Abstract
TiO2 can be integrated with antimony-doped tin oxide to obtain composite materials with high electroconductivity. Based on this, spherical TiO2@Sb–SnO2 (TSS) has been prepared by homogeneous precipitation combined with a high-temperature calcination process. The morphology, structure and composition of TSS were characterized by scanning electron microscope, Brunauer–Emmet–Teller surface area analyzer, X-ray diffractometer and transmission electron microscope with energy-dispersive X-ray spectrometer, respectively. Effects of dropping conditions, introducing sulfate, pH value, calcination temperature and holding time on the conductivity of TSS were investigated by measuring resistivity, Zeta potential and particle size, meanwhile the calcination action and conductive mechanism by thermogravimetric analysis, X-ray photoelectron spectroscopy and electron spin resonance. The results revealed that the core–shell structured TSS was formed, and the resistivity of composite powder was below 4.0 Ω cm under the optimum conditions. Our analysis indicates that the conductive channel mechanism is the main conductive mechanism of the as-prepared composite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Core–shell (CS) nanoparticles (NPs) were first reported in early 1990’s [1], which have improved optical, catalytic, electronic, magnetic properties and other physicochemical properties in various applications, for example, catalysis [2,3,4], lithium ion battery [5], soft magnetic composites [6] and targeted drug delivery [7]. In order to exhibit extraordinary or new properties, the research directions have been diversifying in different shapes [8,9,10], multi shells [5] and so on, ascribing to the synergism between core and shell materials. Therefore, the appropriate design of CS-structured materials with different properties is the primary objective of material investigators.
The reported preparation methods of CS composite powder mainly conclude mechanical aggregation method (ball-milling dispersion [11] and ultrasonic dispersion [12]) one-step hydrothermal synthesis [9], polymer-pyrolysis method [13] and matrix coating methods including co-precipitation [14,15,16], polymeric precursor method [17, 18] (Pechini route [19]), homogeneous precipitation [2, 20, 21], etc. Among these methods, powder coating technology has impacts on a great variety of composite powder [22], in which homogeneous precipitation method has been extensively employed because it can efficiently regulate the size and shape of particles for the synthesis of superfine oxides and other composite materials by slow hydrolysis of the precipitant in the reaction mixture [2].
Inorganic pigment fillers have the broad use in the production of paints, plastics, ceramics and many other applied materials to improve, enhance and endow new functions [23]. However, there are electrostatic hazards due to impact, extrusion and friction with each other of these materials, such as fire, explosion and electromagnetic interference caused by electrostatic discharge [24] as well as adsorption of dust and product bonding resulted from electrostatic attraction. Thus, research and development as well as application of conductive filler are crucial. It has been reported that the inorganic/inorganic particle-coating technology for surface modification was carried out to improve electrical conductivity of the matrix fillers, which was based on cheap mineral fillers, such as kaolinite [14], talc [15], barite [16], clay [19], wollastonite [25] and titanium dioxide (TiO2) [26]. Among them, pigment-grade TiO2 with the particle diameter of 200–300 nm, typically of rutile phase, has been widely acted as the matrix of composite powder because of its superior scattering property (compared with all other white pigments) and unique chemical durability as well as innocuity [27, 28]. Meanwhile, antimony-doped tin dioxide (ATO) is well-known transparent conductive oxide because of high conductivity, optical transparency, excellent IR blocking property, chemical and thermal stability [10, 11, 19], while the high cost and the dark gray color of ATO materials result in an undesirable appearance to the final product. Hence, with TiO2 as the core and ATO as a conductive nanoshell of the coated composite, the advantages of the two materials can be fully utilized to prepare light colored conductive inorganic composite fillers. The composite materials have a more great application prospect than traditional conductive materials including metal [29], carbon materials [30], metal oxides [31] and so on.
In the present work,we adopted homogeneous precipitation to fabricate the preliminary CS precursor that was subsequently calcined to obtain the CS-structured TiO2@Sb–SnO2 (TSS) composite conductive powder. We also have discussed the influence of main factors on the structure and resistivity of the samples based on the selection of optimal parameters, including the conditions of dropping and calcination, the kinds and amounts of sulfate as well as pH value. And the conductive mechanism of composite powder was finally investigated.
Experimental
Materials
All the chemicals used are of analytical grade without further purification, including tin(IV) chloride pentahydrate(SnCl4·5H2O), antimony trichloride(SbCl3), sodium sulfate(Na2SO4), potassium sulfate(K2SO4), ammonium sulfate((NH4)2SO4), urea(CON2H4), cetyltrimethyl ammonium bromide(CTAB, C16H33(CH3)3NBr), hydrochloride acid(HCl) and anhydrous ethanol(C2H5OH). TiO2(Jinzhou titanium industry Co. Ltd., China).
Preparation of TiO2@Sb–SnO2 composite
TiO2@Sb–SnO2 conductive nanocomposite was prepared as follows. In a typical procedure, 0.15 mol of urea and 0.16 mmol (0.075 wt%) CTAB were dissolved in 80 mL deionized water and stirred for 30 min; then 0.025 mol TiO2 was added under continuously stirred conditions. The suspension containing TiO2 (Suspension A) was ultrasound dispersed for 1 h, and then transferred to a 250 mL three-necked, round-bottomed flask. Meanwhile, 4.5 mmol (NH4)2SO4 were taken in the flask. When the temperature rise to 60 °C, 0.20 mol L−1 mixed hydrochloric acid solution of SnCl4·5H2O and SbCl3 (Solution B, n(Sn)/n(Sb) = 12) were added dropwise slowly to the above mixture by peristaltic pump. After that, the seriflux was refluxed under vigorous stirring for 6–10 h at 95 °C to pH = 5. The resulting pale yellow precipitates were washed two to three times with deionized water and anhydrous ethanol, respectively, to remove Cl− (test by AgNO3). The precursors were subsequently dried at 90 °C for 12 h and were finally calcined at 550 °C for 2 h in the air at a heating rate of 15 °C min−1 to obtain TSS composite particles. The fabrication process of conductive composite powder is shown in Fig. 1.
Sample characterization
Surface morphology and microstructure of the samples prepared were observed with a field emission scanning electron microscope (FESEM, S-4800, Hitachi Co., Japan). TEM, HRTEM and TEM elemental mapping images were obtained from a transmission electron microscope (TEM, JEM-2100F, JEOL Co., Japan) equipped with an energy-dispersive X-ray spectrometer (EDS) analyzer. The crystal phases of the synthesized samples were acquired with an X-ray diffractometer (XRD, Ultima IV, Rigaku Co., Japan) with Cu Kα radiation and 2θ ranging from 10° to 80° at a scanning rate of 0.2° s−1. Surface compositions and chemical states analysis of composite powers were examined by X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Scientific Co., USA). Electron paramagnetic resonance (ESR) studies of the uncalcined precursors and calcined samples were tested by JEOL JES-FA200 ESR spectrometer. The particle size distribution of powder and Zeta potential of diluted samples was measured on the Nano-ZS90 particle size analyzer. Thermogravimetric analysis (TGA) measurements (Pyris Diamond, Perkin-Elmer Co., USA) were carried out on the uncalcined precursors from 30 °C to 900 °C in the air atmosphere at a heating rate of 10 °C min−1. The Brunauer–Emmet–Teller (BET) specific surface area of samples was measured at 77 K using a surface area analyzer (Autosorb-iQ-MP/RX, Quantachrome, USA). The brightness of different samples was measured by CM-3600d whiteness instrument (Minolta Co., Japan).
The resistivity of the coated TiO2 powder was measured on the RTS-8 four-point probe resistivity measurement system (Guangzhou, China). Because of the powdery samples, powder density and contacts among granules have influence on the measured resistivity of samples. Therefore, the TSS powder was pressed into sheets (d < 4 mm) on 769YP-24B tablet compressing machine (Tianjin, China) with a Ф20 mm mold under 30 MPa pressure.
Results and discussion
Morphology and crystal structure of TSS powder
The SEM images of uncoated TiO2 and TSS and TEM images of TSS are displayed in Fig. 2. The uncoated TiO2 particles are quasi-spheroid with a smooth surface and a particle size of about 200–300 nm (Fig. 2a). It can be observed from Fig. 2b, c that the surface of TiO2 becomes rough, and there are numerous fine nanoparticles coated upon the outer surface of a matrix core in a dispersed state, forming a dense and continuous ATO coating layer, while the overall morphology of TiO2 core remains unchanged. Figure 2c shows that there is a difference between the shell layer and core of the composite powder, and the thickness of coating shell is roughly 15 nm. As indicated by elemental mapping analysis (Fig. 2e–h), Ti, O, Sn and Sb elements do exist and are uniformly distributed in the powder. As shown in XPS full-scanned spectra (Figure S1), the peaks designated to Sn and Sb can be observed in both the precursor and TSS composite compared with pure TiO2, which confirms that there are Sn and Sb elements on the surface of TiO2 core again. Meanwhile, the HRTEM image (Fig. 2d) of the selected area displays that the crystal plane spacing at 0.333 nm and 0.262 nm corresponds to the (110) and (101) of SnO2 (JCPDS, No. 41-1445), respectively, which further proves that the typical core–shell structured TSS has been formed successfully.
BET method was used to measure the specific surface area of uncoated TiO2 and TSS. The results are shown in Table 1, in which the specific surface area increased from 25.22 m2 g−1 of TiO2 to 48.11 m2 g−1 of TSS with a growth rate of 190%. It indicates that the new tiny interface is formed in the material with the ATO coating on the surface of TiO2, leading to its surface becomes rough, which corresponds to the SEM images.
As for the conductivity of samples, the resistivity of pure TiO2 reaches up to 45 MΩ cm and that of ATO is 1.6 Ω cm. After coating the conductive shell of ATO on TiO2 core by homogeneous precipitation method, the as-prepared TSS composite has the improved conductivity with resistivity of about 4.0 Ω cm. For another, concerning that the light colored requirement for the final product, the whiteness value (\( L^{*} \)) of three samples (TiO2, TSS and ATO) was tested by brightness tester. \( L^{*} \) represents the change from bright (\( L^{*} \) = 100) to dark (\( L^{*} \) = 0) of the different samples. The actual colors of three samples are shown in Fig. S2. The \( L^{*} \) of uncoated TiO2 is 98.34, almost pure white, while that of ATO with deep green is 49.21. The \( L^{*} \) of greyish TSS conductive composite is reduced to 86.79, which meets the whiteness demand of 70–95 for inorganic filler.
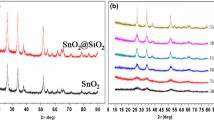
Figure 3 exhibits the XRD patterns of uncalcined precursor and TSS at different calcination temperature. As shown in Fig. 3a, b, the characteristic peaks of all samples obviously appear at 2θ in 27.4°, 36.1°, 41.2°, 54.3°, 56.3° and 68.9°, which correspond to (110), (101), (111), (211), (220) and (301) crystal planes of rutile TiO2 (JCPDS, No. 87-0920), respectively. After calcining, diffraction peaks at (110), (101), (211) and (200) are assigned well to the tetragonal crystalline phase of SnO2 (JCPDS, No. 41-1445), but diffraction peaks of related antimony compounds phases, such as Sb2O3 or Sb2O5, are not observed in any of the samples, which indicates that Sb ions is completely doped into the SnO2 with generating interstitial or substitutional solid solution [11]. However, the uncalcined precursor only has characteristic peaks of TiO2 matrix and unconspicuous diffraction peak of SnO2. The results revealed that TiO2 is coated with amorphous precipitates during the coating process. The peaks of SnO2 became sharper and more intense with the increase of calcination temperature at the same calcination time (Fig. 3b), indicating that there is the higher degree of crystallinity with the calcination.
Calcination action analysis
The first process of the preparation of TSS conductive powder was to form the precursor, whose nucleus was coated with a layer of precipitate at low temperature, in which part of Sb(III) in the shell layers was transformed into Sb(V), but the doped crystalline metal oxide was not formed [32]. Figure 4 shows TG analysis (TGA) curves of drying precursor powders under atmosphere of N2 and air, respectively. The weightlessness trends of the two curves are similar. The TGA curve under air atmosphere, for example, shows three-step weight loss. The precursor powder loses 1.79% of its mass below 130 °C, including adsorbed water. The second weight loss rate is 2.93% up to 350 °C, and there are water loss reactions, mainly referring to Sn(OH)4/SnO2·xH2O → SnO2, Sb(OH)3 → SbO(OH) → Sb2O3, and pyrolysis reactions of the residuals in the precursor [33]. The precursors continue to lose 1.3% of its mass when heated from 350 to 800 °C. Mostly Sb3+ transfer to Sb5+ between 400 and 600 °C, whereas Sb5+ is gradually reduced to Sb3+ to some extent when the temperature exceeds 600 °C, and Sb3+ is still doped in the SnO2 lattice [34]. The particles of coating shell layer have transformed into stable metallic oxide until 800 °C, meanwhile there is no phase transformation with the core remaining rutile TiO2 phase. That is the reason why the mass of samples remains unchanged above 800 °C.
Figure 5a shows that there are two fitted characteristic peaks for the Ti 2p spectrum and the splitting energy between Ti 2p3/2 and Ti 2p1/2 is 5.7 eV, which indicates that Ti exists in the valence state of Ti(IV) in the TSS [35, 36], no valence state change of Ti under the action of calcination. The Sn 3d5/2 and Sn 3d3/2 spin-orbital spectrum (Fig. 5b) of TSS composite also presents characteristic double peaks. The separation between them is 8.4 eV, which agrees well with the previously reported results [11], suggesting a normal state of Sn(IV) in the TSS composite, likewise, no chemical state variation of Sn element.
In Fig. 5c, there is the overlap between O 1s and Sb 3d5/2 lines, including the lattice oxygen species (Olat) of metallic oxides (e.g., TiO2 or SnO2) [37] and the peak assigned to Sb–O. Furthermore, the fitted oxygen vacancy peak can also be seen in the O 1s peak of TSS (Fig. 5c). It is worth noting that the indicator value of the g peak at 2.000 corresponds to oxygen vacancies in the SnO2 lattice of ATO nanoshells [38], whereas the uncalcined precursor has no ESR signal (Fig. 6). Therefore, the analysis of XPS and ESR all indicate that defect reactions occur, accompanied by generating oxygen vacancies during the calcining process of the precursor. Because of the close size of ionic radius between Sb5+ (0.62 Å) and Sb4+ (0.71 Å), Sb5+ can form Sb–O–Sn bonds in the lattice of SnO2, resulting in certain SnO2 crystal deformations [39]. The main reaction equations are as follows:
Moreover, it can be seen from the fitted peak of Sb 3d3/2 (Fig. 5d) that there are two valence states of Sb in the shell layer of TSS, and the peak area of Sb5+ is much larger than that of Sb3+, which verifies that there is the competitive reaction between Sb3+ and Sb5+, mainly Sb5+ in the ATO shell. That is the reason why the ATO-coated composite powder has conductive property. The conductive process depends on the lattice defect of the coated particles [15] and the doped Sb5+ impurity that forms the donor levels [40] in the forbidden band of SnO2 with free electron carriers being provided to the conduction band. After calcining, the color of samples varies from yellow to light gray, which is ascribed to the plasma absorption of electrons in the free conduction band [41].
Electrical conductivity of TSS and optimal process parameters
Electrical conductivity of TSS can be distinctly affected by preparation parameters. We mainly investigated the impacts of dropping conditions, introducing sulfate, pH value, calcination temperature and holding time on the resistivity of TSS to select optimal process parameters in this work. The influence of each parameter was investigated by varying it in the case of other parameters unchanged.
Effect of dropping conditions on resistivity
The integrity of CS structure and size of particles in the shell layer are affected by dropping temperature (Td) and dropping rate (vd). There is no hydrolysis of urea when the temperature is below 60 °C. The low concentration of OH− in the system under this temperature results in less nucleation in the early stage and less effective coating on the surface of TiO2, so the resistivity (ρ) of composite powder is higher while adding Solution B into Suspension A at 55 °C (Fig. 7, Td–ρ). Above 60 °C, the ρ of TSS is gradually raised, since metal cations are not firmly adsorbed on the surface of TiO2 under higher temperature, which contributes to part of Sn4+ and Sb3+ self-nucleation. In addition, the hydrolysis rate of urea increases as raising the temperature (> 60 °C), so the growth rate of crystal nuclei depends upon the hydrolysis rate of urea and the temperature. In the process, the increase of the temperature slows down the nucleation rate together with the expedited growth rate of crystal nuclei. Uniform particles in the coating layer are formed, which improves the conductivity of composite powder, when the nucleation rate is faster than the growth rate of nuclei at 60 °C.
The nucleation rate is much slower than the growth rate due to the low relative saturation of the Sn4+ and Sb3+ in the system under the condition of the slow dropping rate. As a result, the particle size of the coated layer is larger. The relative saturation of the metal cations increases rapidly at the raising rate of adding covered agent. The nucleation and growth of coated particles are not completely independent, resulting in uneven grain size and agglomeration of particles. Effective control of drop velocity can reduce or eliminate the occurrence of conglobation of coated particles in the shell layer. The spherical particles with uniform size are evenly distributed and well dispersed, forming TSS with low ρ at the dropping rate range of 0.2–0.4 mL min−1 (Fig. 7, vd–ρ), which is largely put down to the great consistency of the growth of nuclei in all directions. Moreover, considering that the required time of feeding(td) at the drip speed of 0.2 mL min−1 is much longer than that of 0.4 mL min−1 (Fig. 7, vd–td), so the optimum dropping rate is 0.4 mL min−1 under adding temperature of 60 °C.
Effect of introduction of sulfate ions on size and resistivity
In the preparation process of TSS, the moderate amounts of sulfate radicals (\( {\text{SO}}_{ 4}^{ 2- } \)) introduced can become the induction center of nucleation within a wide pH range during the whole process of homogeneous precipitation and activate the reaction precursor. Multiple sulfate radicals are connected with each other to form two or multiple nuclei [42]. The free-energy barrier of nucleation of Sn4+ and Sb3+ can be reduced by forming new nucleation complexes with \( {\text{SO}}_{ 4}^{ 2- } \). The process is illustrated as follows:
Hydroxyl and chlorine ions in the system cannot be coordinated with metal cations to form stable nucleation precursor complexes without adding sulfate inducer. There was only one nucleation outburst in the system with rapidly producing a large number of small nuclei. Therefore, the precursors formed are colloidal, which makes filtration and washing difficult. There remains a large amount of chlorine ions in the filter cake, so it gets hard after drying. The introduction of \( {\text{SO}}_{ 4}^{ 2- } \) significantly improves the above-mentioned conditions.
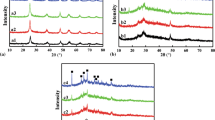
Considering that parts of sulfate, such as Al2(SO4)3 and ZnSO4, will be hydrolyzed into impurity precipitates, Na2SO4, K2SO4 and (NH4)2SO4 are selected as the alternatives of sulfate in this experiment. The best mole ratio of \( {\text{SO}}_{ 4}^{ 2- } \) to Cl− is always 25 among these three sulfates (Fig. 8). However, the potassium ion (K+) or sodium ion (Na+) introduced will destroy the photoelectric performance of the product [17, 43]. Moreover, the radius of K+ is bigger than Na+, so the destructive effects on the conductive properties of the TSS may be stronger. Therefore, the electrical conductivity of the composite powder prepared by adding K2SO4 is worse than that of not adding sulfate. As for (NH4)2SO4, increasing the quantity of ammonium ion (NH4+) can inhibit the hydrolysis of urea, and the effect of NH4+ is greater than that of \( {\text{SO}}_{ 4}^{ 2- } \) when adding a small amount or a large amount of (NH4)2SO4, making the ρ of the TSS powder as-prepared higher. Significant synergy effects between NH4+ and \( {\text{SO}}_{ 4}^{ 2- } \) may increase the conductivity of TSS under the proper amount of (NH4)2SO4. In addition, according to the Darjaguin, Landon, Verwey and Overbeek (DLVO) theory, the soluble sulfate can be used as electrostatic stabilizers, hindering the preferential aggregation of coated particles on the surface of particles [44, 45]. The electric double layer is formed with the charge on the surface of the particles increasing. There is electrostatic repulsion between particles, so as to realize a stable dispersion of particles in the reaction mixture.
What is more, suitable (NH4)2SO4 can also keep the growth of the nuclei at the same rate during in different directions, so it is easy to obtain more regular spherical-coated particles [45]. Figure 9 illustrates the particle size distribution of composite particles is narrow when the mole ratio of \( {\text{SO}}_{ 4}^{ 2- } \) to Cl− is 25. When not adding or introducing small amounts of \( {\text{SO}}_{ 4}^{ 2- } \), the size distribution range of composite powder is larger, which can be ascribed to uncertainty of central nuclear points formed by \( {\text{SO}}_{ 4}^{ 2- } \) multipoint connection. Although the kinetic salt effect becomes very weak in neutral and acidic solution, too much \( {\text{SO}}_{ 4}^{ 2- } \) may still cause a partial shedding of precipitate and coagulation of colloids to a certain extent, so that it leads to wide size distribution and high resistivity of TSS. Therefore, the best molar ratio of \( {\text{SO}}_{ 4}^{ 2- } \) to Cl− is 25 with ammonium sulfate being used as the source of sulfate radicals.
Effect of pH value on Zeta potential and resistivity
As shown in Fig. 10a, when Solution B is added dropwise to the mixture under the temperature of 60 °C [46], the pH value of the system quickly falls to 1–2. After adding Solution B completely, the temperature rises to 95 °C and is retained for 7 h. In this condition, pH maintains at 1.5–2.5. When the reaction time prolongs to 7.5–8 h, the pH alters to 5–6 and reaches to 7–8 invariably as the reaction time is further extended.
In order to maintain the same dispersion condition as the reaction system to evaluate Zeta potential of TiO2 at different pH value, 0.075 wt% CTAB, which is used as long-chain surfactant to generate weak agglomerated or mono-dispersed particles due to its steric hindrance effect [47], was added into 2.5 wt% TiO2 slurry with 4 M hydrochloric acid and 4 M ammonia solution being added to modulate the pH of the dispersion medium. As Fig. 10b shows, the absolute value of ξ at the pH range of 1.5–2.5 is greater than 30 mV [47], indicating that TiO2 has good dispersion in the aqueous solution under this condition, which is conducive to form the uniform coating on TiO2 particles. Furthermore, the starting pH for forming Sn(OH)4 is about 0.22 and the complete precipitation pH of Sn(OH)4 is approximately 2.88. The range of precipitation pH of Sb(OH)3 is from 1.60 to 4.30. Therefore, when the pH value of the system is 1.5–2.5, Sn4+ and Sb3+ ions can be basically hydrolyzed synchronously as the adsorption of hydrolysis products on TiO2 surface. Meanwhile, the ρ value of the prepared composite powder is the lowest (Fig. 10a). The ρ of composite powder increases slowly with the increase of the terminal pH value at the range of 2.5–6.5 because the absolute value of ξ is less than 30 mV, and TiO2 powder is easily agglomerated under the condition, leading to the incomplete coating of the hydrolysate. Although TiO2 powder is in a good dispersion state when pH = 4.5–6.5, Sn4+ and Sb3+ ions are almost completely precipitated, the ρ of composite powder changes little. The partial separation and dissolution of the coating layer occur under the alkaline environment [40], which is the reason why the ρ of TSS increases sharply when the terminal pH varies from approximately 6.5–8.0. Above all, the optimum to prepare precursor composite is that the mixture is stirred for 5.5 h at 95 °C maintaining the pH at 1.5–2.5 after adding the whole capping agent.
Effect of calcination conditions on resistivity
According to the results of TGA, the calcination temperature was selected from 350 to 850 °C. As shown in Fig. 2b, the crystallinity of the precursor gets higher with the rise of calcination temperature. As a consequence, the ρ of the composite powder decreases slowly with it, and it reaches to the lower value at the range of 550–600 °C (Fig. 11). Above 600 °C, the ρ of composite powder shows a slow rising trend mainly because Sb5+ begins to change into Sb3+, which corresponds to TGA, and the coating may burst at high temperature. Moreover, there is also Sb segregation occurs on the crystal surface, hindering carrier migration,
At the calcination temperature of 550 °C, the ρ of TSS decreases significantly with the extension of calcination time, whereas the ρ increases slowly when the calcination time exceeds 2.5 h. In addition, the longer calcination time make the coated particles get coarsened, causing the waste of energy. What is worse, there is little significance for the improvement of its electrical conductivity. Lower calcination temperature and shorter calcination time lead to the poor conductivity of TSS powder because only the adsorbed water is taken off the precursor. In other words, there is no transformation or the incomplete transformation of crystal structure, as well as a low concentration of Sb5+ and few doping defects in coating layer. In conclusion, the obtained TSS has excellent conductivity, with the ρ of about 4.0 Ω cm, when the as-prepared precursor is calcined at the optimum calcination temperature from 550 to 600 °C for 2.5 h.
Conductive mechanism analysis
According to the formula of composite powder resistance [40]:
where ∑Rb and ∑Rc are the self-resistance and contact resistance of conductive particles, respectively, and ∑Rg is the interface resistance in contact with the substrate. The self-resistance of the coated particles is negligible. The different contact states of particles in the shell layer correspond to three equivalent circuit diagrams, respectively, as displayed in Fig. 12.
- (I)
The conductive particles on the surface of TiO2 are discontinuous. Composite particles can be considered as an electrical insulator equivalent to the capacitor effect, which have weak capacitive conductivity.
- (II)
The coated conductive particles are not completely continuous, in which the non-contacting conductive particles form a current path due to the tunnel effect, corresponding to the resistance Rg and the capacitor Cg connected in parallel and then in series with the resistor Rc.
- (III)
The particles in the coating layer contact with each other continuously to form a current path, which is equivalent to the current flowing through a resistance Rc.
There may be three kinds of conductive mechanisms in the TSS composite conductive materials, including the tunnel effect [48], field emission [49] and conductive channel [50] mechanism. Under the condition of low content of ATO (I), the effect of field emission mechanism on the conductivity of the composite becomes significant. The distance between conductive-coated particles of the as-prepared composite is small, mainly forming chain-like conductive channels (III). The conductive channel mechanism plays a leading role. The conductive particles are distributed on the surface of the substrate, a honeycomb or network of conductive networks can be formed (Fig. 12), so the whole composite system is conductive and the purpose of conducting current and eliminating accumulated static charge can be achieved.
Conclusions
In this work, we successfully prepared the core–shell structured TSS via homogeneous precipitation with heat treatment method using urea as appropriate homogeneous precipitant. In the process, coating solution containing tin and antimony ions was added dropwise at a speed of 0.4 mL min−1 into the TiO2 matrix Suspension at 60 °C. Meantime, (NH4)2SO4 was used as nucleating agent and electrostatic stabilizers, as well as CTAB as dispersant. Following experiment began at 95 °C for 5.5 h, controlling the pH value of terminal point at 1.5–2.5. The obtained precursors were finally calcined at 550 °C for 2.5 h in the air to synthesis the composite powder with good conductivity, whose resistivity is below 4.0 Ω cm and the specific surface area has been increased from 25.22 m2 g−1 to 48.11 m2 g−1. Furthermore, the material in a shell of the precursor was calcined to form the shell of antimony-doped tin oxide, in which defect reactions occurred, and Sb was doped into the SnO2 lattice with generating free electron carriers and oxygen vacancies, while forming interstitial or substitutional solid solution. ATO particles contact with each other on the surface of TiO2 to form a conductive path. This present method provides a novel idea for low cost preparation of TiO2 or mineral-matrix light color composite conductive powder and other core–shell functional composite materials.
References
Purbia R, Paria S (2015) Yolk/shell nanoparticles: classifications, synthesis, properties, and applications. Nanoscale 7:19789–19873
Bayal N, Jeevanandam P (2012) Synthesis of CuO@NiO core-shell nanoparticles by homogeneous precipitation method. J Alloy Compd 537:232–241
Kusiora A, Zycha L, Zakrzewskab K, Radecka M (2019) Photocatalytic activity of TiO2/SnO2 nanostructures with controlled dimensionality/complexity. Appl Surf Sci 471:973–985
Jia B, Cao P, Zhang H, Wang G (2019) Mesoporous amorphous TiO2 shell-coated ZIF-8 as an efficient and recyclable catalyst for transesterification to synthesize diphenyl carbonate. J Mater Sci 54:9466–9477. https://doi.org/10.1007/s10853-019-03595-5
Yang S, Huang Y, Han G, Liu J, Cao Y (2017) Synthesis and electrochemical performance of double shell SnO2@amorphous TiO2 spheres for lithium ion battery application. Powder Technol 322:84–91
Zhang Q, Zhang W, Peng K (2019) In-situ synthesis and magnetic properties of core-shell structured Fe/Fe3O4 composites. J Magn Magn Mater 484:418–423
Zheng C, Wang Y, Phua SZF, Lim WQ, Zhao Y (2017) ZnO-DOX@ZIF-8 core–shell nanoparticles for pH-responsive drug delivery. ACS Biomater Sci Eng 3:2223–2229
Zhang S, Chen S, Yang F et al (2019) High-performance electrochromic device based on novel polyaniline nanofibers wrapped antimony-doped tin oxide/TiO2 nanorods. Org Electron 65:341–348
Li N, Li Y, Li W, Ji S, Jin P (2016) One-Step hydrothermal synthesis of TiO2@MoO3 core–shell nanomaterial: Microstructure, growth mechanism, and improved photochromic property. J Phys Chem C 120:3341–3349
Du Y, Yan J, Meng Q, Wang J, Dai H (2012) Fabrication and excellent conductive performance of antimony-doped tin oxide-coated diatomite with porous structure. Mater Chem Phys 133:907–912
Hu Y, Zhong H, Wang Y, Lu L, Yang H (2018) TiO2/antimony-doped tin oxide: Highly water-dispersed nano composites with excellent IR insulation and super-hydrophilic property. Sol Energy Mater Solar Cells 174:499–508
Zhao N, He C, Liu J et al (2014) Dependence of catalytic properties of Al/Fe2O3 thermites on morphology of Fe2O3 particles in combustion reactions. J Solid State Chem 219:67–73
Li YQ, Mei SG, Byon YJ, Wang JL, Zhang GL (2013) Highly solar radiation reflective Cr2O3–3TiO2 orange nanopigment prepared by a polymer-pyrolysis method. ACS Sustain Chem Eng 2:318–321
Hu P, Yang H, Ouyang J (2012) Synthesis and characterization of Sb-SnO2/kaolinites nanoparticles. Appl Clay Sci 55:151–157
Wang LS, Lu HF, Hong RY, Feng WG (2012) Synthesis and electrical resistivity analysis of ATO-coated talc. Powder Technol 224:124–128
Hu Y, Zhang H, Yang H (2008) Synthesis and electrical property of antimony-doped tin oxide powders with barite matrix. J Alloy Compd 453:292–297
Li X, Qian JH, Xu JS, Sun YD, Liu L (2019) Synthesis and electrical properties of antimony-doped tin oxide-coated TiO2 by polymeric precursor method. Mat Sci Semicon Proc 98:70–76
Yu H, Chen F, Ye L, Zhou H, Zhao T (2019) Enhanced photocatalytic degradation of norfloxacin under visible light by immobilized and modified In2O3/TiO2 photocatalyst facilely synthesized by a novel polymeric precursor method. J Mater Sci 54:10191–10203. https://doi.org/10.1007/s10853-019-03636-z
Sladkevich S, Kyi N, Gun J, Prikhodchenko P, Ischuk S, Lev O (2011) Antimony doped tin oxide coating of muscovite clays by the Pechini route. Thin Solid Films 520:152–158
Liu H, Zhang J, Gou J, Sun Y (2017) Preparation of Fe2O3/Al composite powders by homogeneous precipitation method. Adv Powder Technol 28:3241–3246
Montañez MK, Molina R, Moreno S (2014) Nickel catalysts obtained from hydrotalcites by coprecipitation and urea hydrolysis for hydrogen production. Int J Hydrogen Energ 39:8225–8237
Villegas M, Sierra T, Caballero AC, Fernandez JF (2007) Ti-based nanocoatings on Al2O3 powders. Ceram Int 33:875–878
Yang Z, Qi Y, Zhang J (2019) A novel perspective for reflective cooling composites: influence of the difference between the effective refractive index of polymeric matrix and inorganic functional particles. Constr Build Mater 223:928–938
Schwindt N, Pidoll U, Markus D, Klausmeyer U, Papalexandris MV, Grosshans H (2017) Measurement of electrostatic charging during pneumatic conveying of powders. J Loss Prev Proc 49:461–471
Wang C, Wang D, Yang R, Wang H (2019) Preparation and electrical properties of wollastonite coated with antimony-doped tin oxide nanoparticles. Powder Technol 342:397–403
Li X, Qian JH, Xu JS, Xing JJ, Tao E (2018) Synthesis, characterization and electrical properties of TiO2 modified with SiO2 and antimony-doped tin oxide. J Mater Sci Mater El 29:12100–12108
Heller A, Jarvis K, Coffman SS (2018) Association of type 2 diabetes with submicron titanium dioxide crystals in the pancreas. Chem Res Toxicol 31:506–509
Wang Y, Jamal R, Wang M, Yang L, Liu F, Abdiryim T (2017) A donor-acceptor-donor-type conjugated polymer-modified TiO2 with enhanced photocatalytic activity under simulated sunlight and natural sunlight. J Mater Sci 52:4820–4832. https://doi.org/10.1007/s10853-016-0717-7
Redmond PL, Hallock AJ, Brus LE (2005) Electrochemical ostwald ripening of colloidal Ag particles on conductive substrates. Nano Lett 5:131–135
Chung DDL (2001) Electromagnetic interference shielding effectiveness of carbon materials. Carbon 39:279–285
Flahaut E, Peigney A, Laurent C, Marlière C, Chastel F, Rousset A (2000) Carbon nanotube-metal-oxide nanocomposites: microstructure, electrical conductivity and mechanical properties. Acta Mater 48:3803–3812
Rockenberger J, Uz F, Tischer M, Tröger L (2000) Near edge X-ray absoption fine structure measurements and extended X-ray absorption fine structure measurements of the valence state and coordination of antimony in doped nanocrystalline SnO2. J Chem Phys 112:4292–4304
Siciliano P (2000) Preparation, characterisation and applications of thin films for gas sensors prepared by cheap chemical method. Sens Actuat B Chem 70:153–164
Sun K, Liu J, Browning ND (2002) Correlated atomic resolution microscopy and spectroscopy studies of Sn(Sb)O2 nanophase catalysts. J Catal 205:266–277
Tian BZ, Li CZ, Zhang JL (2012) One-step preparation, characterization and visible-light photocatalytic activity of Cr-doped TiO2 with anatase and rutile bicrystalline phases. Chem Eng J 191:402–409
Wan HC, Yao WT, Zhu WK, Tang Y, Ge HL, Shi XZ, Duan T (2018) Fe-N co-doped SiO2@TiO2 yolk–shell hollow nanospheres with enhanced visible light photocatalytic degradation. Appl Surf Sci 444:355–363
Li XY, Wu Y, Zhu W, Xue FQ, Qian Y, Wang CW (2016) Enhanced electrochemical oxidation of synthetic dyeing wastewater using SnO2–Sb-doped TiO2-coated granular activated carbon electrodes with high hydroxyl radical yields. Electrochim Acta 220:276–284
Ou G, Xu Y, Wen B et al (2018) Tuning defects in oxides at room temperature by lithium reduction. Nat Commun 9:1302–1310
Cui YH, Feng YJ, Liu ZQ (2009) Influence of rare earths doping on the structure and electro-catalytic performance of Ti/Sb–SnO2 electrodes. Electrochim Acta 54(21):4903–4909
Hu P, Yang H (2010) Controlled coating of antimony-doped tin oxide nanoparticles on kaolinite particles. Appl Clay Sci 48:368–374
Baena JPC, Agrios AG (2014) Transparent conducting aerogels of antimony-doped tin oxide. ACS Appl Mater Interfaces 6:19127–19134
Hek HD, Stol RJ, Bruyn PLD (1978) Hydrolysis-precipitation studies of aluminum(III) solutions. 3. The role of the sulfate ion. J Colloid Interf Sci 64:72–89
Colemana JP, Freeman JJ, Madhukar P, Wagenknecht JH (1999) Electrochromism of nanoparticulate-doped metal oxides: optical and material properties. Displays 20:145–154
Wen L, Sun X, Xiu Z, Chen S, Tsai C-T (2004) Synthesis of nanocrystalline yttria powder and fabrication of transparent YAG ceramics. J Eur Ceram Soc 24:2681–2688
Lv Y, Zhang W, Liu H, Sang Y, Qin H, Tan J, Tong L (2012) Synthesis of nano-sized and highly sinterable Nd:YAG powders by the urea homogeneous precipitation method. Powder Technol 217:140–147
Tan J, Shen L, Fu X, Hou W, Chen X (2004) Preparation of nanometer-sized (1−x)SnO2·xSb2O3 conductive pigment powders and the hydrolysis behavior of urea. Dyes Pigm 61:31–38
Huo W, Zhang X, Gan K, Chen Y, Xu J, Yang J (2019) Effect of zeta potential on properties of foamed colloidal suspension. J Eur Ceram Soc 39:574–583
Beliatis MJ, Martin NA, Leming EJ, Silva SRP, Henley SJ (2011) Laser ablation direct writing of metal nanoparticles for hydrogen and humidity sensors. Langmuir 27:1241–1244
Yanagi K, Udoguchi H, Sagitani S et al (2010) Transport mechanisms in metallic and semiconducting single-wall carbon nanotube networks. ACS Nano 4:4027–4032
Peng Q, Kalanyan B, Hoertz PG et al (2013) Solution-processed, antimony-doped tin oxide colloid films enable high-performance TiO2 photoanodes for water splitting. Nano Lett 13:1481–1488
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 21878024) and Innovation Team Project of Liaoning Province (Nos. LT2015001 and 2018479-14).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Qian, J., Xing, J. et al. Fabrication of core–shell structured TiO2@Sb–SnO2 with improved electroconductivity. J Mater Sci 55, 3871–3883 (2020). https://doi.org/10.1007/s10853-019-04229-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04229-6