Abstract
The changes in the softening and melting behaviors of ferrous burden in the cohesive zone and the characteristics of the slag–iron–coke interface in a blast furnace were investigated by simulating an actual blast furnace under hydrogen-rich conditions. According to the variation in the transient shrinkage of the burden under different atmospheres, the shrinkage start temperature of the sinter was higher than that of the pellets. The negative shrinkage rate of the pellets was greater than that of the sinter. Additionally, the softening start temperature in the blast furnace decreased under hydrogen-rich conditions, giving the blast furnace a broader range of softening zones. The softening start temperatures of the pellets and sinter decreased from 1102 to 949 °C and 1152 to 1080 °C, respectively. The hydrogen-rich traditional blast furnace conditions narrowed the melting zone temperature range and shifted it toward the high-temperature zone, significantly improving the burden layer permeability. However, under the hydrogen-rich oxygen blast furnace conditions, there were a decrease in the melting start temperature, a shift of the melting zone location to the low-temperature zone, and an increase in the burden layer permeability and pressure difference. A comparison of the slag–iron–coke interface characteristics under different atmospheric conditions showed that the carbon content in metallic iron decreased under hydrogen-rich traditional blast furnace conditions compared with traditional blast furnace conditions. Contrastingly, under hydrogen-rich oxygen blast furnace conditions, the carbon content in metallic iron increased compared with oxygen blast furnace conditions. These findings provide guidance for the development of low-carbon ironmaking processes in blast furnaces.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Saving energy and reducing CO2 emissions have become the focus of social concern as global warming intensifies [1, 2]. The steel industry, as an important industrial sector, has made significant contributions to global development and economic growth; however, its energy structure is dominated by coal, and its annual CO2 emissions account for approximately 4%–7% of the total global CO2 emissions [3]. Therefore, to decrease carbon emissions and adapt to the global “low carbon economy requirements”, technological innovations in steel industry, including hydrogen (H2) metallurgy [4, 5], have been developed. H2 metallurgy, a revolutionary technology to decrease energy consumption and carbon emissions, is gaining increasing attention in the steel industry [6, 7], and various steel companies are actively investing in its research and development. Energy consumption and CO2 emissions in the steel industry are primarily concentrated in the ironmaking process [8]. Approximately 95% of hot metal is produced in blast furnaces, which are expected to remain the dominant ironmaking process for decades because of their production cost and energy efficiency advantages [9]. Based on existing blast furnace (BF) ironmaking technology, using H2 to replace carbon partially as a reducing agent is an effective way to achieve low-carbon ironmaking and green sustainable development.
As an excellent reducing agent and clean energy source, H2 is the most promising energy medium in the twenty-first century. Its powerful diffusion and reduction capabilities improve the thermodynamic and kinetic conditions of burden reduction, and related researchers [10] have reported an increase in the reduction rate with increasing H2 content in the reduction gas. In addition, H2 reduction of iron oxides will increase the H2O content in their blast furnaces, and at a certain temperature, a gasification reaction will occur between H2O and carbon, destroying the coke structure. The high content of H2O will accelerate the deterioration of coke in the blast furnace [11].
To achieve low-carbon smelting in blast furnaces, reasonable control of the shape, thickness, and location of the cohesive zone significantly influences the stable operation of blast furnaces [12]. As H2 is a very effective reducing agent for indirect reduction, it also plays an important role in the formation and properties of the cohesive zone. Qie et al. [13] investigated the effect of H2 addition on the softening and melting reduction behaviors of ferrous burden in a gas-injection blast furnace and found that the softening start temperature of the burden decreased with increasing H2 content in the reducing gas, whereas the melting and dripping temperatures of the burden increased with increasing H2 content in the reducing gas. Overall, from a synthetic consideration of the effect of H2 addition on the reduction rate, amount of melt, burden microstructure, and energy efficiency in gas-injection blast furnaces, it was found that the optimal H2 content was 10%–15%. Lan et al. [14] analyzed the mechanism affecting the softening and melting behaviors of the ferrous burden after hydrogen-rich smelting and found that an increase in the H2 content in the reducing gas led to a downward movement and narrowing of the cohesive zone, effectively increasing the permeability. Additionally, hydrogen-rich smelting enhances the melting temperature of the slag, and a large amount of slag accumulates at the coke reaction interface, hindering the carburization of metallic iron. Pan et al. [15] studied the effects of different degrees of reduction on the cohesive zone and permeability of the mixed burden. With an increase in the reduction degree, the thickness of the cohesive zone decreased, the location of the cohesive zone shifted downward, and the permeability of the mixed burden increased significantly. Moreover, the weight of the drops and the carbon content of the iron in the drops decreased. In recent years, computer models have been widely used in the study of blast furnaces. Li et al. [16, 17] studied the effect of the inner states and overall performance of a hydrogen-rich blast furnace using a three-dimensional steady-state multi-fluid model. It showed that with increasing peripheral opening extent and hydrogen enrichment, the head of the cohesive zone decreased while the root of the cohesive zone increased slightly, leading to a narrower cohesive zone which resulted in reduced tuyere pressure. In addition, hydrogen enrichment improves the energy efficiency of the blast furnace. Tang et al. [18] conducted a study on mathematical simulation of blast furnace with hydrogen injection under constant pulverized coal injection conditions. With increased hydrogen injection, burden reduction was promoted, and the cohesive zone became thinner and its position moved down, resulting in enhanced permeability.
Only a few studies have investigated the effect of H2 volume fraction on the softening and melting behaviors of the burden. However, under actual blast furnace conditions, the gas composition and temperature to which the burden is subjected during descent are constantly changing, and studies on the softening and melting behaviors of the ferrous burden under simulated actual blast furnace hydrogen-rich conditions have not yet been reported. In addition, the softening and melting characteristics of the burden will change with the increase in the reduction potential of the atmosphere. To address these shortcomings, this study provides in-depth research on the softening and melting properties of the ferrous burden within the cohesive zone under simulated blast furnace hydrogen-rich smelting conditions to provide a theoretical basis for production.
2 Experimental
2.1 Experimental samples
Industrially manufactured raw materials and fuels were used in this experiment. Burdens with a diameter of 10.0–12.5 mm were selected by the breaking and sieving method. Tables 1 and 2 list the chemical compositions of the burden and coke, respectively.
2.2 Experimental apparatus and conditions
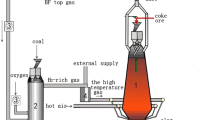
Figure 1 shows a schematic of the softening–melting furnace used in the experiments. The temperature, shrinkage ratio, and pressure difference of the burden layer were recorded during the entire softening–melting experiment. To simulate the blast furnace charging conditions, approximately 500 g of burden was loaded into a graphite crucible (ϕ94 mm × 210 mm), and the thickness of the burden layer was measured and recorded. Concurrently, 80 and 40 g of coke with diameter of 10.0–12.5 mm were placed below and above the burden, respectively. The loading values were 1.0 kg/cm2. Table 3 lists the experimental conditions with a definite temperature profile and reduction atmosphere at different temperature ranges after the preparation work was completed. The heating rate was 9 °C/min below 900 °C and 5 °C/min above 900 °C. The samples were maintained at 900 °C for 30 min to simulate the thermal reserve zone. The gas flow was 5 L/min of N2 below 200 °C and 12 L/min of mixed reducing gas above 200 °C. The gas composition was determined for four different reducing experiments according to the mathematical model of the multizone constraints [19]. Case 1 simulated a traditional blast furnace (TBF), Case 2 simulated a hydrogen-rich TBF, Case 3 simulated an oxygen blast furnace (OBF), and Case 4 simulated a hydrogen-rich OBF. In the OBF process, two rows of tuyeres were arranged on the hearth and lower shaft of the BF. Oxygen and hydrogen-rich reducing gas were injected into the furnace hearth tuyeres. In addition, the preheated top gas deprived of CO2 and H2O was recycled and blasted into the furnace shaft and hearth tuyeres. Table 4 lists the calculated main process parameters. When the first drop of burden was observed from the sight glass, the heating was stopped, and the reducing gas was switched to N2 (5 L/min) as a protective gas and cooled to room temperature. Finally, the microstructure, elemental composition, and distribution of residues were examined by scanning electron microscopy and energy dispersive spectroscopy (SEM-EDS, JEOL, JSM-7800F, Japan).
To evaluate the softening and melting properties of the burden, some characteristic temperatures were defined in this study. T10% represents the softening start temperature when the burden shrinks by 10%, and T40% represents the softening end temperature when the burden shrinks by 40%. Ts represents the melting start temperature at which the pressure difference of the burden increases sharply. Td represents the melting end temperature at which iron starts to drip. ΔTS, ΔTM, and ΔTSM are defined as the softening zone (T40%–T10%), melting zone (Td–Ts), and cohesive zone (Td–T10%), respectively. ΔPmax denotes the maximum pressure drop.
Shrinkage occurs during softening of the burden. The shrinkage and shrinkage rate (SR) of the burden can be calculated using Eqs. (1) and (2), respectively [20].
where shrinkage rate denotes the slope of the temperature–shrinkage curve, %/°C; \(H_{0}\) denotes the initial height of the burden, mm; \(H_{t}\) is the transient height of the burden, mm; and T represents the temperature at a given moment, °C. The permeability index (S) can be calculated using Eq. (3):
where \({\Delta P}_{T}\) is the pressure difference at temperature T; and \({\Delta P}_{S}\) is the pressure difference at temperature Ts.
3 Results and discussion
3.1 Ferrous burden softening behavior under different atmospheres
Figure 2 shows the characteristics of the softening behavior of the burden under different atmospheres. Figure 2a shows that the T10% of the pellets under TBF and hydrogen-rich TBF conditions decreased from 1102 to 1062 °C, T40% decreased from 1172 to 1148 °C, and the ΔTS increased from 70 to 86 °C. Under OBF and hydrogen-rich OBF conditions, T10% decreased from 1014 to 949 °C, T40% decreased from 1149 to 1146 °C, and the ΔTS increased from 135 to 197 °C. Figure 2b shows that the T10% of the sinter under TBF and hydrogen-rich TBF conditions decreased from 1152 to 1137 °C, T40% increased from 1225 to 1229 °C, and the ΔTS increased from 73 to 92 °C. Under OBF and hydrogen-rich OBF conditions, T10% decreased from 1106 to 1080 °C, T40% decreased from 1269 to 1255 °C, and the ΔTS increased from 163 to 175 °C. Therefore, under hydrogen-rich conditions, the softening start temperature of the burden decreased to provide the blast furnace with a broader range of softening zones. As the H2 content in the reducing gas increased, the reduction potential of the gas increased, shortening the Fe2O3 to Fe3O4 reduction process. This led to an increase in the reduced FeO and metallic iron at a lower temperature, resulting in a lower softening start temperature of the burden [13]. Additionally, the reduced metallic iron was present in smaller particles, which are prone to crystal stock [21], and the effect of high-temperature sintering decreased the softening start temperature. The low-melting-point phase that forms early in the pellet is fayalite (2FeO·SiO2), while the sinter forms a large amount of the high-melting-point compound nCaO·mSiO2 [22]. According to the CaO–SiO2–FeO ternary phase diagram, 2FeO·SiO2 starts melting at approximately 1200 °C, while the melting temperature of nCaO·mSiO2 is above 1300 °C, which leads to the softening start temperature of the pellets being lower than that of the sinter. Compared with the sinter, the pellet generates more internal liquid phase, resulting in a scouring effect on the outer iron shell and a lower softening end temperature.
Figure 3 shows the variation in the transient shrinkage of the burden under different atmospheres. The curves in Fig. 3 were divided into several stages by comparing the calculated slopes of the tangents to the curves at different temperatures. Table 5 lists the calculated SRs. The first stage exhibited a swelling in the shrinkage curve of the burden, which is mainly caused by the change in the lattice (Fe2O3 → Fe3O4) and thermal swelling during the reduction process [23, 24]. The negative SR of the pellets under TBF and hydrogen-rich TBF conditions decreased from 0.01596 to 0.00998%/°C. The negative SR of the pellets under OBF and hydrogen-rich OBF conditions decreased from 0.00813 to 0.00314%/°C. The negative SR of the sinter under TBF and hydrogen-rich TBF conditions decreased from 0.00839 to 0.00736%/°C. The negative SR of the sinter under OBF and hydrogen-rich OBF conditions decreased from 0.00503 to 0.00269%/°C. Furthermore, the negative SR of the pellets was greater than that of the sinter, and the increased basicity effectively inhibited swelling and iron whisker growth [24], resulting in a decrease in the negative SR of the sinter in the first-stage temperature range. However, with increasing H2 content in the reducing gas, the burden can quickly progress through the reduction stages of Fe2O3 → Fe3O4 → FeO, thus decreasing the structural damage to the burden and the swelling caused by phase change, which leads to a gradual decrease in the SR [25, 26]. Additionally, the shrinkage start temperature of the sinter was higher than that of the pellets because hematite, which is the main mineral component of the pellets, is more easily reduced than calcium ferrate, the main mineral component of the sinter. In the second stage, as the temperature increased, the burden was reduced by losing oxygen atoms, causing it to shrink. It is noteworthy that the rapid reduction of iron oxides to metallic iron under hydrogen-rich conditions and the higher plasticity of metallic iron at high temperatures than the gangue phase significantly accelerate the shrinkage of the burden, improving the SR under the TBF and OBF conditions. In the third stage, the SR was higher than those in the first two stages. This is mainly because of the large increase in shrinkage caused by the increase in temperature and reduction potential. In the fourth stage of pellet formation, the SR decreased. Meanwhile, in the third and fourth stages of the pellet formation, the SR under hydrogen-rich conditions was lower than that under TBF and OBF conditions. This can be attributed to the plasticity of the reduced iron metal reaching its limit under load. On the other hand, the metallic iron shell contributed to the enhancement of the deformation resistance of the burden owing to its increased reduction potential, which hinders the shrinkage of the slag–iron mixture in the cohesive zone. This led to a decrease in the SR and improved the permeability of the burden, which is helpful for improving the melting performance of the burden and optimizing the blast furnace operation. In the fifth stage of pellet formation and the fourth stage of sintering, the slag–iron phase permeated within the burden, resulting in dripping and an accelerated SR. In addition, under hydrogen-rich conditions, the carbon content in the metallic iron decreased, resulting in reduced droplet weight [15], which causes a decrease in the SR.
3.2 Ferrous burden melting behavior under different atmospheres
Figure 4 shows the characteristics of the burden melting behavior under different atmospheres. Figure 4a shows that the Ts of the pellets under TBF and hydrogen-rich TBF conditions increased from 1257 to 1468 °C, Td increased from 1431 to 1472 °C, and ΔTM decreased from 174 to 4 °C. Therefore, under hydrogen-rich conditions, FeO is rapidly reduced to metallic iron, the low-melting-point phase is decreased [27, 28], and the dripping temperature of the burden is increased, which narrows the temperature range of the melting zone and shifts it to the high-temperature zone, significantly improving the permeability of the blast furnace. Under OBF and hydrogen-rich OBF conditions, the Ts of the pellets decreased from 1475 to 1451 °C, Td decreased from 1475 to 1452 °C, and ΔTM varied slightly. The hydrogen-rich OBF conditions increased the area of the melting zone, but its temperature range remained unchanged. Figure 4b shows that under TBF and hydrogen-rich TBF conditions, the Ts of the sinter increased from 1329 to 1391 °C, Td decreased from 1492 to 1491 °C, and ΔTM decreased from 163 to 100 °C. Consequently, the melting zone range was shortened under hydrogen-rich conditions, but the melting end temperature remained unchanged. Under OBF and hydrogen-rich OBF conditions, the Ts of the sinter decreased from 1418 to 1355 °C, Td decreased from 1478 to 1455 °C, and ΔTM increased from 60 to 100 °C. Therefore, under hydrogen-rich OBF conditions, the melting zone was broadened and moved upward, which is not conducive to the blast furnace permeability. The increase in the 2CaO·SiO2 phase in the sinter hindered the physical contact of metallic iron with coke, resulting in significantly higher dripping temperatures than those of the pellets.
To explain the phenomenon that the melting zone of the burden moves upward under hydrogen-rich OBF conditions, the pellets were reduced to 1100 °C under four conditions: (1) OBF under loaded condition; (2) hydrogen-rich OBF under loaded condition; (3) OBF without load; and (4) hydrogen-rich OBF without load. For conditions 1 and 2, the reduction degrees were measured to be 89.64% and 95.91%, respectively, which showed that a large amount of FeO was still present in the burden. For conditions 3 and 4, the reduction degrees were 94.93% and 99.89%, respectively. Therefore, in the presence of load, the shrinkage of the burden layer hindered the indirect reducing effect of the reducing gas. Moreover, interrupted samples of the burden at the melting start temperature were obtained by shutting down the furnace and quenching it under N2 protection. The chemical composition of the residual slag in the interrupted samples was then analyzed, as shown in Table 6. It shows that the FeO content in the residual slag was higher under hydrogen-rich OBF conditions than under OBF conditions. The phase composition was also obtained by combining the CaO–SiO2–FeO ternary phase diagram with the slag phase composition, as shown in Fig. 5. From the isothermal section in Fig. 5a, it can be seen that the slag phase is in the liquid region at 1400 °C under different atmospheric conditions. From the polythermal projection in Fig. 5b, it can be seen that the liquidus temperature of the slag phase under the hydrogen-rich OBF conditions is lower than that under the OBF conditions.
Under OBF conditions, the tuyere is blown with oxygen instead of preheated air, and the top gas is returned for use after removing CO2 so that the atmosphere contains little N2 and its reduction potential (CO + H2) is enhanced. However, under hydrogen-rich conditions, the H2 content in the gas will be further increased, which leads to a further increase in the degree of reduction of the burden. However, under hydrogen-rich OBF conditions, the FeO content in the slag at the melting start temperature increases. From Sect. 3.1, it can be seen that under hydrogen-rich conditions, the softening start temperature of the burden gradually decreases, so that it is easy to generate a low-melting-point phase containing FeO at a lower temperature, and the liquid phase generation is the main factor leading to the softening of the ferrous burden. The rapid shrinkage of the burden layer makes it difficult for the reducing gas to enter the liquid slag to react with FeO, and thus the reduction of FeO in the liquid slag relies on the direct reduction of solid carbon. However, the reaction with the burden in a hydrogen-rich atmosphere is accompanied by the generation of large amounts of H2O. At the same temperature, the reaction rate of coke with H2O is approximately 2–4 times faster than that with CO2, and the effect of H2O on coke gasification is stronger than that of CO2 [29,30,31,32]. The increased ash content in the surface layer of the coke hinders direct contact between the coke and the FeO-containing liquid slag, which leads to a decrease in the melting start temperature of the cohesive zone.
3.3 Maximum pressure difference under different atmospheres
Figure 6 shows the maximum pressure difference (ΔPmax) and temperature at the maximum pressure difference (ΔPmax-temperature) of the burden under different atmospheres. The volume fraction of H2 in the reducing gas significantly affects the maximum pressure difference. The ΔPmax of the pellets under TBF and hydrogen-rich TBF conditions decreased from 6.5 to 2.0 kPa, and the ΔPmax-temperature increased from 1394 to 1470 °C. Under OBF and hydrogen-rich OBF conditions, the ΔPmax increased from 1.0 to 1.7 kPa, and the ΔPmax-temperature decreased from 1476 to 1451 °C. The ΔPmax of the sinter under TBF and hydrogen-rich TBF conditions decreased from 11.6 to 2.1 kPa, and the ΔPmax-temperature increased from 1397 to 1452 °C. Under OBF and hydrogen-rich OBF conditions, the ΔPmax increased from 7.7 to 9.4 kPa, and the ΔPmax-temperature decreased from 1448 to 1427 °C. Therefore, under hydrogen-rich TBF conditions, a large amount of FeO in the burden is rapidly reduced to metallic iron, which increases the melting point of the slag, lowering the ΔPmax and increasing the ΔPmax-temperature. However, under hydrogen-rich OBF conditions, the ΔPmax and ΔPmax-temperature of the burden exhibited opposite trends, which is detrimental to the production of the blast furnace. However, the reaction efficiency of the furnace body and the utilization rate of gas are improved due to the hydrogen-rich OBF conditions [33]. Nevertheless, the increase in pressure difference cannot be ignored and should be more critically considered during the production process, requiring more delicacy during blast furnace operation.
3.4 Permeability under different atmospheres
The value of S reflects the permeability of the cohesive zone. The smaller the S value, the better the permeability of the cohesive zone. S is strongly related to the pressure difference, and the higher the pressure difference, the lower the gas permeability. Figure 7 shows the variation in the S value of the burden under different atmospheres, which shows that the S value of the pellets under TBF and hydrogen-rich TBF conditions decreased from 282.3 to 4.8 kPa °C, and the S value under OBF and hydrogen-rich OBF conditions was 0 kPa °C. The viscosity coefficient of H2 is significantly lower than that of the other gases, which enhances the permeation of gas. With increasing H2 content in the reducing gas, the reduction potential of the gas was strengthened, and iron oxides were reduced to metallic iron at a lower temperature, avoiding the premature formation of the low-melting-point phase and effectively enhancing the permeability of the burden layer. In Sect. 3.2, it showed that narrowing the melting zone greatly improved the permeability of the cohesive zone. The S value of the sinter under TBF and hydrogen-rich TBF conditions decreased from 892.8 to 148.8 kPa °C, whereas the S value under OBF and hydrogen-rich OBF conditions increased from 177.7 to 214.9 kPa °C. Consequently, it is observed that the burden layer permeability gradually deteriorates under hydrogen-rich OBF conditions. Qie et al. [13], Lan et al. [14], and Yang et al. [34] demonstrated that an increasing H2 content in the gas is accompanied by a corresponding decrease in its S value. Zhou et al. [35] and Umadevi et al. [36] reported that the microstructure of the pellets is a polycrystalline structure of hematite bridges containing a large number of open pores. Reducing gases can quickly pass through these pores into the pellet core, which may lead to better permeability of the pellet than the sinter during the melting stage. Large-scale pellet smelting in a blast furnace is beneficial to improving the gas permeability of the cohesive zone.
3.5 Location of cohesive zone under different atmospheres
Figure 8 shows the variations in the location of the cohesive zone under different atmospheres. The ΔTSM of the pellets under TBF and hydrogen-rich TBF conditions increased from 329 to 410 °C, and the ΔTSM under OBF and hydrogen-rich OBF conditions increased from 461 to 503 °C. The ΔTSM of the sinter increased from 340 to 354 °C under TBF and hydrogen-rich TBF conditions, and the ΔTSM under OBF and hydrogen-rich OBF conditions increased from 372 to 375 °C. As the temperature increased, the range of increase in Td was smaller than that in T10% owing to carburization and dripping of metallic iron [37], which, to some extent, suppressed the increase in the melting end temperature, thus narrowing the temperature range of the melting zone. After blast furnace hydrogen-rich smelting, the softening zone becomes broader; however, the temperature ranges of the melting zone, which has the greatest influence on permeability, decrease, and the melting zone shifts toward the high-temperature zone, indicating that the enhanced permeability improves the productivity of the blast furnace.
3.6 Characterization of residues under different atmospheres
Figure 9 shows the microstructural analysis results of the slag–iron–coke interface in the residual graphite crucible of the pellet under different atmospheres as determined using SEM–EDS. A comparison of the slag–iron–coke interface characteristics showed that the slag was completely separated from the iron, leaving the coke closely intertwined with the slag–iron reaction interface. Under TBF conditions, metallic iron was in close contact with the coke surface, resulting in carburization, and the generation of carburized iron decreased the dripping temperature. However, under hydrogen-rich TBF conditions, an increase in H2 content in the reducing gas led to the generation of large amounts of H2O during the reduction of iron oxides, the gasification reaction consuming the solid carbon on the surface of the coke, and the ash generated by it packaging the coke to hinder contact with metallic iron, which was not conducive to the occurrence of the carburization reaction. Qie et al. [13] also showed that the carbon content of metallic iron decreases with increasing H2 content in the reducing gas.
Under OBF conditions, a slag layer exists between the coke and metallic iron, preventing direct contact between the metallic iron and the coke. The FeO content in the slag under hydrogen-rich OBF conditions was significantly higher than that under other conditions, and the low-melting-point phase containing FeO decreased the melting temperature of the slag and improved its fluidity. Consequently, the slag and coke surface ash induced fast melting, increasing the contact between metallic iron and coke and allowing carburization to occur, which resulted in a decrease in its dripping temperature, further verifying the reason for the elevated melting zone discussed in Sect. 3.2.
4 Conclusions
-
1.
Under hydrogen-rich TBF and OBF conditions, large amounts of FeO and metallic iron were generated at relatively low temperatures, and the softening start temperature decreased, imparting a broader range of softening zones to the blast furnace. Both the softening start and end temperatures of the pellets are lower than those of the sinter, respectively.
-
2.
Under hydrogen-rich TBF and OBF conditions, the swelling during burden reduction decreases. In addition, the plasticity of the generated metallic iron is greater than that in the gangue phase, thus increasing the SR at the start of the burden layer shrinkage. In the subsequent shrinkage process, further shrinkage of the burden is hindered by the large amount of metallic iron shell generation, resulting in a lower SR under hydrogen-rich TBF and OBF conditions compared with regular TBF and OBF conditions.
-
3.
When comparing hydrogen-rich TBF with TBF conditions, the temperature range of the melting zone narrows and moves towards the high-temperature zone, which significantly improves the burden layer permeability. Meanwhile, when comparing hydrogen-rich OBF with OBF conditions, the gasification reaction between H2O and coke leads to ash hindering contact between solid carbon and slag. This decreases the melting start temperature, broadens the range of the melting zone, and shifts it to the low-temperature zone, which is not conducive to the burden layer permeability.
-
4.
The characteristics of the slag–iron–coke interface under different atmospheres show that the carbon content in metallic iron decreased under hydrogen-rich TBF compared with TBF conditions, while the carbon content in metallic iron increased under hydrogen-rich OBF compared with OBF conditions.
References
W.Q. Xu, B. Wan, T.Y. Zhu, M.P. Shao, J. Clean. Prod. 139 (2016) 1504–1511.
K.D. Xu, Iron and Steel 45 (2010) No. 3, 1–12.
X.C. Tan, H. Li, J.X. Guo, B.H. Gu, Y. Zeng, J. Clean. Prod. 222 (2019) 823–834.
K.H. Ma, J.Y. Deng, G. Wang, Q. Zhou, J. Xu, Int. J. Hydrogen Energy 46 (2021) 26646–26664.
Y.B. Chen, H.B. Zuo, Ironmak. Steelmak. 48 (2021) 749–768.
J. Tang, M.S. Chu, F. Li, C. Feng, Z.G. Liu, Y.S. Zhou, Int. J. Miner. Metall. Mater. 27 (2020) 713–723.
X.Y. Zhang, K.X. Jiao, J.L. Zhang, Z.Y. Guo, J. Clean. Prod. 306 (2021) 127259.
G.Y. Sun, B. Li, W.S. Yang, J. Guo, H.J. Guo, Energies 13 (2020) 1986.
Z.J. Liu, J.L. Zhang, T.J. Yang, ISIJ Int. 55 (2015) 1146–1156.
A. Heidari, N. Niknahad, M. Iljana, T. Fabritius, Materials 14 (2021) 7540.
J. Zhao, H.B. Zuo, C. Ling, W.T. Guo, J.S. Wang, Q.G. Xue, J. Iron Steel Res. Int. 27 (2020) 743–754.
X.L. Liu, T. Honeyands, G. Evans, P. Zulli, D. O’Dea, Ironmak. Steelmak. 46 (2019) 953–967.
Y.N. Qie, Q. Lyu, X.J. Liu, J.P. Li, C.C. Lan, S.H. Zhang, C.J. Yan, Metall. Mater. Trans. B 49 (2018) 2622–2632.
C.C. Lan, S.H. Zhang, X.J. Liu, Q. Lyu, M.F. Jiang, Int. J. Hydrogen Energy 45 (2020) 14255–14265.
Y.Z. Pan, H.B. Zuo, B.X. Wang, J.S. Wang, G. Wang, Y.L. Liu, Q.G. Xue, Ironmak. Steelmak. 47 (2020) 322–327.
J. Li, S.B. Kuang, R.P. Zou, A.B. Yu, Metall. Mater. Trans. B 53 (2022) 4124–4137.
J. Li, S.B. Kuang, L.L. Jiao, L.L. Liu, R.P. Zou, A.B. Yu, Fuel 323 (2022) 124368.
J. Tang, M.S. Chu, F. Li, Z.D. Zhang, Y.T. Tang, Z.G. Liu, J. Yagi, J. Clean. Prod. 278 (2021) 123191.
Y.H. Han, J.S. Wang, Y.Z. Li, X.F. She, L.T. Kong, Q.G. Xue, J. Univ. Sci. Technol. Beijing 33 (2011) 1280–1286.
K.H. Ma, J. Xu, J.Y. Deng, D.D. Wang, Y. Xu, Z.H. Liao, C.F. Sun, S.F. Zhang, L.Y. Wen, Metall. Mater. Trans. B 49 (2018) 2308–2321.
X.W. An, J.S. Wang, R.Z. Lan, Y.H. Han, Q.G. Xue, J. Iron Steel Res. Int. 20 (2013) No. 5, 11–16.
M. Hayashi, K. Suzuki, Y. Maeda, T. Watanabe, ISIJ Int. 56 (2016) 220–225.
S. Hayashi, Y. Iguchi, Ironmak. Steelmak. 32 (2005) 353–358.
H.T. Wang, H.Y. Sohn, Ironmak. Steelmak. 38 (2011) 447–452.
L.Y. Yi, Z.C. Huang, T. Jiang, L.N. Wang, T. Qi, Powder Technol. 269 (2015) 290–295.
F. Li, M.S. Chu, J. Tang, Z.G. Liu, C. Feng, Y.T. Tang, JOM 69 (2017) 1751–1758.
H.C. Chuang, W.S. Hwang, S.H. Liu, Mater. Trans. 50 (2009) 1448–1456.
S. Ueda, T. Kon, T. Miki, S.J. Kim, H. Nogami, ISIJ Int. 55 (2015) 2098–2104.
P. Wang, Y.Q. Zhang, H.M. Long, R.F. Wei, J.X. Li, Q.M. Meng, S.C. Yu, ISIJ Int. 57 (2017) 643–648.
P. Wang, S. Yu, H. Long, R. Wei, Q. Meng, Y. Zhang, Ironmak. Steelmak. 44 (2017) 595–600.
W.T. Guo, Q.G. Xue, Y.L. Liu, Z.C. Guo, X.F. She, J.S. Wang, Q.Q. Zhao, X.W. An, Int. J. Hydrogen Energy 40 (2015) 13306–13313.
C.C. Lan, Q. Lyu, X.J. Liu, M.F. Jiang, Y.N. Qie, S.H. Zhang, Int. J. Hydrogen Energy 43 (2018) 19405–19413.
Z.G. Zhao, X.B. Yu, Y.S. Shen, Y.T. Li, H. Xu, Z.J. Hu, Energy Fuels 34 (2020) 15048–15060.
G.Q. Yang, J.L. Zhang, Y.X. Chen, Q.Y. Wu, Z.Y. Zhao, J. Zhao, Iron and Steel 47 (2012) No. 9, 14–18.
K. Zhou, J.Q. Song, Z.X. You, H.E. Xie, X.W. Lv, ISIJ Int. 60 (2020) 1409–1415.
T. Umadevi, P. Kumar, N.F. Lobo, M. Prabhu, P.C. Mahapatra, M. Ranjan, ISIJ Int. 51 (2011) 14–20.
H.S. Kim, J.G. Kim, Y. Sasaki, ISIJ Int. 50 (2010) 1099–1106.
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. U1960205 and 51804024), China Baowu Low Carbon Metallurgy Innovation Foundation (BWLCF202101 and BWLCF202104) and China Minmetals Science and Technology Special Plan Foundation (2020ZXA01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the author(s).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyu, Bb., Wang, G., Yang, F. et al. Softening and melting behaviors of ferrous burden in hydrogen-rich blast furnace cohesive zone. J. Iron Steel Res. Int. 30, 2366–2377 (2023). https://doi.org/10.1007/s42243-023-00951-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-023-00951-3