Abstract
40Cr10Si2Mo steel is widely utilized because of its excellent mechanical properties, with grain size being a critical factor determining subsequent phase transformation processes and material microstructure performance. This paper reports the use of high-temperature laser scanning confocal microscopy (HT-LSCM) for in situ observation experiments to systematically investigate the growth of austenite grains and the martensitic phase transformation mechanism in 40Cr10Si2Mo steel during an 1800-second isothermal hold at temperatures ranging from 900 °C to 1250 °C. A dynamic model of austenite grain growth is established to optimize the parameters of the austenitic process. The results indicate that the austenite grain size increases continuously with increasing temperature and prolonged time. The Dong model predicts grain sizes that align well with experimental values. Austenite grains grow through grain boundary migration and grain annexation, whereas the precipitation and dissolution of M(Cr, Mo)23C6 affect grain growth. With prolonged time, some grain boundaries extend into new boundaries through subgrain rotation. The fine grains at lower temperatures reduce the initial temperature of the martensite transition (Ms), and the primary martensite nucleates along the grain boundaries of the prior austenite. The secondary martensite is attached to the primary martensite nucleus at a certain angle and grows in parallel while inhibiting the phase transition of the surrounding untransformed austenite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat-resistant stainless steel is widely used in applications such as steam turbines, internal combustion engines, and turbine blades because of its excellent thermal strength, thermal hardness, fatigue strength, wear resistance, and oxidation–corrosion resistance.[1,2,3] In recent years, with the development of the automotive industry and more extreme operating conditions, greater demands have been placed on the service life and mechanical properties of heat-resistant steels for engine valves. 40Cr10Si2Mo is a 9 to 12 pct Cr martensitic heat-resistant stainless steel that exhibits outstanding thermal stability and resistance to oxidation and fatigue.[4] This steel is prone to thermal embrittlement during hot working at 1050 °C to 1100 °C, with production processes being unstable and complex, where the heating temperature and holding time play crucial roles in austenite grain growth and martensitic phase transformation behavior. Coarse austenite grains and uneven grain size distributions can lead to increased material brittleness and decreased strength and toughness and adversely affect subsequent phase transformation processes and processing performance.[5,6] Therefore, it is necessary to understand the relationship between the austenitic grain growth mechanism and martensitic phase transformation in depth and to optimize the austenitizing parameters to meet the requirements of the current internal combustion engine performance for martensitic heat-resistant stainless steel and improve the material process performance.
Many studies have employed high-temperature laser scanning confocal microscopy (HT-LSCM) to observe in situ the grain growth and microstructural evolution of materials. Lin et al.[7] investigated the inhibitory effect of adding trace alloying element Mg on the grain growth of austenite in low-carbon steel. Austenite grain growth was observed in situ at 1300 °C. The results demonstrated that finely dispersed Mg inclusions can inhibit grain boundary migration in austenite. Wang et al.[8] conducted in situ observation experiments at different temperatures and holding times to study the growth of austenite grains and subsequent bainite and martensite transformations. It was concluded that the average austenite grain size increases with increasing temperature. With increasing cooling rate, the duration of the phase transformation decreases, and the transformation shifts from bainite to martensite. Kinetic calculations of austenite grain growth can directly reflect the influence of the heating temperature and holding time on grain growth in materials and analyze the mechanisms of grain growth and microstructural evolution. Zhou et al.[9] studied the growth of grains during the austenitization process of 700 MPa-grade high-strength steel. The results showed that with increasing holding time, austenite grains gradually grow, and the amount of undissolved precipitates decreases. Moreover, an equation for predicting the size and distribution of austenite grains was established. The predicted values are in good agreement with the experimental data, indicating that uniform and fine initial austenite grain sizes were obtained. Liu et al.[10] investigated the influence of different process parameters on the austenite grain growth behavior of medium-carbon alloy steel. AIN particles were observed via field emission scanning electron microscopy (FESEM), and the amount of AIN precipitation was detected via electron probe microanalysis (EPMA). The results showed that with increasing holding temperature and holding time, the average grain size of austenite in the test steel continuously increases, with the heating temperature having a more significant effect on the austenite grain size. A predictive model describing the growth of austenite grains during the heating process of medium-carbon alloy steel was established, and the model’s high accuracy was verified.
The heating temperature and holding time of austenite grain growth and the subsequent cooling process of martensitic transformation play decisive roles in the microstructure and properties of 40Cr10Si2Mo steel. Therefore, the effects of the heating temperature and holding time on the austenitic grain growth and martensitic transformation mechanism of 40Cr10Si2Mo steel were dynamically analyzed on the basis of in situ observations via high-temperature confocal laser scanning microscopy. A dynamic grain growth model was established to guide subsequent production process formulation and optimization.
Experimental
Experimental Material
In the experiment, a hot-rolled annealed 40Cr10Si2Mo martensitic heat-resistant steel bar was analyzed and its chemical composition is shown in Table I. The initial microstructure and elemental distribution of the steel are depicted in Figure 1, showing M23C6 carbides rich in Cr and Mo distributed in the ferrite matrix.
Experimental Methods
A phase diagram simulation of 40Cr10Si2Mo steel was conducted via JMatPro 7.0 thermodynamic software to determine the relationship between the phase composition and temperature. The sample size for the differential thermal analysis was Φ3 mm × 0.5 mm, and the equipment model used was a combination of synchronous thermal analysis infrared GC instruments (TG/DSC–FTIR–GCMS). The heating rate was set to 10 °C/min, with a sample weight of 38 mg.
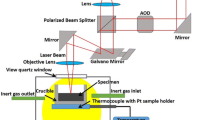
The in situ observation experiments were conducted in the VL2000DX-SVF18SP HT-LSCM, with sample dimensions of Φ6 mm × 3 mm. Before the experiment, the samples were ground and ultrasonically cleaned with an acetone solution. HT-LSCM was employed for in situ observation of the microstructural evolution of 40Cr10Si2Mo steel during heating, holding, and cooling processes. The experimental process is shown in Figure 2. The sample was heated at a rate of 5 °C/s to different holding temperatures ranging from 900 °C to 1250 °C, with an interval increment of 50 °C. After 1800 seconds, the mixture was quickly cooled to room temperature in an argon-vacuum atmosphere.
In accordance with the Jeffries equivalent area method, the grain size under different austenitizing conditions was measured via Nano Measurer software, and the grain size change curve was plotted via Origin software. After grinding and polishing, the quenched steel sample was etched with 10 g FeCl3 + 30 mL HCl + 120 mL H2O at room temperature to observe the quenched microstructure, and the scanning microstructure was observed under a Zeiss Gemini 300 scanning electron microscope. After the experiment, the material was analyzed via EBSD, where EBSD data were obtained at a step size of 0.26 μm under an accelerating voltage of 20 kV, and the microstructure of the material was reconstructed via Aztec Crystal EBSD postprocessing software.
Results
Phase Transition of 40Cr10Si2Mo Steel During Heating
The temperature–phase distribution curve for 40Cr10Si2Mo steel is shown in Figure 3(a). As the heating temperature increased, the BCC ferrite in the initial structure gradually transformed into FCC austenite, whereas the carbides continued to precipitate or dissolve. Below 850 °C, the ferrite phase constituted approximately 90 to 93 pct. Beyond 880 °C, austenite began to form, marking the start of the austenite transformation temperature (Ac1) for the steel. At approximately 920 °C, the austenite transformation was essentially complete, representing the termination temperature (Ac3) for the austenite transformation of steel. High-temperature ferrite phases began to appear at 1350 °C. The temperature range of 1350 °C to 1360 °C represents the coexistence region of high-temperature ferrite and austenite, with 1360 °C being the melting point of 40Cr10Si2Mo steel. Additionally, the M23C6 and M2C phases exist at room temperature, with the M23C6 phase dissolving at approximately 1000 °C, the M2C phase dissolving at approximately 180 °C, and the M7C3 phase existing between 980 °C and 1100 °C.
Figure 3(b) shows the DSC–TG curve and derivative curve of the DSC during the heating process of 40Cr10Si2Mo steel. The phase transition points of the steel during continuous heating were determined through DSC differential thermal analysis. The DSC derivative curve exhibits endothermic peaks at approximately 900 °C and 970 °C and exothermic peaks at approximately 1020 °C and 1080 °C. Combining Figure 3(a) and relevant studies, it can be inferred that austenite transformation begins at approximately 900 °C; the heat absorption peak near 970 °C is caused by the precipitation of M7C3; M23C6 precipitates at approximately 1020 °C; and the exothermic peak near 1080 °C is due to the dissolution of the M7C3 phase. The simulation results from JMatPro 7.0 software are consistent with the experimental results obtained from DSC measurements.
Austenite Grain Growth Behavior
Figure 4 shows in situ observations of the morphologic evolution of austenite grain growth at different holding times for 40Cr10Si2Mo steel at temperatures of 900 °C, 1000 °C, and 1100 °C. The grain size increased with increasing heating temperature and holding time. Higher holding temperatures require greater activation energy for grain growth, thereby accelerating the grain growth process.[11] At a heating temperature of 900 °C, the temperature–phase composition curve for 40Cr10Si2Mo steel lies within the two-phase region, with a composition of ferrite and austenite (α + γ). With prolonged holding time, ferrite gradually transformed into austenite, and under these conditions, the austenite grain boundaries were not visible, as shown in Figures 4(a) through (c). Upon heating the steel to 900 °C, oxidation caused the sample surface to darken. As the holding time increased, the arrangement of the metal atoms at the grain boundaries became irregular, and the oxidized atoms easily diffused to other locations, resulting in a relatively bright white color.
Figures 4(d) through (f) shows the morphology of austenite grains at 1000 °C after different holding times. In Figure 4(e), the austenite grains are relatively small, with new austenite grains nucleating and growing at the grain boundaries, whereas smaller austenite grains are unevenly distributed around larger austenite grains. As the holding time increased, carbides continued to precipitate along the austenite grain boundaries. When the holding time reached 30 minutes, the carbides aggregated and coarsened at the grain boundaries, as shown in Figure 4(f). In addition to the precipitates visible at the beginning of heating, numerous fine carbide particles appeared within the austenite grains during the holding process, gradually coarsening over time, as depicted in Figures 4(d) through (f). Combined with the temperature–phase composition diagram, it is evident that at 1000 °C, carbides primarily exist in the forms of M7C3 and M23C6.
At a holding temperature of 1100 °C, the evolution of the austenite grain morphology after a 30-minute holding period is depicted in Figures 4(g) through (i). Compared with the morphology at the preset temperature, the grain boundaries of the austenite grains became more distinct after holding (Figure 4(h)). With prolonged holding, the grain boundaries became more pronounced, appearing narrower and straighter. In the case of 40Cr10Si2Mo steel held at 1100 °C for 15 minutes, most austenite grain boundaries were curved. As the holding time increased, the austenite grains notably enlarged, and the grain boundary angles gradually approached 120 deg, as illustrated in Figures 4(h) and (i). Local carbide small particles gradually coalesced into larger particles, as indicated by the red rectangle in Figure 4(h). Additionally, the extension and migration of austenite grain boundaries led to the formation of larger grains, as depicted by the blue rectangle in Figure 4(i). The gradual merging of small grains and the migration of some grain boundaries illustrate the growth process and trend of austenite.
Figure 5 shows the morphologic evolution of austenite grains in 40Cr10Si2Mo steel after a 30-minute holding time at 1250 °C. The microstructural evolution during the heating process is evident. For the undeformed sample, temperature was the primary driving factor for microstructural changes during the heating stage. When the temperature exceeds Ac1 (880 °C), nucleation and growth of austenite occur at the interfaces of carbides and ferrites in 40Cr10Si2Mo steel.[12] When the heating temperature was just reached, the austenite grain boundaries were not obvious (Figure 5(a)). Owing to continuous heating, austenite grain boundaries are clear when heat preservation starts at 1250 °C for 2 minutes, and the thermal etching of the material at the austenite grain boundaries requires sufficient heat accumulation.[13] When the heat was held for 5 minutes, the microstructure contained equiaxed grains with straight grain boundaries (Figure 5(c)), and grain boundary migration was obvious during the heat preservation process, as shown by the dotted lines in Figures 5(c) through (f). During the process of grain boundary migration, some of the original grain boundaries gradually disappeared, and the old boundaries were replaced. In addition to the grain boundaries expanding outward, the small grains were separated by the surrounding larger grains, as shown by the arrows in Figure 5(d). This is because the grain boundaries between adjacent grains are crystal structure defects that are associated with energy changes. High temperatures reduce the activation energy for crystal defect motion, leading to grain boundary movement.[14] Partial twins that pass through or occupy the entire grain were also found in the austenite grains, which can be regarded as annealing twins.[15] According to Wang et al.,[16] with decreasing bedding fault energy, the critical stress of twin nucleation decreases, which makes it easy to meet the energy conditions, and the formation of twins becomes easier. The presence of more alloying elements in 40Cr10Si2Mo steel reduces the stacking fault energy, increasing the likelihood of intragranular twinning. The appearance of twins causes grain segregation or refinement, increases resistance to dislocation motion, and enhances the strength of the steel. The MS-B81 particles gradually coarsened from the dispersed small particles during the heat preservation process, and the simulation results obtained with JMatPro7.0 software confirmed the existence of MS-B81 particles.
Phase Transition of 40Cr10Si2Mo Steel During Cooling
Figure 6 shows in situ observation images of the martensitic phase transformation during the cooling process of 40Cr10Si2Mo steel at a quenching temperature of 1200 °C. As the temperature rapidly decreased, the microstructure transformed from austenite to martensite. At 208.4 °C, the martensite phase transformation began, and the prior austenite grain boundaries became visible (Figure 6(a)). The transformation was essentially complete when the temperature reached 120.9 °C. Previous studies have reported that the martensite start temperature (Ms) of 40Cr10Si2Mo steel, measured through thermal expansion experiments, is approximately 187 °C, which is lower than the Ms point determined through in situ observations. The nucleation and growth of martensite occur rapidly after the phase transformation, but the transformation does not initiate simultaneously in all the grains.[17] Martensite nucleates at the grain boundaries, grows within the grains, and stops at the grain boundaries. With decreasing temperature, an increasing amount of lath martensite appears, with most laths extending across the entire grain. The formation of martensite stimulates the nucleation of the surrounding unconverted austenite, and the secondary martensite grows in parallel. The arrows in Figures 6(b) and (c) distinguish the packets (groups of parallel laths with the same habit plane) and blocks (groups of laths with the same direction) within the prior austenite grain (PAG) boundaries.[18] The results indicate that martensite plates nucleate at the austenite grain boundaries and extend outward in different directions. As the temperature decreases, the volume fraction of martensite gradually increases.
At 164.8 °C, lath martensite appeared simultaneously at certain angles. Some lath martensite grains grew in parallel with each other (Figure 6(d)). As the temperature continued to decrease, the increase in primary martensite was accompanied by the appearance of secondary martensite. Secondary martensite refers to thin laths of martensite formed around primary martensite, which appeared at a certain angle to the primary martensite (Figure 6(d)). Surface undulations caused by martensitic phase transformation gradually appeared at the boundaries of the PAG, with most martensite ceasing growth upon encountering grain boundaries (Figure 6(e)). When the temperature decreased to 120.9 °C, the nucleation and growth of martensite became very weak, and the phase transformation rate gradually decreased. The distortion caused by the martensitic transformation hindered the martensitic transformation of the surrounding austenite, and part of the region was retained as retained austenite (RA), where elements such as carbon in the ferrite diffused to the RA (Figure 6(e)). The growth rate of lath martensite was relatively fast, and martensitic phase transformation selectively began in the PAG but was short lived. The latent heat generated by martensitic phase transformation was also one of the reasons for the selective occurrence of martensitic phase transformation. The selective occurrence of martensitic phase transformation was also related to the distortion caused by martensitic phase transformation. The transformation of martensite in untransformed austenite is strongly inhibited by the transformation of martensite in the surrounding area.[19,20]
Discussion
Austenite Grain Growth Kinetics
Figure 7(a) presents the grain size statistics obtained with Nano Measurer software via the Jeffries equivalent circle method. According to the curve, after the holding temperature was reached, the austenite grains rapidly grew, with the curve of the grain size over time exhibiting exponential growth, and the rate of growth gradually decreased. After approximately 600 seconds of holding, the rate of grain growth reached its minimum. With increasing heating temperature, the austenite grain size increased from 41.37 μm at 1000 °C to 201.76 μm at 1250 °C. Figure 7(a) shows that there was little variation in the grain size between 1050 °C and 1100 °C. However, when the temperature changed from 1100 °C to 1150 °C, there was a significant increase in the grain size, reaching approximately 75 μm. Combined with the analysis in Section III–A, it can be inferred that carbides precipitate in the steel between 1050 °C and 1100 °C, hindering austenite grain boundary migration. When the temperature exceeds 1100 °C, the carbide particles gradually dissolve, leading to rapid growth of the austenite grains.
The Beck equation[21] is a commonly used model for describing the relationship between austenite grain growth and time at a constant temperature. It is expressed as follows:
In the equation, D represents the average grain size of austenite (μm), t represents the holding time (s), n represents the grain growth exponent, and K is a constant that depends on the material. Nonlinear curve fitting was conducted for the experimental data of D and t obtained for 40Cr10Si2Mo steel, and the corresponding K and n values are listed in Table II. The relationship between the grain size and holding time is consistent with the corresponding model under all heating temperatures fitted by the Beck model. However, this model assumes that the initial grain size is zero at the beginning of holding, leading to errors.
During the heating process, the growth of austenite grains in steel is a physical metallurgical process controlled by thermal activation, diffusion, and interfacial reactions. The growth of grains during heating essentially involves the diffusion of atoms across interfaces at grain boundaries within the steel.[22] However, the Beck equation does not consider the influence of the heating temperature or the initial grain size of austenite at the beginning of holding. Therefore, the Dong equation[23] is adopted for fitting:
In the equation, d is the austenite grain size (μm); d0 represents the initial grain size of austenite (μm); Q is the activation energy for grain growth (J/mol); R is the molar gas constant, 8.314 J/(mol K); t is the holding time (s); T is the heating temperature (K); and A, m, and n are constants that depend on the material. Taking the natural logarithm of both sides of Eq. [2] yields Eq. [3] as follows:
The size of the austenite grains is related to the heating temperature, heating rate, and composition of the steel. By statistically analyzing the initial grain sizes at different isothermal temperatures and fitting them accordingly, the relationship between the initial grain size (d0) and the isothermal temperature can be obtained, as shown in Figure 7(b). When the holding time is less than 600 seconds, the grain growth shows a power function rule. However, when the holding time exceeds 600 seconds, the grain size can be considered to remain constant. Therefore, when regressing the model, a two-stage approach is considered with a time node.
First, the linear relationship between the grain size and holding temperature at different temperatures for 40Cr10Si2Mo steel at the same moment is obtained. The grain sizes at 0, 100, 200, and 300 seconds are substituted into Eq. [3]. By empirically setting m from 1 to 7, linear regression is performed via Origin software to obtain the linear relationship between \(\ln (d^{m} - d_{0}^{m} )\) and \((1/T)\) for different m values. The slope and intercept of the curve are obtained, denoted as \(( - Q/R)\) and \(\ln A + n\ln t\), respectively. The mean square error (the square root of the sum of the squares of the differences between the fitted and actual values) is introduced. The parameter m and the mean square error are then obtained through nonlinear curve fitting via Origin software. The parameters are presented in detail in Table III. According to the calculation results, a nonlinear relationship between m and the mean square error is plotted, as shown in Figure 7(c). When m is approximately 2.52, the minimum mean square error is 0.00568662072, indicating that m is closest to 2.52 at this point. At this time, Q is determined to be 103,615.8 J/mol.
Under the obtained value of m = 2.52, the linear relationship between \(\ln (d^{m} - d_{0}^{m} )\) and lnt at the same temperature and different times is calculated. The slope value represents n. When \(n = 0.711\) is obtained by fitting, it is most consistent with the rule of the growth rate constant \(A = 4.368 \times 10^{7}\). The grain growth model is derived as Eq. [4] (t ≤ 600 seconds). When the holding time exceeds 600 seconds, the change in the grain size becomes negligible. The average change in the grain size at the end of the holding period is 1.725 μm. Therefore, if the holding time exceeds 600 seconds, the grain size is considered to be the same as that at 600 seconds, indicating no further grain growth. The resulting model is shown in Eq. [5].
The model is compared with the actual values, where the scatter points represent the measured grain sizes and the curves represent the fitted results, as shown in Figure 7(d). The maximum error between the measured points and the theoretical curves is 9.28 μm, the minimum error is 0.27 μm, and the average error is 1.54 μm. The model is well validated, as the measured data points fall on the fitted curves.
Austenite Grain Growth Mechanism
Figure 8 shows the SEM morphology of 40Cr10Si2Mo steel cooled for 1800 seconds at different quenching temperatures. As the holding temperature increased, the austenite grain size of the steel increased, and carbides gradually precipitated or dissolved along the grain boundaries. When the quenching temperature was 900 °C, the room-temperature structure consisted of ferrite (F), martensite (M), retained austenite (RA), and carbides (Figure 8(a)). Thermodynamic calculations indicate that at this temperature, the 40Cr10Si2Mo steel is in a two-phase region, and the original austenite grain boundaries are not clear. As the temperature increased to 950 °C, the carbides in the 40Cr10Si2Mo steel began to aggregate and precipitate along the grain boundaries. Since this quenching temperature exceeds the Ac3 of steel, the prior austenite grain boundaries are visible in the room-temperature structure (Figure 8(b)). When the temperature further increased to 1000 °C, the carbides in the 40Cr10Si2Mo steel dissolved significantly and compared with those in the 950 °C sample, the grain size noticeably increased (Figure 8(c)). When the quenching temperature exceeded 1050 °C (Figure 8(d)), the microstructure at room temperature indicates that the carbides had completely diffused into the matrix, and grain growth was no longer hindered by the carbides; thus, grains began to grow rapidly. Therefore, the change in austenite grain size is closely related to the coarsening or dissolution of carbides. The significant increase in the austenite grain size at 1150 °C may have been related to the disappearance of the M23C6 carbides.
Figure 9 shows the SEM morphology and EDS analysis results for 40Cr10Si2Mo steel at different quenching temperatures, where the carbide type observed is M(Cr, Mo)23C6. Alloying elements (such as Cr and Mo) can form tiny carbides with C atoms in steel, pinning the grain boundaries to hinder the growth of austenite.[24] Figure 3(a) shows that between 600 °C and 800 °C, C atoms still existed in the form of carbide particles, and the change in temperature had little effect on the microstructure. A comparison of the data in Figures 1(a) and 9(a) reveals that the M(Cr, Mo)23C6 particles were very stable when 40Cr10Si2Mo steel was heated from room temperature to 900 °C and their size and distribution hardly changed. Above 1050 °C, the coarsening of the austenite grains was related to the redissolution of the M(Cr, Mo)23C6 particles. The atomic sizes of alloying elements such as Cr and Mo differ significantly from those of Fe, facilitating the formation of a solute drag effect.[25,26] The reconcentration of many solute atoms at grain boundaries or subgrain boundaries can prevent the migration of the grain boundaries, thereby inhibiting recrystallization. M(Cr, Mo)23C6 particles precipitate first at the grain boundaries and dislocation lines, pinning the austenite grain boundaries and hindering the growth of the austenite grains. Grain boundary migration causes the austenite grains to grow. When the grain boundary contacts M(Cr, Mo)23C6 particles, the surface energy increases and only when the thermal activation energy exceeds this increased surface energy can the M(Cr, Mo)23C6 particles be cut by or bypassed around the grain boundary. Thus, M(Cr, Mo)23C6 particles significantly slow the formation of austenite, impeding grain growth.[27,28]
The process of austenitization occurs simultaneously with the coarsening and dissolution of precipitated phases. When the dissolution temperature of the second-phase particles is lower than the austenitization temperature, the particles gradually dissolve. Conversely, if the austenitization temperature does not reach the dissolution temperature of the second phase, Ostwald ripening[29] occurs, whereby smaller particles dissolve, while larger particles coarsen. The coarsening and dissolution of second-phase particles affect the size and microstructure of austenite grains after cooling.[30,31,32] The solute concentration around small particles is greater than that around large particles, causing solute atoms to diffuse from small to large particles, resulting in the redissolution of small particles and the growth of large particles. Therefore, with a sufficiently long holding time at 1000 °C, fine precipitates redissolved continuously, forming larger carbide particles, which hindered grain growth.
The growth of grains in alloys is facilitated by the migration of grain boundaries, which do not occur simultaneously. The migration direction is relatively random, and the migration distance varies at the same time. The probability of migration is greater for smaller grain boundaries and curved grain boundaries, with a higher probability of migration toward the center of curvature.[33] Figures 10(a) and (b) show that at higher temperatures, when atoms have sufficient diffusion ability, they diffuse from the concave side of the grain to the convex side, causing the interface to move toward the center of curvature; this results in the continuous growth of grains on the convex side, whereas grains on the concave side shrink and disappear until the grain boundaries become flat. When the driving force for interface movement is zero, a relatively stable state can be achieved. Grain boundary migration always occurs toward the center of curvature. As the grain boundaries migrate, small grains (those with fewer than 6 boundaries) are gradually absorbed into adjacent larger grains (those with more than 6 boundaries), and the grain boundaries tend to become straight. The junction angle of three grains tends toward 120 deg, putting the grain boundaries in equilibrium.[34] Although grains do not grow into regular hexagons, they still follow the general laws of grain growth. When the grains grow to a larger size, the original grain boundaries gradually disappear, and new boundaries appear, which may be due to grain growth at this time through subcrystalline rotation.[35] As the grains grow larger, the proportion of high-angle grain boundaries decreases, whereas that of low-angle grain boundaries increases. At the same temperature, as the grains grow larger, the migration rate of the grain boundaries rapidly decreases. When the driving force for grain boundaries is small and migration is difficult, subgrains near the grain boundaries form new, larger grains through rotation. In summary, Figure 10(c) schematically illustrates the mechanism of austenite grain growth in 40Cr10Si2Mo steel. At lower temperatures (900 °C), some ferrite transforms into austenite, and the austenite grain boundaries are not obvious. With prolonged holding time, the volume fraction of austenite increases. When the temperature increases to 1000 °C, small M(Cr, Mo)23C6 particles continuously dissolve into the matrix, whereas large M(Cr, Mo)23C6 particles segregate along the grain boundaries. Greater thermal activation energy is required for grain growth. When the temperature further increases to 1100 °C, exceeding the dissolution temperature of the M(Cr, Mo)23C6 particles, grain growth accelerates, and the junction angle of the grain boundaries tends toward 120 deg. With a sufficiently long holding time, subgrain rotation at some grain boundaries results in the formation of new, larger grains.
Martensitic Transformation Mechanism
Different prior austenite grain sizes affect the martensitic transition temperature and phase transition of supercooled austenite during cooling. The transformation from austenite to martensite results in volume expansion and strain energy consumption. The formation of extremely high dislocation densities results in crystal defect storage energy consumption.[36,37] These energy consumptions occur within the range of the difference in free enthalpy between the two phases (the driving force for phase transformation) and do not require additional shear energy. Therefore, the martensitic transformation in steel can be quickly completed under the influence of the driving force for phase transformation.[38] The experimental results indicate that the starting temperature of the martensitic transformation (Ms) is lower in finer grains at lower temperatures.
Figure 11 shows in situ observations, inverse pole figures (IPFs), and reconstructed parent phase austenite maps of 40Cr10Si2Mo steel under different austenitization parameters (See Supplementary video). Higher quenching temperatures result in larger grain sizes and greater driving forces for martensitic phase transformation. Concurrently, the migration rate of the phase interfaces also increases, leading to faster martensitic phase transformation. The prior austenite grain sizes obtained through EBSD reconstruction are consistent with the in situ observation results. During the early stages of martensitic transformation, the nucleation and growth of martensite grains in different parent phase austenite grains do not interfere with each other, resulting in a lower volume fraction of martensite. As the temperature decreases, the martensitic phase transformation gradually increases, and martensite plates constrain each other.[39]
Figure 11(g) shows that martensite nuclei do not initially exist in the parent phase but rather preferentially form at the triangular boundary of the prior austenite and then grow along the austenite grain boundaries into the interior of one side of the grain. The new phase α has a K–S relationship with the parent phase austenite (γ), maintaining a coherent relationship, whereas the austenite grains on the other side of the grain boundary have a different phase and do not provide suitable conditions for growth.[40] The growth of martensite is a process of phase interface migration. Owing to the relative tolerance and other factors, the increase in elastic strain energy blocks the martensite growth process, and the martensite finally stops growing. In different regions, the free-nucleated primary martensite (FM) grows into large areas along the inertial plane from the grain boundary to the grain interior, followed by a small secondary martensite (SM) with an angular distribution among the primary martensite. The growth of martensite nuclei occurs through a nondiffusional, collectively cooperative displacement mechanism. During the lattice reconstruction process from austenite to martensite, defects such as dislocations, stacking faults, and interfaces provide defect energy to assist in nucleation, providing favorable conditions for structural fluctuations and energy fluctuations that serve as nucleation sites.[41,42] The martensitic transformation mechanism of 40Cr10Si2Mo steel is illustrated in Figure 12. Martensite nucleates along the PAG boundaries and grows between grains until it encounters other lath martensite or grain boundaries, where it stops. Simultaneously, martensite nucleates at already formed primary lath martensite sites and grows parallel to a certain angle direction, forming new secondary lath martensite. The formation of secondary lath martensite also inhibits the phase transformation of the surrounding untransformed austenite, promoting the conversion of retained austenite.
Conclusion
This study, which is based on in situ observations via HT-LSCM, presents an investigation of the grain evolution and martensitic phase transformation of 40Cr10Si2Mo steel during 1800 seconds of isothermal holding at temperatures ranging from 900 °C to 1250 °C. This work establishes a kinetic equation for grain growth, providing theoretical support for optimizing the process performance of steel. The main conclusions are as follows:
-
(1)
The austenite grain size of 40Cr10Si2Mo steel increases from 41.37 to 201.76 μm when the heating temperature increases from 1000 °C to 1250 °C. The activation energy of grain growth increases. The grain size increases with time as a power function, and the growth rate is the smallest after 600 seconds of insulation.
-
(2)
An austenitic grain growth kinetics model of 40Cr10Si2Mo steel was obtained by fitting the Beck equation and the Dong equation. The results show that the Dong dynamic model has higher prediction accuracy and is more suitable for predicting austenite grain growth under different holding temperatures and holding times.
-
(3)
At 900 °C, the ferrite portion of 40Cr10Si2Mo steel transforms into austenite, with indistinct grain boundaries, and as the holding time increases, the volume of austenite increases. When the temperature reaches 1000 °C, Ostwald ripening occurs, M(Cr, Mo)23C6 precipitates or dissolves continuously and a relatively high thermal activation energy can meet the conditions for grain growth. When the temperature increases above 1100 °C, M(Cr, Mo)23C6 is completely dissolved, grain growth accelerates, the grain boundary intersection angle tends to 120 deg, and after a long enough holding time, parts of the grain boundaries form larger grains through subcrystalline rotation.
-
(4)
Owing to the K–S relationship, the primary martensite nucleates along the grain boundary of the prior austenite and grows between grains. As the interface moves, the martensite eventually stops growing due to the increase in elastic strain energy caused by the relative tolerance and other factors. The secondary martensite nucleates at the formed first lath martensite and grows parallel to it in a certain angular direction. The formation of secondary lath martensite also inhibits the phase transition of the surrounding untransformed austenite.
References
G.K. Ahiale, J.S. Yoo, S. Lee, D.Y. Park, and Y.-J. Oh: Mater. Sci. Eng. A, 2018, vol. 725, pp. 290–98.
Y. Zhang, D. Zou, T. Wei, J. Li, L. Tong, and W. Zhang: Mater. Res. Express, 2020, vol. 7, p. 036513.
Y. Zhao, H.-L. Liu, L.-L. Wei, and L.-Q. Chen: Tungsten, 2023, vol. 5, pp. 467–80.
Q. Wang, Q. Wang, Z. Du, Z. He, X. Dang, Z. Qi, and C. Yang: J. Iron. Steel Res. Int., 2023, vol. 30, pp. 760–71.
C. Celada-Casero, J. Sietsma, and M.J. Santofimia: Mater. Des., 2019, vol. 167, p. 107625.
D.M. Xu, G.Q. Li, X.L. Wan, R.D.K. Misra, J.X. Yu, and G. Xu: Mater. Sci. Eng. A, 2020, vol. 773, p. 138722.
C.-K. Lin, Y.-H. Su, W.-S. Hwang, G.-R. Lin, and J.-C. Kuo: Mater. Sci. Technol., 2018, vol. 34, pp. 596–606.
Q. Wang, Q. Ye, W. Wang, X. Deng, and Z. Wang: IOP Conf. Ser. Mater. Sci. Eng., 2019, vol. 562, p. 012126.
X.-G. Zhou, X. Li, C.-Y. Zeng, S.-W. Wu, and Z.-Y. Liu: Trans. Indian Inst. Met., 2023, vol. 76, pp. 3115–25.
Z. Liu, Y. Bao, M. Wang, X. Li, and F. Zeng: Int. J. Miner. Metall. Mater., 2019, vol. 26, pp. 282–90.
R.A.M. Napitupulu: IOP Conf. Ser. Mater. Sci. Eng., 2017, vol. 237, p. 012038.
T. Garcin, K. Ueda, and M. Militzer: Metall. Mater. Trans. A, 2017, vol. 48A, pp. 796–808.
Y. Xu, J. Liu, Y. Zhao, and Y. Jiao: Philos. Mag., 2021, vol. 101, pp. 77–95.
G. Ji, X. Gao, Z. Liu, and K. Zhang: J. Iron. Steel Res. Int., 2019, vol. 26, pp. 292–300.
S. Mahajan, C.S. Pande, M.A. Imam, and B.B. Rath: Acta Mater., 1997, vol. 45, pp. 2633–38.
S. Wang and L.E. Murr: Metallography, 1980, vol. 13, pp. 203–24.
R. Chen, Z. Zheng, N. Li, J. Li, and F. Feng: Mater. Charact., 2018, vol. 144, pp. 400–10.
C. Du, J.P.M. Hoefnagels, S. Kölling, M.G.D. Geers, J. Sietsma, R. Petrov, V. Bliznuk, P.M. Koenraad, D. Schryvers, and B. Amin-Ahmadi: Mater. Charact., 2018, vol. 139, pp. 411–20.
Y. Dong, L. Xiang, C. Zhu, Y. Du, Y. Xiong, X. Zhang, and L. Du: J. Mater. Res. Technol., 2023, vol. 27, pp. 5411–23.
C. Wang, K.F. Lin, Y.L. Zhao, T. Yang, T.L. Zhang, W.H. Liu, C.H. Hsueh, H.C. Lin, J.J. Kai, and C.T. Liu: Mater. Sci. Eng. A, 2020, vol. 786, p. 139371.
P.A. Beck, J.C. Kremer, and L. Demer: Phys. Rev., 1947, vol. 71, pp. 555–655.
Y. Zhang, X. Li, Y. Liu, C. Liu, J. Dong, L. Yu, and H. Li: Mater. Charact., 2020, vol. 169, p. 110612.
D. Dong, F. Chen, and Z. Cui: J. Mater. Eng. Perform., 2016, vol. 25, pp. 152–64.
J.M. Zhang, C.Y. Huo, Q.R. Ma, and Y.R. Feng: Int. J. Press. Vessels Pip., 2018, vol. 165, pp. 29–33.
V.K. Devra and J. Maity: Philos. Mag. Lett., 2020, vol. 100, pp. 245–59.
T. Luo, D. Mangelinck, F. Serrano-Sánchez, C. Fu, C. Felser, and B. Gault: Acta Mater., 2022, vol. 226, p. 117604.
C. Dong, Z. Liu, Z. Chen, H. Bao, X. Wang, and Z. Liu: J. Alloys Compds, 2020, vol. 825, p. 154106.
C. Sun, J. Li, J. Zhang, W. Yan, and S. Li: J. Iron. Steel Res. Int., 2023, vol. 30, pp. 2000–09.
B.V. Ivanskii and R.D. Vengrenovich: Phys. Met. Metallogr., 2016, vol. 117, pp. 756–65.
L. Yang, Y. Li, Z. Xue, and C. Cheng: China Foundry, 2017, vol. 14, pp. 421–28.
J.-S. Park, Y.-S. Ha, S.-J. Lee, and Y.-K. Lee: Metall. Mater. Trans. A, 2009, vol. 40A, pp. 560–68.
P. Gong, E.J. Palmiere, and W.M. Rainforth: Acta Mater., 2015, vol. 97, pp. 392–403.
H. Zhang, M. Long, P. Tang, S. Ai, W. Guo, D. Chen, and J. Wu: Steel Res. Int., 2023, vol. 94, p. 2300103.
Y. Zhang, G. Shen, Z. Wang, J. Gu, and J. Zhang: J. Mater. Res. Technol., 2023, vol. 26, pp. 7381–94.
B.R. Patterson and Y. Liu: Metall. Trans. A, 1992, vol. 23, pp. 2481–82.
M.J. Santofimia, L. Zhao, R. Petrov, C. Kwakernaak, W.G. Sloof, and J. Sietsma: Acta Mater., 2011, vol. 59, pp. 6059–68.
D.P. Koistinen and R.E. Marburger: Acta Metall., 1959, vol. 7, pp. 59–60.
O. Fabrichnaya, C. Ullrich, M. Wendler, G. Savinykh, and D. Rafaja: J. Alloys Compds, 2016, vol. 686, pp. 511–21.
W. Huang, L. Lei, and G. Fang: Mater. Charact., 2020, vol. 163, p. 110307.
Y.Z. Li, M. Wang, and M.X. Huang: Mater. Sci. Eng. A, 2021, vol. 811, p. 141061.
Z. Li, Q. Yuan, S. Xu, Y. Zhou, S. Liu, and G. Xu: Materials, 2023, vol. 16, p. 16103840.
M. Sinha, A. Karmakar, B. Syed, and S. Ghosh: Metall. Mater. Trans. A, 2020, vol. 51A, pp. 3435–46.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Number 52174371), the National Key Research and Development Program of China (Grant Number 2021YFB3501003), and the Shaanxi Provincial Science and Technology Department Enterprise Joint Fund (Grant Number 2021JLM-33).
Data Availability
The raw data required to reproduce these findings cannot be shared at this time, as the data also form part of an ongoing study. The processed data required to reproduce these findings cannot be shared at this time, as the data also form part of an ongoing study.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
(AVI 36427 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, T., Wang, Q., Du, Z. et al. Research on the Austenite Grain Growth Behavior and Martensitic Phase Transformation Mechanism of 40Cr10Si2Mo Steel via In Situ Observation. Metall Mater Trans B 55, 4044–4058 (2024). https://doi.org/10.1007/s11663-024-03229-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-024-03229-5