the same heat treatment. Here, it is important to consider that the powder was heat treated in a rotating tube furnace at a speed of 10 rpm and the particles sintered after heat treatment suggesting limited mixing of powders (see Fig. 1). In addition, owing to the cold argon gas flow in our experimental setup, powder particles situated in the top layers of the powder mass experienced lower temperatures during heat treatment compared to those located in the center of the mass. Accordingly, it may also be expected that the cooling rates would vary by position during furnace cooling. Thus, it is reasonable to assume that certain regions in the powder pool were subjected to lower temperatures throughout the heat treatment cycle. Finally, due to the variations in the temperatures of the particles during heat treatment, the microstructure of particles varied within each powder type. This microstructural heterogeneity (between powder particles) in the HT-2 and HT-3 powders was also confirmed through peak splitting in the XRD peaks \((10\overline{1 }0)\) and (0002) for α’ phase (see Fig. 3).
Powder Mechanical Properties
Mechanical testing of AR and heat treated powders demonstrated that effective control of mechanical properties is possible with control of powder microstructure through powder heat treatment. Nanoindentation is a valuable tool for probing the mechanical properties of small micro-scale powder particles as a function of the developed microstructure. The high hardness of HT-1 powder (versus AR powder) was due to the heat treatment (540 °C, 5 h) chosen for this study, which is a commonly used aging heat treatment for increasing the strength and ductility of solution-treated materials (Ref 44). Consequently, applying HT-1 heat treatment likely resulted in the precipitation of fine β phase dispersoids (although undetectable in SEM micrographs) around α’ and precipitate strengthening of the HT-1 powder. Accordingly, the high hardness in HT-2 powder can be attributed to precipitate strengthening, however, with detectable β precipitates around α’. Additionally, the presence of AA grains restricts the mobility of dislocations during nanoindentation by grain boundary strengthening. In contrast to HT-1 and HT-2 powders, the nanohardness for the HT-3 powder was very similar to the AR powder hardness due to the heterogeneous microstructure developed in this powder material. Here, HT-3 powder comprised particles with both PA grains with coarse β precipitates and AA grains with fine β precipitates. As mentioned above, AA grains with fine β precipitates (similar to HT-2 powder microstructure) may be harder than α’, due to precipitate strengthening in addition to solid solution strengthening, and grain boundary strengthening which are also characteristic for the α’ microstructure. Alternatively, PA grains are expected to be softer than α’ due to the absence of grain boundary strengthening (large grains) and solid solution strengthening. Moreover, the β phase precipitates are not small and are more ductile than the α phase due to a larger number of slip systems (BCC structure in β phase compared to HCP structure in α phase). Owing to the above reasons and the fact that nanohardness for each powder type represents an average of eight powder particles, the nanohardness values were similar. Lastly, it is important to note that both HT-2 and HT-3 powders were post-processed using a mortar and pestle to break up the sintered powder. Although this process involved very low applied force it is possible that it may have introduced some amount of cold work into the powders. Thus, it is possible that the hardness values reported for these powders represent a slight overestimate of the hardness immediately after heat treatment.
CS Coating Microstructure
Microstructure characterization of AR and heat treated CS coatings revealed that the majority of the CS coating microstructure can be controlled by controlling the powder microstructure through powder heat treatment. The microstructure of the CS coating was a function of both the powder microstructure and the CS process. The severe plastic deformation associated with the CS process was primarily concentrated in regions close to the particle–particle interface ((Ref 45)) and as such the resultant coatings exhibited inhomogeneity in the coating microstructure. Due to the high strain rate (up to 108–109/s (Ref 46, 47)) near the particle interface, adiabatic shear instability (ASI) is initiated resulting in dynamically recrystallized ultra-fine grains (UFGs) near the particle interfaces. Regarding this, ASI occurs due to the high temperature (0.8–0.9Tm (Ref 48)) associated with the high strain rates and is further enhanced due to the low thermal conductivity of titanium (Ref 45). UFGs are typically submicron (order of few hundred nm) in size and difficult to delineate in regular SEM imaging (Ref 20). Consequently, these regions were characterized by a featureless microstructure (e.g., Fig. 9(b and d)). Here, UFG formation near particle–particle interfaces has been reported for CS CP-titanium (Ref 48, 49) and Ti-6Al-4V (Ref 22, 24) previously.
Particle interiors experience limited plastic strain and retain their original microstructure. This simply demonstrates the solid-state nature of the cold spray process. Microstructure changes (e.g., recrystallization, melting, phase transformation) that are commonly observed in other thermal spray processes do not occur during cold spray because of the low process temperatures. While stagnation gas temperature is relatively high (400 °C), the particles are expected to reach temperatures of only 250–330 °C (Ref 20) and will only spend a few seconds at this temperature. Thus, while α’ decomposition can occur at temperatures as low as 400 °C, an extended soak time (> 2 h) would be required to observe changes in the structure. Furthermore, titanium and titanium alloys, in general, exhibit minimal microstructural change at stress relieving heat treatment temperatures (i.e., ~ 540–550 °C).
Powder microstructure can influence the microstructure characteristics near the particle–particle interfaces of the corresponding coatings. As observed from Figs. 9(b, d), 10(c), and 12, powder particles with a dual-phase microstructure resulted in CS particles comprising elongated α and elongated β, whereas the single-phase powder particles (α’) resulted in CS particles exhibiting featureless microstructure. Furthermore, α’ on account of its needle-like grain morphology (fine grain boundary spacing) promotes dynamic recrystallization (Ref 22, 50) resulting in a featureless microstructure near particle interfaces. Alternately, the AA and PA grains had much larger grain boundary spacing, and thus, were simply elongated along the direction of plastic flow.
CS Coating Properties
Our evaluation of the deposition quality and mechanical properties of coatings produced by heat treated powders showed that the deformation characteristics of AR powder can be effectively altered by powder heat treatment. Powder comprising PA grains with coarse precipitates may promote deformation during the CS process. Further, the mechanical properties of CS coatings can be tailored by controlling the powder microstructure through powder heat treatment.
Porosity
Porosity of the CS coatings is largely dependent on particle velocity and deformation characteristics which in turn depend on the powder microstructure and temperature. Powder particle velocities are significantly influenced by particle size, morphology, and CS processing conditions. In the current study, average particle size varied slightly between the various powder treatment methods. It is well known that small particles are easily accelerated in the gas stream and experience high particle velocities (Ref 20). Accordingly, the HT-2 powder had a higher average particle size (although the distribution is bimodal) and would therefore have a lower average particle velocity. Lower particle velocities translate to lower particle deformation (Ref 20) during deposition resulting in a porous coating. Moreover, the HT-2 and HT-3 powders were post-processed with mortar and pestle to break up the sintered powder particles. It was reported that cold spray deposition of cryomilled Al 7075 powder resulted in increased coating porosity compared to coatings produced using un-milled powder, due to insufficient deformation of the highly cold-worked cryomilled powder (Ref 51). Thus, although insignificant, post-processing of the HT-2 powder may have partially contributed to the high porosity. However, it is surprising to note that the HT-3 coating demonstrated similar levels of porosity to the AR coating and lower average porosity versus the HT-1 coating (Figs. 8 and 13(a)), even with higher average powder particle size and mortar/pestle post-processing. This may be attributed to the markedly different microstructure of the HT-3 powder when compared to AR and HT-1 powders. In this regard, it has been shown that powders comprising a dual-phase microstructure with coarse α and β phase grains demonstrate a higher degree of deformation compared to powder counterparts comprising α’ (Ref 20, 25). Here, powder particles with coarse grain microstructure typically possess low hardness and yield strengths. Accordingly, lower particle velocities are required to deposit coatings with similar levels of porosity. Overall, from the above it may be inferred that the HT-3 powder microstructure comprising PA grains with coarse β precipitates were partly responsible for the better deformation and low porosity of the HT-3 coatings.
Mechanical Properties
Microhardness testing and nanoindentation provide a first glimpse at the mechanical performance of CS coatings, whereas the former provides a macroscopic mechanical response the latter illustrates the local mechanical properties. The nanoindentation maps represented in Fig. 14 showed that nanohardness is strongly influenced by (i) inter-particle porosity and cohesive strength, (ii) the location of indents with respect to the particle interface and associated degree of local plastic strain experienced by the particle, and (iii) the microstructure of the feedstock powder. In general, regions experiencing severe plastic deformation (near particle interfaces) exhibit high hardness due to strain hardening and grain boundary strengthening, the latter arising from the UFGs present due to dynamic recrystallization (featureless microstructure in Figs. 9(b and d), and 14).
Microhardness of the coating is more representative of the inter-particle strengths and porosity of the coating (Ref 25, 52). Thus, the HT-2 coating exhibits a lower average hardness and more importantly an increased hardness variability than all other coatings (Fig. 13(b)). This is despite the higher inherent powder hardness. The increased variability can be attributed to the higher porosity in the coating. Thus, indentations made near pores resulted in low hardness and those in regions away from pores resulted in high hardness due to the high hardness associated with the microstructure of the heat treated powder and deformation induced by cold spray. Owing to the same reasons, decreased average hardness but an increased variation in the hardness values is observed for HT-1 coating. On the other hand, HT-3 coating exhibited similar porosity and comparable hardness variability to the AR coating. The HT-3 coating, however, exhibited lower average hardness compared to the AR coating. This is particularly interesting considering that the average nanohardness for the HT-3 powder after heat treatment (Figure 7) was comparable to the AR powder. Recall, after heat treatment the HT-3 powder microstructure was heterogenous, with some powders exhibiting a harder NA and AA with fine β precipitate microstructure, while others showed a softer PA with coarse β precipitate microstructure. This may suggest that the deposition efficiency of the softer powder was somewhat higher than the harder powders. Thus, the lower hardness (compared to the AR coating) for the HT-3 coating may be attributed to the coarse α + β microstructure in the feedstock powder (Figs. 4h and 6c).
In summary, high-temperature heat treatment of powders (see Section Heat Treated Powder Morphology and Microstructure) resulted in sintering and microstructural variability between powder particles for the same heat treatment. Consequently, the full impact of powder treatment was not fully realized. In the future, efforts in this direction should involve developing a heat treatment method that would limit powder sintering while maintaining uniform temperature throughout the powder. One way to accomplish this would be to simply utilize a high speed rotating tube furnace with potential to reach speeds up to 40–50 rpm or employ a fluidized bed furnace. In addition, close atmosphere control is critical as the potential for oxidation and alpha case formation is high in Ti-6Al-4V powder (Ref 33). Finally, if powder microstructure variability can be avoided and particles comprising a coarse α + β microstructure are produced via HT-3 heat treatment, then CS coatings with good quality may be produced at low particle velocities. This will allow production of cost-effective CS Ti-6Al-4V coatings (sprayed using nitrogen or nitrogen/helium mixtures).
Conclusions
Plasma-atomized Ti-6Al-4V powder was heat treated to three different temperatures (HT-1, HT-2, and HT-3) and subsequently deposited on Ti-6Al-4V substrates using a high-pressure CS system. AR and HT-1 powders were characterized by a homogeneous microstructure (between powder particles) consisting of the α’ phase. Alternately, HT-2 and HT-3 powders were characterized by a heterogeneous microstructure comprising NA, AA with fine β, and PA with coarse β grains. The heterogeneity originated from the inefficient mixing and non-uniform thermal gradient experienced by the HT-2 and HT-3 powder mass during heat treatment. In general, at low/moderate heat treatment temperatures (i.e., HT-1 and HT-2) α’ dissociated to either NA or AA and fine perceptible β precipitates. With the increase in heat treatment temperatures (HT-3), the α and β phases coarsened to PA and coarse β precipitates. In addition to altering powder microstructures, heat treatment significantly influenced the powder properties. A powder comprising PA and coarse β precipitates (HT-3 powder) possessed lower hardness compared to α’ (AR powder) due to the larger grain size and absence of solution strengthening effect. Barring the HT-2 powder coating all coatings were deposited with good deposition quality (porosity < 1%). Particles comprising PA and coarse β precipitates within HT-3 coating exhibited significant flattening suggesting softening of powder promotes deformation during CS process. Moreover, the as-processed powder microstructure was preserved in the CS coating to a large extent confirming control of CS coating microstructure is possible via powder heat treatment. Finally, powder microstructure also significantly influenced CS coating microstructure (near particle boundaries) and mechanical properties.
Our study shows that CS coating microstructure and mechanical properties can be effectively controlled via powder heat treatment. This is noteworthy as utilization of such a technique may be beneficial for repair applications where the repaired structure needs to retain the microstructure and chemistry of the base material. Additionally, heat treatment shows promise at producing powders with enhanced deformation characteristics (HT-3 powder). This will provide an opportunity at producing cost-effective CS coatings.
References
B. Dutta, F.H.S. Froes, The additive manufacturing (AM) of titanium alloys, Titanium powder metallurgyed. (Elsevier, 2015) p. 447-468
C. Widener, M. Carter, O. Ozdemir, R. Hrabe, B. Hoiland, T. Stamey, V. Champagne and T.J. Eden, Application of high-pressure cold spray for an internal bore repair of a navy valve actuator, J. Therm. Spray Technol., 2016, 25(1–2), p 193–201.
R. Jones, N. Matthews, C. Rodopoulos, K. Cairns and S. Pitt, On the use of supersonic particle deposition to restore the structural integrity of damaged aircraft structures, Int. J. Fatigue, 2011, 33(9), p 1257–1267.
V. Champagne and D. Helfritch, Critical assessment 11: structural repairs by cold spray, Mater. Sci. Technol., 2015, 31(6), p 627–634.
A. Moridi, S.M. Hassani-Gangaraj, M. Guagliano and M. Dao, Cold spray coating: review of material systems and future perspectives, Surf. Eng., 2014, 30(6), p 369–395.
M. Rokni, C. Widener, G. Crawford and M. West, An investigation into microstructure and mechanical properties of cold sprayed 7075 Al deposition, Mater. Sci. Eng., A, 2015, 625, p 19–27.
H. Assadi, H. Kreye, F. Gärtner and T. Klassen, Cold spraying–A materials perspective, Acta Mater., 2016, 116, p 382–407.
F. Gärtner, T. Stoltenhoff, J. Voyer, H. Kreye, S. Riekehr and M. Kocak, Mechanical properties of cold-sprayed and thermally sprayed copper coatings, Surf. Coat. Technol., 2006, 200(24), p 6770–6782.
P.S. Phani, D.S. Rao, S. Joshi and G. Sundararajan, Effect of process parameters and heat treatments on properties of cold sprayed copper coatings, J. Therm. Spray Technol., 2007, 16(3), p 425–434.
C. Borchers, F. Gärtner, T. Stoltenhoff and H. Kreye, Microstructural bonding features of cold sprayed face centered cubic metals, J. Appl. Phys., 2004, 96(8), p 4288–4292.
D. Goldbaum, J.M. Shockley, R.R. Chromik, A. Rezaeian, S. Yue, J.-G. Legoux and E. Irissou, The effect of deposition conditions on adhesion strength of Ti and Ti6Al4V cold spray splats, J. Therm. Spray Technol., 2012, 21(2), p 288–303.
M.V. Vidaller, A. List, F. Gaertner, T. Klassen, S. Dosta and J.M. Guilemany, Single impact bonding of cold sprayed Ti-6Al-4V powders on different substrates, J. Therm. Spray Technol., 2015, 24(4), p 644–658.
A.W.-Y. Tan, W. Sun, A. Bhowmik, J.Y. Lek, I. Marinescu, F. Li, N.W. Khun, Z. Dong and E. Liu, Effect of coating thickness on microstructure, mechanical properties and fracture behaviour of cold sprayed Ti6Al4V coatings on Ti6Al4V substrates, Surf. Coat. Technol., 2018, 349, p 303–317.
A.W.-Y. Tan, W. Sun, Y.P. Phang, M. Dai, I. Marinescu, Z. Dong and E. Liu, Effects of traverse scanning speed of spray nozzle on the microstructure and mechanical properties of cold-sprayed Ti6Al4V coatings, J. Therm. Spray Technol., 2017, 26(7), p 1484–1497.
P. Vo, E. Irissou, J.-G. Legoux and S. Yue, Mechanical and microstructural characterization of cold-sprayed Ti-6Al-4V after heat treatment, J. Therm. Spray Technol., 2013, 22(6), p 954–964.
W.Y. Li, C. Zhang, X. Guo, J. Xu, C.J. Li, H. Liao, C. Coddet and K.A. Khor, Ti and Ti-6Al-4V coatings by cold spraying and microstructure modification by heat treatment, Adv. Eng. Mater., 2007, 9(5), p 418–423.
X.-T. Luo, Y.-K. Wei, Y. Wang and C.-J. Li, Microstructure and mechanical property of Ti and Ti6Al4V prepared by an in-situ shot peening assisted cold spraying, Mater. Des., 2015, 85, p 527–533.
M. Garrido, P. Sirvent and P. Poza, Evaluation of mechanical properties of Ti6Al4V cold sprayed coatings, Surf. Eng., 2018, 34(5), p 399–406.
D. Boruah, B. Robinson, T. London, H. Wu, H. de Villiers-Lovelock, P. McNutt, M. Doré and X. Zhang, Experimental evaluation of interfacial adhesion strength of cold sprayed Ti-6Al-4V thick coatings using an adhesive-free test method, Surf. Coat. Technol., 2020, 381, p 125130.
V.S. Bhattiprolu, K.W. Johnson, O.C. Ozdemir and G.A. Crawford, Influence of feedstock powder and cold spray processing parameters on microstructure and mechanical properties of Ti-6Al-4V cold spray depositions, Surf. Coat. Technol., 2018, 335, p 1–12.
H. Zhou, C. Li, H. Yang, X. Luo, G. Yang, W. Li, T. Hussain and C. Li, Pores structure change induced by heat treatment in cold-sprayed Ti6Al4V coating, J. Therm. Spray Technol., 2019, 28(6), p 1199–1211.
V.S. Bhattiprolu, K.W. Johnson and G.A. Crawford, Influence of powder microstructure on the microstructural evolution of as-sprayed and heat treated cold-sprayed Ti-6Al-4V coatings, J. Therm. Spray Technol., 2019, 28(1–2), p 174–188.
A. Birt, V. Champagne Jr., R. Sisson Jr. and D. Apelian, Microstructural analysis of Ti–6Al–4V powder for cold gas dynamic spray applications, Adv. Powder Technol., 2015, 26(5), p 1335–1347.
A. Birt, V. Champagne, R. Sisson and D. Apelian, Microstructural analysis of cold-sprayed Ti-6Al-4V at the micro-and nano-scale, J. Therm. Spray Technol., 2015, 24(7), p 1277–1288.
V.N.V. Munagala, V. Akinyi, P. Vo and R.R. Chromik, Influence of powder morphology and microstructure on the cold spray and mechanical properties of Ti6Al4V coatings, J. Therm. Spray Technol., 2018, 27(5), p 827–842.
J.Y. Lek, A. Bhowmik, A.W.-Y. Tan, W. Sun, X. Song, W. Zhai, P.J. Buenconsejo, F. Li, E. Liu and Y.M. Lam, Understanding the microstructural evolution of cold sprayed Ti-6Al-4V coatings on Ti-6Al-4V substrates, Appl. Surf. Sci., 2018, 459, p 492–504.
D. Goldbaum, R.R. Chromik, S. Yue, E. Irissou and J.-G. Legoux, Mechanical property mapping of cold sprayed Ti splats and coatings, J. Therm. Spray Technol., 2011, 20(3), p 486–496.
D. Goldbaum, R.R. Chromik, N. Brodusch and R. Gauvin, Microstructure and mechanical properties of Ti cold-spray splats determined by electron channeling contrast imaging and nanoindentation mapping, Microsc. Microanal., 2015, 21(3), p 570.
A. Sabard, H. de Villiers Lovelock and T. Hussain, Microstructural evolution in solution heat treatment of gas-atomized Al alloy (7075) powder for cold spray, J. Thermal Spray Technol., 2018, 27(1), p 145–158.
A. Sabard, P. McNutt, H. Begg and T. Hussain, Cold spray deposition of solution heat treated, artificially aged and naturally aged Al 7075 powder, Surf. Coat. Technol., 2020, 385, p 125367.
W.A. Story and L.N. Brewer, Heat treatment of gas-atomized powders for cold spray deposition, Metall. and Mater. Trans. A., 2018, 49(2), p 446–449.
H. Chandler, Heat treater's guide: practices and procedures for nonferrous alloys, ASM Int. (1996)
V.S. Bhattiprolu and G.A. Crawford, Microstructural evolution and mechanical behavior of heat treated Ti-6Al-4V powders, Metallogr. Microstruct. Anal., 2018, 7(6), p 761–768.
W.C. Oliver and G.M. Pharr, An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments, J. Mater. Res., 1992, 7(6), p 1564–1583.
E. ASTM, 2109-01, Test Methods for Determining Area Percentage Porosity in Thermal Sprayed Coatings
F. Kaschel, R. Vijayaraghavan, A. Shmeliov, E. McCarthy, M. Canavan, P. McNally, D. Dowling, V. Nicolosi, M. Celikin, Mechanism of stress relaxation and phase transformation in additively manufactured Ti-6Al-4V via in situ high temperature XRD and TEM analyses. Acta Materialia (2020)
T. Schmidt, F. Gärtner, H. Assadi and H. Kreye, Development of a generalized parameter window for cold spray deposition, Acta Mater., 2006, 54(3), p 729–742.
M. Smagorinski and P. Tsantrizos, Production of spherical titanium powder by plasma atomization, Adv. Powder. Metall. Part. Mater., 2002, 3, p 3–248.
T. Ahmed and H. Rack, Phase transformations during cooling in α+ β titanium alloys, Mater. Sci. Eng., A, 1998, 243(1–2), p 206–211.
Z.Z. Fang, Sintering Adv. Mater. (Elsevier, 2010)
W. Xu, M. Brandt, S. Sun, J. Elambasseril, Q. Liu, K. Latham, K. Xia and M. Qian, Additive manufacturing of strong and ductile Ti–6Al–4V by selective laser melting via in situ martensite decomposition, Acta Mater., 2015, 85, p 74–84.
B. Vrancken, L. Thijs, J.-P. Kruth and J. Van Humbeeck, Heat treatment of Ti6Al4V produced by Selective Laser Melting: Microstructure and mechanical properties, J. Alloy. Compd., 2012, 541, p 177–185.
R. Pederson, O. Babushkin, F. Skystedt and R. Warren, Use of high temperature X-ray diffractometry to study phase transitions and thermal expansion properties in Ti-6Al-4V, Mater. Sci. Technol., 2003, 19(11), p 1533–1538.
J. Bray, Properties and selection: nonferrous alloys and special purpose materials, ASM Metals Handbook, 92, (1990)
C. Lee and J. Kim, Microstructure of kinetic spray coatings: a review, J. Therm. Spray Technol., 2015, 24(4), p 592–610.
M. Rokni, C. Widener and G. Crawford, Microstructural evolution of 7075 Al gas atomized powder and high-pressure cold sprayed deposition, Surf. Coat. Technol., 2014, 251, p 254–263.
X.-T. Luo, C.-X. Li, F.-L. Shang, G.-J. Yang, Y.-Y. Wang and C.-J. Li, High velocity impact induced microstructure evolution during deposition of cold spray coatings: a review, Surf. Coat. Technol., 2014, 254, p 11–20.
G. Bae, K. Kang, J.-J. Kim and C. Lee, Nanostructure formation and its effects on the mechanical properties of kinetic sprayed titanium coating, Mater. Sci. Eng., A, 2010, 527(23), p 6313–6319.
K. Kim, M. Watanabe, J. Kawakita and S. Kuroda, Grain refinement in a single titanium powder particle impacted at high velocity, Scripta Mater., 2008, 59(7), p 768–771.
F.J. Humphreys, M. Hatherly, Recrystallization and related annealing phenomena. (Elsevier, 2012)
R. Ghelichi, S. Bagherifard, D. Mac Donald, M. Brochu, H. Jahed, B. Jodoin and M. Guagliano, Fatigue strength of Al alloy cold sprayed with nanocrystalline powders, Int. J. Fatigue, 2014, 65, p 51–57.
D. Goldbaum, J. Ajaja, R.R. Chromik, W. Wong, S. Yue, E. Irissou and J.-G. Legoux, Mechanical behavior of Ti cold spray coatings determined by a multi-scale indentation method, Mater. Sci. Eng., A, 2011, 530, p 253–265.
Acknowledgments
This work was supported by US Army Research, Development and Engineering Command under grant number W15QKN-16-C0094 and in part by the U.S. Army Research Laboratories under the grant number W911NF-19-20329. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the U.S. Government.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhattiprolu, V.S., Johnson, K.W. & Crawford, G.A. Influence of Powder Heat Treatment on the Microstructure and Mechanical Properties of Cold Sprayed Ti-6Al-4V Coatings. J Therm Spray Tech 30, 2050–2068 (2021). https://doi.org/10.1007/s11666-021-01276-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-021-01276-x