Abstract
Filled-skutterudite materials are promising candidates for thermoelectric applications in the intermediate-temperature range to recover waste heat. In this work, mechanical alloying and spark plasma sintering (SPS) techniques were used to consolidate double-filling InxLa0.25Co4Sb12 (x = 0, 0.1, 0.3, 0.5) samples. X-ray diffraction, scanning electron microscopy, and energy-dispersive x-ray spectroscopy were used to analyze the microstructure of the InxLa0.25Co4Sb12 SPS-ed samples. The microstructure results revealed the main phase of the CoSb3 skutterudite structure with a small amount of InSb and CoSb2 attributed to secondary phases. Electrical resistivity remarkably decreased to 9.7 μΩ m at room temperature for the In0.5La0.25Co4Sb12 sample, mainly due to the effective impurity of the InSb nanoinclusions. Moreover, the InSb nanoinclusions yielded a substantial depression in lattice thermal conductivity due to phonon scattering. The reduction in lattice thermal conductivity was approximately 62% for the In0.5La0.25Co4Sb12 sample compared with the La0.25Co4Sb12 sample, resulting in a remarkable enhancement in the dimensionless figure-of-merit (ZT) of 1.25.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, thermoelectric (TE) applications have become a promising technology for energy harvesting.1 Waste heat can be converted into useful energy via TE generation.2 A skutterudite TE material is a good candidate for TE materials in the temperature range of 300–800 K. Furthermore, filled-skutterudite TE materials have a low lattice thermal conductivity, excellent mechanical strength, and good thermal, oxidation, and chemical stability.3 However, the binary skutterudite CoSb3 system may not be suitable for TE applications, owing to its low carrier concentration, high electrical resistivity, and high thermal conductivity.4 Generally, carrier effective mass, carrier concentration, and carrier mobility play an important role in TE characteristics. Three different approaches are used to improve skutterudite TE material: filling the voids,5,6,7 doping or substitution at antimony or cobalt sites,8,9 and nanostructuring.10,11,12
A heavy filler atom is a promising strategy to enhance the performance of skutterudite systems, owing to its high carrier concentration and low lattice thermal conductivity. In general, the atomic radius of the filler should be smaller than the void radius of skutterudite Co4Sb12 (1.892 Å)13,14 to be easily filled into the void space. Several experiments have been conducted on skutterudite materials by adding fillers, such as Ba, Bi, Ce, Hf, In, La, Li, Sr, Tl, and Yb,15,16,17,18,19,20,21,22,23,24,25 and the TE properties improved by enhancing the carrier concentration and mobility, as well as minimizing the lattice thermal conductivity. For example, Li et al.26 investigated the effect of In-filling into skutterudite material. They found that In addition increased the carrier concentration due to the supply of electrons. The thermoelectric figure-of-merit (ZT) increased to the highest value of 0.67 for In0.35Co4Sb12 at 600 K. Sesselmann et al.27 examined the effect of changing the heat treatment parameters on In-filled skutterudite. The results showed a major reduction in lattice thermal conductivity due to minor changes in the synthesis parameters. Consequently, the maximum value ZT of 0.7 for the In0.2Co4Sb12 sample at 675 K was achieved. Deng et al.28 studied InxCo4Sb12 -filled skutterudite compounds. The results showed the highest power factor of 31.3 μW/cmK2 at 616 K and the lowest thermal conductivity of 2.193 W/mK at 568 K. The enhanced dimensionless ZT was 0.88 for the In0.5Co4Sb12 sample at 665 K. Mallik et al. studied the influence of In addition into a Co4Sb12 compound. The results showed a considerable reduction in thermal conductivity probably due to the rattling of In-filling and the presence of InSb as the secondary phase at the grain boundaries. The thermoelectric ZT achieved the highest values of 1.12 and 0.96 for the In0.25Co4Sb12 and In0.4Co4Sb12 compounds, respectively.29,30 The same compound of In0.25Co4Sb12 filling skutterudite was obtained by He et al.31 who achieved a high ZT of 1.2 at 575 K. Lee et al.32 studied multi-filled skutterudite InxYbyLa0.3-x-yCo4Sb12 compounds. The results showed that the double-filling skutterudite was most effective in reducing thermal conductivity. A maximum ZT of 0.9 was obtained for the In0.1Yb0.2Co4Sb12 sample at 782 K. Khovaylo et al.33 investigated the effect of In-filled skutterudite. The results showed a substantial contribution of phonon scattering due to the InSb impurity phase that precipitated in nanometer-sized regions along the grain boundaries, and, consequently, a considerable reduction in a thermal conductivity. Thus, a maximum ZT ≈ 1.5 was recorded for the In1Co4Sb12 sample at 725 K.
The current work aims to investigate the double-filling of InxLa0.25Co4Sb12 materials to boost the carrier concentration and improve the TE transport properties. A double-filled skutterudite system was synthesized with La as the dominating filling element and In as the auxiliary filler. The In atom can successfully fill the La0.25Co4Sb12 skutterudite void because the atomic radius of In (1.55 Å) is smaller than the void radius of skutterudite Co4Sb12 (1.892 Å).13 The results show a remarkable reduction in lattice thermal conductivity for the In0.5La0.25Co4Sb12 sample compared with the La0.25Co4Sb12 sample.
Experimental
Sample Preparation and Measurements
High-purity powder of Co (99.8%), Sb (99.5%), La (99.9%), and In (99.99%) were purchased from Alfa Aesar, and all the preparation processes are discussed in the literature.34 The samples were cut into various parts for characterization. X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy-dispersive x-ray spectroscopy (EDX) measurement data were collected for the SPS-ed samples. The setup of the characterization devices is described in the literature.34,35
The temperature dependency of electrical resistivity and Seebeck coefficient was measured using a commercial instrument (ZEM-3; Ulvac-Riko), and thermal diffusivity was measured using a laser flash apparatus (TC-7000H; Ulvac-Riko). The thermal conductivity was calculated from the thermal diffusivity, D, and specific heat, Cp, using k = D Cp d, where d is the sample density. All the TE properties were measured at a temperature range between 300 and 800 K, and then the ZT value was calculated.
Results and Discussion
Microstructure Analysis of InxLa0.25Co4Sb12 (0 ≤ x ≤ 0.5) Skutterudites
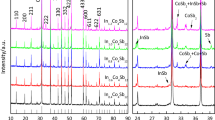
Figure 1 shows the XRD patterns of the InxLa0.25Co4Sb12 SPS-ed samples. The analysis of XRD patterns revealed the major phase of CoSb3 skutterudite for all SPS-ed samples. A small peak of impurity corresponded to CoSb2 secondary phase. The formation of CoSb2 phase was mainly due to the deficiency of the Sb atom and the short SPS sintering time. Moreover, the structural studies found a peak corresponding to the InSb nanoinclusion phase, as shown in Fig. 1. This nanoinclusion impurity began to occur when x ≥ 0.3, due to the excess In in the skutterudite structure. This result agrees with previous experimental research, which revealed that the maximum limit of In-filling into the CoSb3 cages is 1.37 at.% (x = 0.22).30,36 Furthermore, the Rietveld refinement for the InxLa0.25Co4Sb12 (0.1 ≤ x ≤ 0.5) samples demonstrated the skutterudite lattice structure obtained by using Jana software, as shown in Fig. 2. The results show the lattice parameters increased from 9.0411 Å for the La0.25Co4Sb12 sample to 9.0580 Å for the In0.3La0.25Co4Sb12 sample. Thus, the In atom successfully filled the skutterudite void because the atomic radius of In (1.55 Å) was smaller than the void radius of skutterudite Co4Sb12 (1.892 Å).13 The results of the lattice parameters and the nominal and actual compositions of the SPS-ed samples are listed in Table I.
The SEM morphologies of the InxLa0.25Co4Sb12 (0.1 ≤ x ≤ 0.5) SPS-ed samples are shown in Figs. 3a, 4, and 5(a). The samples exhibited a relatively high density of up to 99% according to the microstructures. However, pores were produced during SPS sintering, as evidenced by the surface morphology of the In0.1La0.25Co4Sb12 sample, which had the lowest density of about 95%. The SEM–EDX images showed skutterudite CoSb3 as the primary phase for all SPS-ed samples, as well as CoSb2 corresponding to the secondary phase, which match the XRD results. Moreover, the SEM–EDX results reveal that the InSb nanoinclusions were randomly distributed on the skutterudite matrix and agglomerated at the grain boundaries for the In0.5La0.25Co4Sb12 sample, as shown in Fig. 5a and d. Furthermore, the formation of the InSb nanoinclusions had a strong influence on reducing the lattice thermal conductivity due to the scattering of the charge carriers at the interfaces. Additionally, the La atoms were inhomogeneously distributed and appeared in all the samples, as shown by the elemental mapping in Figs. 3d, 4, and 5d, which may effectively scatter the phonons to further decrease the lattice thermal conductivity.37,38,39,40
Thermoelectric Properties
The temperature dependency of electrical resistivity for the InxLa0.25Co4Sb12 SPS-ed samples is shown in Fig. 6. At room temperature, the electrical resistivity rapidly decreased with increasing the In–filler fraction content, which clearly indicated that the In-filler acted as n-type to increase the electron concentration and that more electrons were donated to the conduction band. Furthermore, the electrical resistivity of the In0.1La0.25Co4Sb12 sample decreased with the increasing temperature, indicating non-degenerate semiconducting transport behavior, whereas, for the higher In content of the In0.3La0.25Co4Sb12 and In0.5La0.25Co4Sb12 samples, the electrical resistivity increased with temperature, displaying semimetal-like behavior. These results are consistent with published reports for skutterudites.41,42 The InSb phase was highly conductive,30,43 and, on reaching the solubility limit of In (x = 0.22), a substantial reduction in the electrical resistivity was achieved mainly due to the increasing volume fraction of InSb. Thus, the lowest electrical resistivity value of 9.67 μΩ m was obtained for the In0.5La0.25Co4Sb12 sample at room temperature, which was lower than that reported by He et al.31 for In0.25Co4Sb12, approximately 12 μΩ m at the same temperature due to the existence of the InSb phase with relatively high conductivity, as described above. Moreover, this reduction in electrical resistivity was attributed to the contribution of the In-filler atoms that acted as electron donor on the CoSb3 skutterudite system that increased the carrier concentration. Additionally, the addition of In above the filling fraction limit (x > 0.22) saturated the material and allowed the formation of the InSb nanoinclusion phase at the grain boundaries of the Co4Sb12 skutterudite matrix, as the results showed a considerable reduction in the electrical resistivity.
Figure 7 shows the variation of the Seebeck coefficient versus temperature difference for the InxLa0.25Co4Sb12 SPS-ed samples. Over the entire studied temperature range, all the double-filled samples showed a negative Seebeck coefficient, indicating that the n-type semiconductor behavior and the majority carriers were electrons. However, the Seebeck coefficient results of the single-filler La0.25Co4Sb12 sample revealed a positive sign (p-type) semiconducting material, and these tendencies agreed well with the literature data44 with the La fraction content form (0.1–0.5). Moreover, the In atoms acted as electron donors that led to decreasing the hole concentration of the La0.25Co4Sb12 p-type skutterudite. The electrical conductivity of the In-filled skutterudites was inversely proportional to the Seebeck coefficients. Furthermore, the absolute Seebeck coefficient decreased with the In–filling fraction at room temperature, whereas the electrical conductivity increased owing to the higher electron carrier concentrations. The Seebeck coefficient almost monotonically increased with the temperature for x = 0.3 and x = 0.5. However, a nonmonotonic behavior was revealed for the x = 0.1 sample, hence achieving a maximum value of the Seebeck coefficient over recorded samples of 252 μVK−1 at 495 K. This value is higher than the 205 μVK−1 at 673 K obtained for the In0.4Co4Sb12 compound.30 Visnow45 found that the Seebeck coefficient for the In0.113Co4Sb12 compound was ~ 255 μVK−1 at 623 K, which is almost similar to the current values. However, the InSb nanoinclusion phase had a negative influence on the Seebeck coefficient, mainly due to its high conductivity, as mentioned above, and this agreed with previous published articles.30,43
The temperature dependency of the power factor for the InxLa0.25Co4Sb12 SPS-ed samples is presented in Fig. 8. The power factor can be calculated by using the equation \(PF={S}^{2}\sigma \), where S is the Seebeck coefficient, and σ is the electrical conductivity. The power factor was considerably improved with In-addition fraction content, and also the temperature, with the highest peak before declining for all samples. The highest power factor value of 3.85 10–3 W/mK2 was obtained for the In0.5La0.25Co4Sb12 sample at 543 K, mainly due to the considerable reduction in electrical resistivity. This result is higher than that reported in the literature of 3.25 10−3 W/mK2 for the In0.3La0.5Co4Sb12 compound at the same temperature,46 3.5 10−3 W/mK2 for the In0.25Co3.9La0.1Sb12 compound at 600 K,47 ~ 3.2 10−3 W/mK2 for In0.1Yb0.1La0.1Co4Sb12 compound,48 and 2.33 10−3 W/mK2 for In0.05Pr0.05Co4Sb12 at 609 K.42
Figure 9(a) shows the temperature dependency of the InxLa0.25Co4Sb12 samples as a function of thermal conductivity (k). The thermal conductivity was considerably reduced and showed nonmonotonic behavior with increasing In–filling fraction content. The lowest value of thermal conductivity of 1.27 W/mk was obtained for the In0.1La0.25Co4Sb12 sample at 495 K, which is substantially lower than the reported values of 1.6 W/mk for the In0.05Co4Sb12 compound at 580 K.31 This result is mainly attributed to the highest porosity of the In0.1La0.25Co4Sb12 sample, which is consistent with the literature.49,50,51 For the higher In–addition fraction content (x = 0.3 and x = 0.5) samples, the thermal conductivity was high mainly due to the existence of InSb impurities with a high thermal conductivity ~ 40 W/mK.30,43 The temperature dependency of lattice thermal conductivity (kl) for the InxLa0.25Co4Sb12 samples is shown in Fig. 9b. The equation kl = ktotal – ke is used to compute the lattice thermal conductivity, where ke is the electronic contribution, and ktotal is the total thermal conductivity. Moreover, the Wiedemann–Franz law (ke = L.σ.T, where L = 2.44 10−8 V2/K2), can be used to determine the electronic contribution. The Lorenz number as the function of temperature dependence can be calculated via a single parabolic band model equation 52:
where KB is the Boltzmann constant, e is the electron charge, \(\lambda \) is the scattering parameter, \(\upxi \)=\({\mathrm{E}}_{\mathrm{F}}/{\mathrm{K}}_{\mathrm{B}}\mathrm{T}\) is the reduced Fermi energy, EF is the Fermi energy, and Fn(\(\upxi \)) is the Fermi integral defined by:
In this calculation, the scattering parameter is set to be − 0.5 by assuming the acoustic phonon scattering is the dominant carrier scattering mechanism at the measurement temperature range. The reduced Fermi energy is extracted from the measured Seebeck coefficient values using52:
By using the extracted reduced Fermi energy, the Lorenz numbers were calculated to be 2.08, 1.61, 1.62, and 1.67 × 10−8 V2/K2 for the proportions of x = 0, 0.1, 0.3, and 0.5, respectively, at room temperature. In general, the calculated Lorenz numbers are smaller than the degenerate limit of the Lorenz number (2.44 × 10−8 V2/K2) typically observed in TE materials with carrier concentrations between generate and non-degenerate semiconductors. The lattice thermal conductivity parameters were considerably reduced with the In–addition fraction content. Furthermore, the InSb nanoinclusion phase induced more interfaces into the system, which led to a substantial decrease in the lattice thermal conductivity through the interface scattering of phonons. The results show ~ 62% reduction of the lattice thermal conductivity compared with the La0.25Co4Sb12 sample. The lowest value of lattice thermal conductivity, 0.83 W/m K, was achieved for the In0.5La0.25Co4Sb12 sample at 639 K, which is three times lower than that of samples selected from the published reports.53,54 The lowest value of lattice thermal conductivity was obtained for the highest In–filling fraction content x = 0.5 sample, which agrees with previous published reports.26,27,33 In addition, the lattice thermal conductivity was minimized due to the strain field scattering of high-energy phonons.37,38,39,40 This result agrees with the previous study by Nolas et al.,55 who found that an uneven distribution of the filler can introduce a point-defect-type phonon scattering and scatter a large spectrum of phonons.
The temperature dependency of the InxLa0.25Co4Sb12 SPS-ed samples as a function of the thermoelectric ZT is shown in Fig. 10. The results show a clear trend and remarkable improvement of ZT with In–filler fraction content and temperature. The formation of the InSb nanoinclusion phase at the grain boundaries of the skutterudite matrix has a substantial effect on reducing the lattice thermal conductivity, while maintaining good electrical transport properties. Consequently, the maximum ZT = 1.25 obtained for In0.5La0.25Co4Sb12 sample was considerably improved compared with the In-free La0.25Co4Sb12 sample. This result is higher than the 0.79 obtained for the In0.1Co4Sb12 compound,56 0.9 attained in the In0.1Yb0.2Co4Sb12 compound at 782 K32 and ~ 1.2 obtained for In0.25Co4Sb12 compound.31 The In-filler boosts the TE properties of La0.25Co4Sb12 skutterudite material at a medium-temperature range.
Conclusions
MA and SPS techniques were used to successfully fabricate InxLa0.25Co4Sb12 skutterudite compounds. The major phase of the CoSb3 skutterudite structure was observed by the XRD patterns for SPS-ed samples with minor amounts of CoSb2 and InSb as the secondary phases. The InSb nanoinclusion impurity remarkably reduced the electrical resistivity and lattice thermal conductivity of the InxLa0.25Co4Sb12 skutterudites. The In0.5La0.25Co4Sb12 sample achieved the lowest electrical resistivity and lattice thermal conductivity of 9.67 μΩ m and 0.83 W/m K, respectively. Furthermore, the In-filler dramatically boosted the power factor, reaching a maximum value of 3.85 10−3 W/m K2 at 543 K for the In0.5La0.25Co4Sb12 sample due to a considerable reduction in electrical resistivity. The ZT was greatly improved to 1.25 at 688 K for the In0.5La0.25Co4Sb12 sample, which was substantially higher than that of the unfilled sample.
References
M.B.A. Bashir, S. Mohd Said, M.F.M. Sabri, D.A. Shnawah, and M.H. Elsheikh, Recent advances on Mg2Si1–xSnx materials for thermoelectric generation. Renew. Sustain. Energy Rev. 37, 569–584 (2014).
M. Hamid Elsheikh, D.A. Shnawah, M.F.M. Sabri, S.B.M. Said, M. Haji Hassan, M.B. Ali Bashir, and M. Mohamad, A review on thermoelectric renewable energy: principle parameters that affect their performance. Renew. Sustain. Energy Rev. 30, 337–355 (2014).
D.Y.N. Truong, H. Kleinke, and F. Gascoin, Thermoelectric properties of higher manganese silicide/multi-walled carbon nanotube composites. J. Chem. Soc. Dalt. Trans. 43, 15092–15097 (2014).
K.H. Park, S.C. Ur, I.H. Kim, S.M. Choi, and W.S. Seo, Transport properties of Sn-doped CoSb3 skutterudites. J. Korean Phys. Soc. 57, 1000–1004 (2010).
J.L. Feldman, P. Dai, T. Enck, B.C. Sales, D. Mandrus, and D.J. Singh, Lattice vibrations in La(Ce)Fe4Sb12 and CoSb3: Inelastic neutron scattering and theory. Phys. Rev. B. 73, 14306 (2006).
V. Keppens, D. Mandrus, B.C. Sales, B.C. Chakoumakos, P. Dai, R. Coldea, M.B. Maple, D.A. Gajewski, E.J. Freeman, and S. Bennington, Localized vibrational modes in metallic solids. Nature 395, 876–878 (1998).
M.M. Koza, M.R. Johnson, R. Viennois, H. Mutka, L. Girard, and D. Ravot, Breakdown of phonon glass paradigm in La-and Ce-filled Fe4Sb12 skutterudites. Nat. Mater. 7, 805–810 (2008).
J. Yang, D.T. Morelli, G.P. Meisner, W. Chen, J.S. Dyck, and C. Uher, Effect of Sn substituting for Sb on the low-temperature transport properties of ytterbium-filled skutterudites. Phys. Rev. B. 67, 165207 (2003).
Z. Zhou, C. Uher, A. Jewell, and T. Caillat, Influence of point-defect scattering on the lattice thermal conductivity of solid solution Co(Sb1–xAsx)3. Phys. Rev. B. 71, 235209 (2005).
Y. Tang, Z.M. Gibbs, L.A. Agapito, G. Li, H.-S. Kim, M.B. Nardelli, S. Curtarolo, and G.J. Snyder, Convergence of multi-valley bands as the electronic origin of high thermoelectric performance in CoSb3 skutterudites. Nat. Mater. 14, 1223–1228 (2015).
M. Rull-Bravo, A. Moure, J.F. Fernández, and M. Martín-González, Skutterudites as thermoelectric materials: revisited. Rsc Adv. 5, 41653–41667 (2015).
J.W. Sharp, E.C. Jones, R.K. Williams, P.M. Martin, and B.C. Sales, Thermoelectric properties of CoSb3 and related alloys. J. Appl. Phys. 78, 1013–1018 (1995).
J.C. Slater, Structure of isobutylene. J. Chem. Phys. 41, 3199 (1964).
V. Trivedi, M. Battabyal, P. Balasubramanian, G.M. Muralikrishna, P.K. Jain, and R. Gopalan, Microstructure and doping effect on the enhancement of the thermoelectric properties of Ni doped Dy filled CoSb3 skutterudites. Sustain. Energy Fuels. 2, 2687–2697 (2018).
G. Rogl, A. Grytsiv, P. Rogl, N. Peranio, E. Bauer, M. Zehetbauer, and O. Eibl, N-type skutterudites (R, Ba, Yb)yCo4Sb12 (R = Sr, la, mm, DD, SrMm, SrDD) approaching ZT≈ 2.0. Acta Mater. 63, 30–43 (2014).
L.D. Chen, T. Kawahara, X.F. Tang, T. Goto, T. Hirai, J.S. Dyck, W. Chen, and C. Uher, Anomalous barium filling fraction and n-type thermoelectric performance of BayCo4Sb12. J. Appl. Phys. 90, 1864–1868 (2001).
T. Dahal, Q. Jie, G. Joshi, S. Chen, C. Guo, Y. Lan, and Z. Ren, Thermoelectric property enhancement in Yb-doped n-type skutterudites YbxCo4Sb12. Acta Mater. 75, 316–321 (2014).
H. Li, X. Su, X. Tang, Q. Zhang, C. Uher, G.J. Snyder, and U. Aydemir, Grain boundary engineering with nano-scale InSb producing high performance InxCeyCo4Sb12+z skutterudite thermoelectrics. J. Mater. 3, 273–279 (2017).
C. Chen, L. Zhang, J. Li, F. Yu, D. Yu, Y. Tian, and B. Xu, Enhanced thermoelectric performance of lanthanum filled CoSb3 synthesized under high pressure. J. Alloys Compd. 699, 751–755 (2017).
G. Tan, Y. Zheng, Y. Yan, and X. Tang, Preparation and thermoelectric properties of p-type filled skutterudites CeyFe4–xNixSb12. J. Alloys Compd. 584, 216–221 (2014).
S. Choi, K. Kurosaki, A. Yusufu, Y. Ohishi, H. Muta, and S. Yamanaka, Thermoelectric properties of p-Type Tl-filled skutterudites: TlxFe1.5Co2.5Sb12. J. Electron. Mater. 44, 1743–1749 (2015).
G. Rogl, A. Grytsiv, K. Yubuta, S. Puchegger, E. Bauer, C. Raju, R.C. Mallik, and P. Rogl, In-doped multifilled n-type skutterudites with ZT= 1.8. Acta Mater. 95, 201–211 (2015).
M.H. Elsheikh, M.F.M. Sabri, S.M. Said, Y. Miyazaki, H.H. Masjuki, D.A. Shnawah, N. Abdullah, S. Naito, and M.B.A. Bashir, Microstructural modification of Co4Sb12 skutterudite thermoelectric material through Al exceed doping. Sci. Adv. Mater. 8, 2121–2127 (2016).
O. Raihan, S. Mohd Said, M.F.M. Sabri, S. Rozali, B.D. Long, K. Kimura, K. Tobita, M.F. Fitriani, M.B. Mohd Salleh, and Ali Bashir, Parametric analysis of ball milling condition on thermoelectric performance of Bi0.6FeCo3Sb12 skutterudite. Mater. Res. Exp. 5, 105008 (2018).
M.H. Elsheikh, M.F.M. Sabri, S.M. Said, Y. Miyazaki, H.H. Masjuki, D.A. Shnawah, S. Naito, and M.B.A. Bashir, Rapid preparation of bulk AlxYb0.25Co4Sb12 (x = 0, 0.1, 0.2, 0.3) skutterudite thermoelectric materials with high figure of merit ZT = 1.36. J. Mater. Sci. 52, 5324–5332 (2017).
G. Li, K. Kurosaki, Y. Ohishi, H. Muta, and S. Yamanaka, Thermoelectric properties of indium-added skutterudites InxCo4Sb12. J. Electron. Mater. 42, 1463–1468 (2013).
A. Sesselmann, T. Dasgupta, K. Kelm, E. Müller, S. Perlt, and S. Zastrow, Transport properties and microstructure of indium-added cobalt–antimony-based skutterudites. J. Mater. Res. 26, 1820–1826 (2011).
L. Deng, X.P. Jia, T.C. Su, S.Z. Zheng, X. Guo, K. Jie, and H.A. Ma, The thermoelectric properties of InxCo4Sb12 alloys prepared by HPHT. Mater. Lett. 65, 2927–2929 (2011).
R.C. Mallik, J.-Y. Jung, S.-C. Ur, and I.-H. Kim, Thermoelectric properties of InzCo4Sb12 skutterudites. Met. Mater. Int. 14, 223–228 (2008).
R.C. Mallik, C. Stiewe, G. Karpinski, R. Hassdorf, and E. Müller, Thermoelectric properties of Co4Sb12 skutterudite materials with partial in filling and excess in additions. J. Electron. Mater. 38, 1337–1343 (2009).
T. He, J. Chen, H.D. Rosenfeld, and M.A. Subramanian, Thermoelectric properties of indium-filled skutterudites. Chem. Mater. 18, 759–762 (2006).
J.-K. Lee, S.-M. Choi, W.-S. Seo, Y.-S. Lim, H.-L. Lee, and I.-H. Kim, Thermoelectric properties of Spark Plasma Sintered InxYbyLa0.3–x–yCo4Sb12 skutterudite system. Renew. Energy. 42, 36–40 (2012).
V.V. Khovaylo, T.A. Korolkov, A.I. Voronin, M.V. Gorshenkov, and A.T. Burkov, Rapid preparation of InxCo4Sb12 with a record-breaking ZT= 1.5: the role of the in overfilling fraction limit and Sb overstoichiometry. J. Mater. Chem. A. 5, 3541–3546 (2017).
S.M. Said, M.B.A. Bashir, M.F.M. Sabri, Y. Miyazaki, D.A.A. Shnawah, A.S. Hakeem, M. Shimada, A.I. Bakare, N.N.N. Ghazali, and M.H. Elsheikh, Enhancement of thermoelectric behavior of La0.5Co4Sb12–xTex skutterudite materials. Metall Mater. Trans. A Phys. Metall. Mater. Sci. 48, 3073–3081 (2017).
M. Bashir, A. Bashir, S.M. Said, M. Faizul, M. Sabri, and Y. Miyazaki, Enhancement of thermoelectric properties of Yb0.25Co4Sb12 skutterudites through Ni substitution. Sains Malaysiana. 47, 181–187 (2018).
K.I. Litvinova, A.I. Voronin, M.V. Gorshenkov, D.Y. Karpenkov, A.P. Novitskii, and V.V. Khovaylo, Thermoelectric properties of CexNdyCo4Sb12 skutterudites. Semiconductors 51, 928–931 (2017).
H. Geng, J. Zhang, T. He, L. Zhang, and J. Feng, Microstructure evolution and mechanical properties of melt spun skutterudite-based thermoelectric materials. Materials (Basel). 13, 984 (2020).
R. Wei, G. Huiyuan, Z. Zihao, and Z. Lixia, Filling-fraction fluctuation leading to glasslike ultralow thermal conductivity in caged skutterudites. Phys. Rev. Lett. 118, 245901 (2017).
J. Gainza, F. Serrano-Sánchez, J. Prado-Gonjal, N.M. Nemes, N. Biskup, O.J. Dura, J.L. Martínez, F. Fauth, and J.A. Alonso, Substantial thermal conductivity reduction in mischmetal skutterudites MmxCo4Sb12 prepared under high-pressure conditions, due to uneven distribution of the rare-earth elements. J. Mater. Chem. C. 7, 4124–4131 (2019).
X. Tong, Z. Liu, J. Zhu, T. Yang, Y. Wang, and A. Xia, Research progress of p-type Fe-based skutterudite thermoelectric materials. Front. Mater. Sci. 15, 1–17 (2021).
G. Tang, D. Zhang, G. Chen, F. Xu, and Z. Wang, High temperature thermoelectric and magnetic properties of InxNdyCo4Sb12 skutterudites. Phys. B Condens. Matter. 408, 79–82 (2013).
G. Tang, W. Yang, F. Xu, and Y. He, Synthesis and thermoelectric properties of In and Pr double-filled skutterudites InxPryCo4Sb12. J. Electron. Mater. 43, 435–438 (2014).
G. Busch and E.F. Steigmier, Thermal and electrical conductivities, Hall effect and thermoelectric power of InSb. Helv. Phys. Acta. 34, 1–28 (1961).
K. Liu, X. Dong, and Z. Jiuxing, The effects of La on thermoelectric properties of LaxCo4Sb12 prepared by MA-SPS. Mater. Chem. Phys. 96, 371–375 (2006).
E. Visnow, C.P. Heinrich, A. Schmitz, J. De Boor, P. Leidich, B. Klobes, R.P. Hermann, W.E. Müller, and W. Tremel, On the true indium content of In-filled skutterudites. Inorg. Chem. 54, 7818–7827 (2015).
M.B.A. Bashir, S.M. Said, M.F.M. Sabri, Y. Miyazaki, D.A. Shnawah, M. Shimada, M.F.M. Salleh, M.S. Mahmood, E.Y. Salih, F. Fitriani, and M.H. Elsheikh, In-filled La0.5Co4Sb12 skutterudite system with high thermoelectric figure of merit. J. Electron. Mater. 47, 2429–2438 (2018).
J.-Y. Jung, K.-H. Park, I.-H. Kim, S.-M. Choi, and W.-S. Seo, Thermoelectric and transport properties of in-filled and Ni-doped CoSb3 skutterudites. J. Korean Phys. Soc. 57, 773–777 (2010).
J.K. Lee, S.M. Choi, W.S. Seo, Y.S. Lim, H.L. Lee, and I.H. Kim, Thermoelectric properties of spark plasma sintered InxYbyLa0.3–x–yCo4Sb12 skutterudite system. Renew. Energy. 42, 36–40 (2012).
L. Yang, J.S. Wu, and L.T. Zhang, Synthesis of filled skutterudite compound La0.75Fe3CoSb12 by spark plasma sintering and effect of porosity on thermoelectric properties. J. Alloys Compd. 364, 83–88 (2004).
N. Gostkowska-Lekner, B. Trawinski, A. Kosonowski, B. Bochentyn, M. Lapinski, T. Miruszewski, K. Wojciechowski, and B. Kusz, New synthesis route of highly porous InxCo4Sb12 with strongly reduced thermal conductivity. J. Mater. Sci. 55, 13658–13674 (2020).
F. Fitriani, S.M. Said, S. Rozali, M.F. Mohd, M. Faizul, M. Sabri, L. Bui, T. Nakayama, O. Raihan, M.M.I. Megat Hasnan, M.B.A. Bashir, and F. Kamal, Enhancement of thermoelectric properties in cold pressed nickel doped bismuth sulfide compounds. J. Electron. Mater. 14, 689–699 (2018). https://doi.org/10.1007/s13391-018-0072-8.
D.M. Rowe and C.M. Bhandari, Modern Thermoelectrics (London: Holt. Rinehart Winst, 1983), p. 69.
G. Li, K. Kurosaki, Y. Ohishi, H. Muta, and S. Yamanaka, Thermoelectric properties of indium-added skutterudites InxCo4Sb12. J. Electron. Mater. 42, 1463–1468 (2013).
M.B.A. Bashir, M.F. Mohd Sabri, S.M. Said, Y. Miyazaki, I.A. Badruddin, D.A. Ameer Shnawah, E.Y. Salih, S. Abushousha, and M.H. Elsheikh, Enhancement of thermoelectric properties of Co4Sb12 skutterudite by Al and La double filling. J. Solid State Chem. 284, 121205 (2020).
G.S. Nolas, J.L. Cohn, and G.A. Slack, Effect of partial void filling on the lattice thermal conductivity of skutterudites. Phys. Rev. B. 58, 164 (1998).
L. Wang, K.F. Cai, Y.Y. Wang, H. Li, and H.F. Wang, Thermoelectric properties of indium-filled skutterudites prepared by combining solvothermal synthesis and melting. Appl. Phys. A Mater. Sci. Process. 97, 841–845 (2009).
Acknowledgments
This work was funded by the Deanship of Scientific Research at Jouf University under grant No. (DSR-2021-02-03107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bashir, M.B.A., Salih, E.Y., Said, S.M. et al. Thermoelectric Transport Properties of Double-Filling InxLa0.25Co4Sb12 Skutterudite Materials. J. Electron. Mater. 52, 971–979 (2023). https://doi.org/10.1007/s11664-022-10083-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-10083-1