Abstract
The distribution of iron-bearing granular materials in the throat of a blast furnace (BF) plays a crucial role in influencing their performance at high temperatures. Therefore, it is essential to establish a quantitative relationship between the charging operation of the iron-bearing materials at ambient temperatures and their subsequent softening and melting behaviors at elevated temperatures. In this study, the discrete element method (DEM) is employed to quantify the instantaneous mass segregation of quaternary iron-bearing materials throughout the continuous charging process, starting from the feeding conveyor belt and continuing up to the throat. Subsequently, employing quaternary basicity (R4) as a pivotal bridge, softening and melting experiments (the temperature above 900 °C and in an atmosphere with a mole fraction of 30 pct CO to 70 pct N2) are conducted to assess the influence of physical segregation on chemical performances under simulated BF conditions. The results reveal that the sequence of loading quaternary iron-bearing materials onto the feeding belt causes fluctuations in R4, ranging from 1.08 to 1.75. Moreover, these fluctuations are propagated throughout the charging process, resulting in notable fluctuations in the mass fractions of iron-bearing materials and the R4 at the hopper outlet and the end of the chute. Therefore, the primary factor influencing the flowing characteristics of the granular materials is their distribution within the hopper. Then, the segregation in the throat is further characterized by the presence of two distinct R4 ranges (1.2 to 1.29, and 1.3 to 1.39) observed across a total of 48 equal-area blocks, and the significant difference between these two categories is determined by sinters. Besides, the influence of the R4 on softening and melting temperatures is quantitatively evaluated, resulting in a ‘w’-shaped temperature distribution from the center to the edge of the BF. Our findings provide evidence of the inadequacy of evaluating the softening and melting behaviors of iron-bearing materials solely based on their initial proportions in the structure of iron-bearing materials. Instead, a quantitative examination of the granular segregation in the throat, arising from the BF charging operation, is deemed essential to provide substantial support for well-designed elevated-temperature experiments. This approach enables a more comprehensive understanding of the intricate journey of iron-bearing materials in the BF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The blast furnace (BF) operates as a multi-phase counter-flow reactor, efficiently reducing iron-bearing materials to produce hot metal.[1,2,3,4] The distribution of granular materials within the BF is significantly influenced by the charging operation, thereby affecting gas flow, heat transfer, and reaction efficiency. As a result, extensive efforts have been devoted to achieving a comprehensive understanding of granular segregation behaviors during the charging process via industrial-scale measurements,[5] lab-scale experiments,[6,7,8] mathematical model analysis,[9,10,11] and discrete element method (DEM) simulations.[12,13,14]
The continuous charging process of the BF can be divided into three key stages. In the first stage, iron-bearing granular materials are loaded onto a conveyor belt, where the specific charging sequence induces the onset of granular segregation.[15] In addition, the placement of the main feeding belt influences the trajectory of granular motion within the hopper.[16] Moving to the second stage, the iron-bearing granular materials are charged into a hopper where the hopper’s shape and size directly impact the flow dynamics of the granular materials. For a wedge-shaped hopper, smaller granules demonstrate enhanced efficiency in converting kinetic energy during discharge.[17] Simultaneously, the slope of the lower part of the hoppers affects granular size distribution exclusively during the discharge process.[18] Furthermore, when granular materials are charged into a conical-shaped hopper, mass discharge rates reduce granular segregation during hopper discharge.[19,20] Subsequently, the granules will pass through a Y-shaped tube. However, the motion and segregation behaviors of the granules are not influenced by the angle of the Y-shaped tube.[21] Entering the third stage, the iron-bearing granular materials reach the BF throat through a rotating chute. The trajectory of the flowing granular materials is primarily influenced by centrifugal and Coriolis forces exerted on the granules by the rotating chute.[12,22] The interaction between chute curvature and the Coriolis force led to circumferential mass, size, and porosity segregation[23,24,25,26,27,28,29,30] which is also affected by the effective lengths of chutes,[24] the size ratio,[31] and so on. In addition, the chute shape also played a critical role, with a rectangular-shaped chute promoting a uniform circumferential distribution of granular materials, whereas a trapezoidal chute encouraged aggregation at lower rotating speeds.[25,32]
After the iron-bearing materials are discharged, these materials descend further, and as the temperature in the furnace increases, these materials begin to soften and melt, and form a cohesive zone.[33,34] The cohesive zone, however, exhibits poor permeability, which directly affects the gas flow re-distributed within the furnace.[35] Therefore, softening and melting tests[36,37,38,39,40] are widely used to provide assessments of how iron-bearing materials perform in the high temperature of a BF. For single-component materials, an increase in the basicity of sinters leads to a deterioration in both pressure drop and permeability.[41,42] Conversely, increasing the MgO content of pellets enhances their high-temperature properties.[43] Moreover, it is widely acknowledged that lump ores perform inferior to sinters at high temperatures.[44] In addition, the softening and melting temperatures of the samples increased with increasing basicity under different atmospheres.[45] However, high-temperature interaction between iron-bearing materials can improve the softening and melting characteristics.[46] Specifically, the interaction behaviors between lump ores and sinters[44] can significantly improve the softening temperature of lump ores, and the softening and melting properties of the oxidized pellets are dramatically improved by the interaction between oxidized pellets and metalized pellets.[47] However, there is no obvious interaction between lump ore and pellets, pellets and pellets, and lump ore and lump ore.[48]
Undoubtedly, the granular segregation of iron-bearing materials in the BF throat has a vital impact on their chemical reactions at high temperatures. Although previous studies have provided comprehensive insights into granular segregation behaviors, a critical gap remains in establishing a comprehensive connection between the physical segregations resulting from the charging operation and the subsequent chemical performances in the BF. To address this gap, this study first employs the DEM to simulate the granular flow behaviors of quaternary iron-bearing materials, including sinters, alkaline pellets, acid pellets, and lump ores, during the continuous charging process. The quaternary basicity (extended Vee ratio[49]), denoted as \(R_{4} = \frac{{{\text{wt}}\left( {{\text{CaO}}} \right) + {\text{wt}}\left( {{\text{MgO}}} \right)}}{{{\text{wt}}\left( {{\text{SiO}}_{{2}} } \right) + {\text{wt}}\left( {{\text{Al}}_{{2}} {\text{O}}_{{3}} } \right)}}\), where \({\text{w}}{\text{t}}\left({\text{CaO}}\right)\), \(\text{wt(MgO)}\), \({\text{wt}}\left({{\text{S}}{\text{i}}{\text{O}}}_{2}\right)\), and \(\text{wt(}{\text{Al}}_{2}{{\text{O}}}_{3}\text{)}\) represent the mass fractions of CaO, MgO, SiO2, and Al2O3, respectively, serves as a key link enabling the elaborate design of schemes for subsequent softening and melting experiments to evaluate the performance of iron-bearing materials with different proportions at temperatures above 900 °C and in an atmosphere with a mole fraction of 30 pct CO to 70 pct N2. This study pioneers an innovative approach to bridge the gap between iron-bearing materials’ charging operation and iron-bearing materials’ softening and melting behaviors, shedding light on how the physical behaviors of granular materials during the charging process profoundly influence their chemical performance at elevated temperatures.
Experimental Procedure
Iron-Bearing Granular Materials
The quaternary iron-bearing granular materials include sinters, alkaline pellets, acid pellets, and lump ores. Their physical properties and chemical compositions are summarized in Tables I and II, respectively.
Softening and Melting Experiments
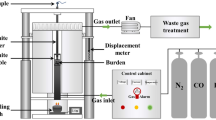
In Figure 1(a), a schematic diagram of the apparatus utilized for the softening and melting experiments is presented. To conduct the experiments, 150 g of iron-bearing granular materials are sandwiched between two layers of 15 g of cokes within a graphite crucible, as depicted in Figure 1(b). The graphite crucible features nine uniformly distributed holes with a diameter of 6 mm at its bottom. Subsequently, the crucible is placed in the furnace and heated from the ambient temperature to 1500 °C as the heating rate is explained in Figure 1(c). Notably, a reducing gas mixture (30 pct CO to 70 pct N2 in mole fraction) instead of an inert gas (100 pct N2), is injected into the furnace once the samples have reached 900 °C. In addition, a displacement sensor records the instantaneous shrinkage of the sandwich-like packed bed, a pressure sensor captures the instantaneous pressure drop, and a weight sensor records the weight of drippings. Further details on the softening and melting experiments can be found in previous studies.[50,51,52,53]
Numerical Simulation Conditions
A 5500 m3 full-scale bell-less top model of BF with two parallel hoppers is established in Figure 2(a). It consists of six main parts arranged from top to bottom: the feeding system, receiving hopper, two parallel hoppers, central throat tube, rotating chute, and throat. In particular, Figure 2(b) provides a further explanation of the feeding system. The granular materials are initially charged onto the B-2 belt (80.52 m in length and 2.20 m in width). Before being discharged into the receiving hopper, they are subsequently conveyed onto the B-1 belt (6.00 m in length) at the end of the B-2 belt. The minimum angle between the two crossed belts is 62.16 deg, and both belts maintain a velocity of 2.00 m/s.
In addition, the charging process of quaternary iron-bearing granular materials is simulated by the DEM.[54] Briefly, the Newton’s equations of motion are used to determine the translational and rotational motions of each particle:
where mi, Ii, ui, and ωi represent the mass, inertial moment, translational velocity, and angular velocity of particle i, respectively. K is the total number of particles in contact with particle i. The forces acting on particles involve the gravitational force, mig, and inter-particle forces in terms of the normal force, \({{\varvec{F}}}_{{\varvec{n}},{\varvec{i}}{\varvec{j}}}\), and tangential force, \({{\varvec{F}}}_{{\varvec{t}},{\varvec{i}}{\varvec{j}}}\), which are determined by the Hertz–Mindlin model.[17] Torque Tt,ij arises from the tangential forces, while Tr,ij is the rolling friction torque, which is generated by the asymmetric normal contact force.
The parameters used in the DEM simulation are summarized in Table III. The diameters of quaternary granular materials in the simulation are enlarged by a factor of five compared to their actual properties to save computational resources. Therefore, these parameters are first validated by comparing the predicted repose angles with the experimental measurements in Figure 3. The simulation results closely match the experiments, with errors mostly below 5 pct, except for a 7.06 pct error for the alkaline pellets. Overall, the parameters in the simulations effectively capture the granular behaviors of these iron-bearing granular materials.
Besides, a charging matrix labeled as \(O_{3,3, 4,4,3,2,1,1}^{39.5, 38, 36,34,32,30,28,30\,\deg }\) is used to charge a batch of granular materials. Specifically, the superscripts represent the chute charging angle, which is the angle between the centerlines of the chute and the BF. The subscripts correspond to the number of revolutions performed at each angle. In addition, the rotational speed of the chute is maintained at 8 rpm/min.
Results and Discussion
Instantaneous Basicity Segregation in the BF Charging Process
The charging process can be divided into three primary stages: materials feeding onto the belt, charging into and discharging out of the hopper, and distribution within the throat through the chute in Figure 4(a). As a result, the mass fraction distributions of quaternary iron-bearing materials in the granular mixtures, along with the calculated R4 distribution, are observed at three locations: the initiation of the feeding belt, the outlet of the parallel hopper, and the end of the chute. These corresponding results are recorded with respect to the dimensionless charging time in Figures 4(b) to (e).
In a batch of granular mixtures, the initial mass fractions of sinters, alkaline pellets, acid pellets, and lump ores are 40, 30, 24, and 6 pct, respectively. The R4 value for this proportion is calculated to be 1.30. During the first charging stage, from t = 0 to 0.3, the quaternary iron-bearing granular materials are sequentially fed onto the belt according to the charging sequences depicted in Figure 4(b). This sequential feeding results in significant fluctuations in the mass fraction and corresponding R4 along the belt. Specifically, as shown in Figure 4(c), sinters are initially fed onto the belt, followed by alkaline and acid pellets starting at t = 0.03. This sequence causes the average R4 to decrease from 1.75 to approximately 1.27. Subsequently, lump ores are introduced at t = 0.09, further reducing the average R4 to 1.08. At this point, the mass fractions of sinters, alkaline pellets, acid pellets, and lump ores are 29.08, 28.40, 27.68, and 14.84 pct, respectively. Upon completion of the charging operations for lump ores, acid pellets, and alkaline pellets on the belt at t = 0.18, 0.23, and 0.27, respectively, the average R4 steadily increases to 1.27, 1.58, and 1.75 in sequence.
Once all granular mixtures have settled within the hopper, the bottom outlet is opened during the second charging stage from t = 0.48 to 0.97 (as shown in Figure 4(d)). It is commonly understood that the granular mixtures above the outlet have priority over those close to the walls for being discharged.[55] Therefore, there are three noteworthy points observed during the discharge process. First, before t = 0.6, the mass fractions of sinters and alkaline pellets rapidly decrease at the outlet of the hopper, while the mass fraction of acid pellets continues to increase, eventually reaching a similar level with average values of 35.82, 32.01, and 27.83 pct respectively. This results in a continuous decrease in R4, followed by slight fluctuations before stabilizing at around 1.3 until t = 0.8. Then, from t = 0.8 to 0.93, the mass fraction of sinters abruptly climbs to approximately 62.38 pct and then decreases to around 25.52 pct, while the mass fractions of alkaline and acid pellets continue to decrease to around 16 pct. Since before the end of the belt feeding, all other iron-bearing materials except for the sinter have been fully fed onto the belt based on the charging sequence, resulting in the sinter becoming the main iron-bearing material on the top of the hopper and leading to R4 first increasing at 1.52 and then decreasing at around 1.11. Finally, some other residual iron-bearing materials remained in the hopper due to their location near the walls at the end of charging, causing huge fluctuations in the mass fractions of sinters and alkaline pellets and temporarily increasing R4 to 1.62.
Simultaneously, the quaternary iron-bearing materials are distributed in the throat of the BF through a rotating chute. During the third charging stage from t = 0.5 to 1.0, the mass fraction and calculated R4 below the chute are recorded in Figure 4(e). The mass fraction and R4 exhibit similar patterns as in the second stage. Therefore, the flow characteristics of iron-bearing granular materials in the BF remain consistent, and these characteristics are determined by the distribution of materials in the hopper.
Mapping the Granular Segregation in the Throat
Throughout the three consecutive stages, the quaternary iron-bearing materials are distributed in the BF throat. The cross section of the throat is divided into eight equal sectors in the circumferential direction and six rings with the same area in the radial direction, as illustrated in Figure 5(a). The mass distributions of iron-bearing granular materials and the calculated R4 in these 48 blocks are quantitatively depicted in Figure 5(b). First, the mass distributions of quaternary iron-bearing granular materials are generally increased in the radial direction. For example, in sector I, the mass of quaternary iron-bearing materials is 491.66 kg in ring I, and it increases to 6388.29 kg in ring V, and then slightly decreases to 5563.44 kg in ring VI. Second, since sinters are the dominant iron-bearing materials in ring I, the calculated R4 varies from 1.40 to 1.55. In the remaining regions, the spatial distribution of R4 mainly falls into two categories. In category C1, R4 ranges from 1.2 to 1.29 across 22 blocks, with an average value of 1.28. On the other hand, in category C2, R4 varies from 1.3 to 1.39 across 18 blocks, with an average value of 1.32. In addition, Figure 5(c) illustrates the distribution of R4 in the throat for these two categories. Category C2 is primarily concentrated in the center, and at the edges of sector I to sector III of the throats. Consequently, the basicity distribution in the throat is non-uniform.
Figure 6 presents the mass fractions of quaternary iron-bearing granular materials for categories C1 and C2. In both categories, sinters have the highest mass fraction, while lump ores have the lowest. Notably, category C2 exhibits a significantly higher mass fraction of sinters, reaching almost 40 pct or even higher, with an average value that exceeds category C1 by 3.28 pct. However, the mass fractions of other iron-bearing granular materials in category C2 are lower compared to category C1. Specifically, for category C1, the mass fractions of alkaline pellets and acid pellets range from 27.14 to 33.37 pct and 23.87 to 27.06 pct, respectively. However, lump ores have a mass fraction only ranging from 3.43 to 7.85 pct. Furthermore, the average mass fractions of alkaline pellets, acid pellets, and lump ores for category C2 are 29.69, 22.84, and 5.79 pct, respectively. These findings indicate that the notable difference between these two categories of iron-bearing materials is determined by sinters.
Hence, in the subsequent section, three typical proportions of iron-bearing materials which include a proportion from each of the two R4 categories and one initial proportion are selected for the elevated-temperature experiments.
Influence of Granular Segregation on Their Performance at Elevated Temperatures
According to the quantitative analysis of the granular segregation in the mapping throat, two typical schemes of quaternary iron-bearing materials with R4 of 1.28 (denoted as Q-1) for category C1 and 1.32 (denoted as Q-2) for category C2, are established in Table IV, in comparison to the scheme with initial R4 of 1.3 (denoted by Q-0) and four single-component schemes (denoted as S-0 to S-4).
The softening and melting behaviors of four single-component schemes and three quaternary-component schemes are measured and compared in Figure 7(a). T10 represents the softening beginning temperature at which the samples exhibit a 10 pct reduction in volume, and T40 represents the softening ending temperature at which the samples feature a 40 pct reduction in volume. Consequently, the softening range is defined as ∆T1 = T40 − T10. Besides, Ts represents the temperature at which the pressure drop of the packed bed reaches 500 Pa, defining the onset of the melting process. Td represents the temperature at which the samples start dripping. As a result, the melting range is defined as ∆T2 = Td − Ts. Furthermore, ∆Pmax is defined as the maximum pressure drop of the packed beds above 900 °C, and the results are compared in Figure 7(b).
As depicted in Figure 7, among the single-component schemes, the lump ore (S-3) and acid pellet (S-2), characterized by lower R4, exhibit lower T10 temperatures at 967.5 °C and 970.9 °C, respectively. As the R4 increases, the T10 temperature correspondingly rises, resulting in comparatively higher T10 temperatures for alkaline pellet (S-1) and sinter (S-0), reaching 1051.5 °C and 1036.7 °C, respectively. Furthermore, the sinter and alkaline pellet feature a wider softening range compared to the acid pellet and lump ore. In terms of melting performance, the lump ore and acid pellet exhibit lower Ts temperatures at 1092.9 °C and 1043.6 °C, respectively, yet possess wider melting ranges of 290.4 °C and 264.5 °C. Conversely, the alkaline pellet and sinter have higher Ts temperatures at 1249.1 °C and 1213 °C, respectively, with the former having a narrower melting range of only 33.2 °C, and no dripping observed for the latter under present experimental conditions. However, all the single-component schemes demonstrate similar ∆Pmax values at approximately 1.5 kPa, although the acid pellet has a slightly higher ∆Pmax value at around 1.8 kPa.
Moreover, for the quaternary iron-bearing materials schemes, both T10, T40, and Ts temperatures increase with higher R4. Despite this, the softening range remains consistent at around 110°C. Conversely, the ∆Pmax values increase as R4 rise, resulting in ∆Pmax values of 2.1, 3, and 4.9 kPa for Q-1, Q-0, and Q-2, respectively. Notably, there is a marginal 2 pct increase in lump ore for Q-1 compared to the initial proportion for Q-0, leading to a decrease in the R4 and a significant reduction in the T10 temperature of the quaternary iron-bearing materials. Besides, the proportion of sinter in the quaternary iron-bearing materials has a direct influence on ∆Pmax, displaying a linear relationship. For instance, comparing Q-0 with Q-1, which has 1 pct less proportion of sinters in the mixture, there is a corresponding decrease of 0.9 kPa in ∆Pmax. Conversely, in the case of Q-2, where the sinter proportion increases by 2 pct compared to Q-0, there is an observed increase of 2.8 kPa in ∆Pmax. In summary, except for the sinter, the basicity increases the temperature at which iron-bearing materials start softening. Furthermore, the characteristic temperatures of quaternary-component schemes consistently surpass those of single-component materials, owing to the interaction behaviors between the iron-bearing materials involved. Additionally, Figure 8 illustrates the distribution of softening and dripping temperatures based on transient R4 in the throat, as well as the distribution simply based on the initial R4 in a BF. The figure reveals that the actual quaternary iron-bearing materials in the throat exhibit lower softening and dripping temperatures compared to the counterparts associated with the initial iron-bearing materials proportion. Furthermore, in regions characterized by higher R4, the softening and dripping temperatures are higher than those characterized by lower R4, creating a distinct ‘w’-shaped temperature distribution from the center to the edge in the BF.
Conclusions
This study presents an innovative approach to establishing a quantitative relationship between the physical segregation of quaternary iron-bearing materials during the charging process and the subsequent chemical performance in the BF using DEM simulation and elevated-temperature experiments, with quaternary basicity as a pivotal bridge. The key findings can be summarized as follows.
-
(1)
Our investigation reveals distinct variations in mass fractions and R4 at different locations in the BF charging system. The distribution of quaternary iron-bearing granular materials on the feeding belt is significantly influenced by the charging sequence. As a result, the corresponding R4 varies from 1.75 to 1.08, then to 1.75. This impact propagates throughout the charging process, leading to notable fluctuations in the mass fractions of iron-bearing materials and the determined R4 at the hopper outlet and the end of the chute. The distribution of granular materials in the hopper is a primary determinant of their flowing characteristics.
-
(2)
The BF throat is divided into 48 equal-area blocks, revealing a radial increase in the mass distributions of quaternary iron-bearing materials. Notably, the R4 in these blocks predominantly fall into two categories, C1 with 22 blocks having an average R4 of 1.28, and C2 with 18 blocks having an average R4 of 1.32. Moreover, the significant difference between these two categories is determined by sinters.
-
(3)
The softening and melting characteristics of single iron-bearing materials reveal that the materials with lower R4 exhibit lower softening beginning temperatures, narrower softening ranges, and wider melting temperature ranges compared to materials with higher R4. Conversely, quaternary iron-bearing materials demonstrate interaction effects leading to higher T10 and Ts temperatures, as well as ∆Pmax values, with these values increasing as the R4 increases. Intriguingly, the softening range temperatures remain consistent across all quaternary iron-bearing material schemes.
-
(4)
In comparison to previous evaluations of softening and melting behaviors solely based on the initial proportions of iron-bearing materials in the mixture, this study demonstrates a ‘w’-shaped temperature distribution from the center to the edge in the BF by considering the influence of granular segregation during the charging operation.
References
D. Proctor, K. Fehling, E. Shay, J. Wittenborn, J. Green, C. Avent, R. Bigham, M. Connolly, B. Lee, T. Shepker, and M. Zak: Environ. Sci. Technol., 2000, vol. 34, pp. 1576–82.
Z. Liu, J. Zhang, H. Zuo, and T. Yang: ISIJ Int., 2012, vol. 52, pp. 1713–23.
S. Kuang, Z. Li, and A. Yu: Steel Res. Int., 2018, vol. 89, p. 1700071.
G. Wang, Z. Liao, Z. Hu, D. Wang, H. Bai, Z. Zou, and J. Xu: Metall. Mater. Trans. B, 2022, vol. 53B, pp. 931–37.
H. Mio, Y. Narita, S. Matsuzaki, K. Nishioka, and S. Nomura: Powder Technol., 2019, vol. 344, pp. 797–803.
Y. Yu and H. Saxén: Chem. Eng. Sci., 2010, vol. 65, pp. 5237–50.
C. Ho, S. Wu, H. Zhu, A. Yu, and S. Tsai: Miner. Eng., 2009, vol. 22, pp. 986–94.
Y. Yu and H. Saxén: Steel Res. Int., 2013, vol. 10, pp. 1018–33.
V. Radhakrishnan and K. Ram: J. Process. Control., 2001, vol. 11, pp. 565–86.
L. Shi, G. Zhao, M. Li, and X. Ma: Appl. Math. Model., 2016, vol. 40, pp. 10254–73.
H. Zhao, M. Zhu, P. Du, S. Taguchi, and H. Wei: ISIJ Int., 2012, vol. 52, pp. 2177–85.
K. Zhou, Z. Jiang, D. Pan, W. Gui, and J. Huang: Steel Res. Int., 2022, vol. 93, p. 2100332.
Y. Xu, J. Xu, Z. Liao, Y. Pei, L. Gao, C. Sun, M. Kou, and L. Wen, Powder Technol., 2019, vol. 343, pp. 422–35.
H. Mio, M. Kadowaki, S. Matsuzaki, and K. Kunitomo: Miner. Eng., 2012, vol. 33, pp. 27–33.
W. Xu, S. Cheng, and C. Li: Ironmak. Steelmak., 2022, vol. 49, pp. 208–16.
W. Xu, S. Cheng, Q. Niu, and G. Zhao: ISIJ Int., 2017, vol. 57, pp. 1173–80.
Z. Liao, J. Xu, C. Sun, Y. Yang, Y. Pei, M. Kou, Z. Hu, L. Meng, and L. Wen: Adv. Powder Technol., 2020, vol. 31, pp. 670–77.
S. Wu, M. Kou, J. Xu, X. Guo, K. Du, W. Shen, and J. Sun: Chem. Eng. Sci., 2013, vol. 99, pp. 314–23.
X. Huang, Q. Zheng, A. Yu, and W. Yan: Powder Technol., 2020, vol. 361, pp. 179–89.
X. Huang, Q. Zheng, D. Liu, A. Yu, and W. Yan: Chem. Eng. Sci., 2022, vol. 253, p. 117579.
J. Chen, H. Zuo, Y. Wang, Q. Xue, and J. Wang: Metall. Mater. Trans. B, 2022, vol. 53B, pp. 3793–3804.
Z. Hong, H. Zhou, J. Wu, L. Zhan, Y. Fan, Z. Zhang, S. Wu, H. Xu, L. Wang, and M. Kou: Steel Res. Int., 2021, vol. 92, p. 2000262.
J. Chen, H. Zuo, H. Zhao, Q. Xue, and J. Wang: Powder Technol., 2022, vol. 409, p. 117845.
J. Xu, S. Wu, M. Kou, L. Zhang, and X. Yu: Appl. Math. Model., 2011, vol. 35, pp. 1439–55.
M. Kou, J. Xu, S. Wu, H. Zhou, K. Gu, S. Yao, and B. Wen: Particuology, 2019, vol. 44, pp. 194–206.
B. Dai, J. Yang, F. Liu, X. Gu, and K. Lin: Powder Technol., 2020, vol. 363, pp. 611–20.
S. Kumar, S. Khatoon, S. Parashar, P. Dubey, J. Yogi, and A. Anand: Powder Technol., 2023, vol. 427, p. 118682.
Z. Deng, Y. Fan, J. Theuerkauf, K. Jacob, P. Umbanhowar, and R. Lueptow: Powder Technol., 2020, vol. 374, pp. 389–98.
T. Zhang, J. Gan, A. Yu, D. Pinson, and Z. Zhou: Powder Technol., 2020, vol. 361, pp. 435–45.
Y. Yang, C. Sun, Z. Liao, C. Leng, Z. You, and J. Xu: Powder Technol., 2022, vol. 411, p. 117954.
C. Li, K. Dong, S. Liu, G. Chandratilleke, Z. Zhou, and Y. Shen: Powder Technol., 2022, vol. 407, p. 117660.
W. Xu, S. Cheng, Q. Niu, and G. Zhao: Ironmak. Steelmak., 2017, vol. 46, pp. 105–12.
L. Jiao, S. Kuang, A. Yu, Y. Li, X. Mao, and H. Xu: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 258–75.
X. Dong, A. Yu, S. Chew, and P. Zulli: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 330–49.
L. Jiao, S. Kuang, Y. Li, X. Mao, H. Xu, and A. Yu: Metall. Mater. Trans. B, 2023, vol. 54B, pp. 734–55.
X. An, J. Wang, R. Lan, Y. Han, and Q. Xue: J. Iron. Steel Res. Int., 2013, vol. 20, pp. 11–16.
P. Nogueira and R. Fruehan: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 829–38.
P. Nogueira and R. Fruehan: Metall. Mater. Trans. B, 2005, vol. 36B, pp. 583–90.
P. Nogueira and R. Fruehan: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 551–58.
B. Lyu, G. Wang, F. Yang, H. Zuo, Q. Xue, and J. Wang: J. Iron. Steel Res. Int., 2023, vol. 30, pp. 2366–77.
P. Tan, J. Zhang, J. Huang, Y. Wang, Z. Liu, and F. Han: Chin. J. Eng., 2023, vol. 45, pp. 890–98.
T. Li, C. Sun, X. Liu, S. Song, and Q. Wang: Ironmak. Steelmak., 2018, vol. 45, pp. 755–63.
F. Silva, L. Lemos, P. DeFreitasNogueira, and M. Bressan: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 69–76.
C. Loo, L. Matthews, and D. O’dea: ISIJ Int., 2011, vol. 51, pp. 930–38.
B. Lyu, G. Wang, L. Zhao, H. Zuo, Q. Xue, and J. Wang: J. Iron. Steel Res. Int., 2023, vol. 30, pp. 227–35.
S. Wu, L. Wang, Y. Lu, and K. Gu: Steel Res. Int., 2018, vol. 89, p. 1800041.
X. She, J. Wang, J. Liu, X. Zhang, and Q. Xue: ISIJ Int., 2014, vol. 54, pp. 2728–36.
S. Wu, H. Han, H. Xu, H. Wang, and X. Liu: ISIJ Int., 2010, vol. 50, pp. 686–94.
G. Park, Y. Kang, and J. Park: ISIJ Int., 2011, vol. 51, pp. 1375–82.
P. Ma, K. Ma, J. Deng, Q. Wu, and J. Xu: ISIJ Int., 2023, vol. 63, pp. 1957–64.
J. Deng, K. Ma, L. Hu, M. Kou, L. Wen, and J. Xu: Ceram. Int., 2020, vol. 46, pp. 11854–60.
K. Ma, J. Xu, J. Deng, M. Kou, and L. Wen: Int. J. Hydrogen Energy, 2019, vol. 44, pp. 19555–62.
K. Ma, J. Xu, J. Deng, D. Wang, Y. Xu, Z. Liao, C. Sun, S. Zhang, and L. Wen: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 2308–21.
P. Cundall and O.L. Strack: Géotechnique, 1980, vol. 30, pp. 331–36.
J. Xu, Z. Hu, Y. Xu, D. Wang, L. Wen, and C. Bai: Powder Technol., 2017, vol. 308, pp. 273–89.
Acknowledgments
The authors gratefully acknowledge funding through projects from the Natural Science Foundation of Chongqing, China (Grant Nos. cstc2019jcyj-msxmX0089, cstc2021ycjh-bgzxm0165, cstb2023nscq-msx0514), and Galen scholarship.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, Z., Wang, K., Yang, Y. et al. Local Influences of Transient Basicity Segregation in Iron-Bearing Materials on Softening and Melting in Blast Furnaces at High Temperatures. Metall Mater Trans B 55, 2617–2625 (2024). https://doi.org/10.1007/s11663-024-03121-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-024-03121-2