Abstract
Titanium metallic powder (2.98 wt% O) of irregular and semi-spherical particles sizes ranging between 0.5 and 3.5 µm was obtained by magnesiothermic reduction of TiO2 and a leaching purification process. Magnesiothermic reduction experiments were carried out to evaluate the influence of temperature and molar ratio of Mg/TiO2. A rotary tube reactor in three different configurations was used to promote solid–liquid and solid–gas model reaction. The best configuration resulted when solid–gas model reaction was promoted. Different mixtures of acid as 6M HCl, 3M HCl, 0.55M HCl plus a mixture of 8 HCl% + 3 HNO3% were used to evaluate the purification of solid titanium metal by dissolution of Mg, MgO, Ni, Fe, magnesium titanates, and titanium oxides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium and its alloys exhibit special properties in comparison with other metals, such as high strength/density ratio, high corrosion resistance, bio-compatibility [1], low thermal expansion coefficient, good performance tribological properties [2], high electrical conductivity, good ductility, excellent fracture resistance, cryogenic properties, shape memory behavior, and hydrogen affinity (for hydrogen storage), and make it an attractive material that fulfills many high-tech requirements for different engineer application [3]. Those inherent properties fulfill both the elevated and the normal requirement for high engineering and daily applications, respectively. However, the present price limits its use for many possible applications, and nowadays, titanium is mostly used for those that require highly engineered materials, such as, aerospace or military applications, prostheses and/or high corrosion-resistant components, etc. The high price of titanium is caused by the highly technical multi-step production process. Norgate [4] has estimated that if a low-cost production process is developed, reducing price should be about 50% and the penetration of titanium into new markets could increase to about 2.2 times. These new uses are entailed in components used daily, which do not require high performance of mechanical properties e.g., jewelry, no-load bearing, etc. The total costs to obtain conventional titanium components are shared: 38% on obtaining the sponge through the Kroll process, 15% on refining the sponge by metallurgical processes, and a final 47% on the machining work. Moreover, in comparison with the steel production, the manufacturing of the titanium sheet is up to 80 times more expensive. This is because more than ¾ of the total material is converted into scrap (the oxidized and contaminated surface regions and the surface crack must be removed prior to any further manufacturing) [5]. An approximation of cost inputs on the aerospace component machined from plate is 14% on sponge, 34% on mill products, and 52% on machining [6]. Table 1 shows a comparison of the relative costs for the different production steps of steel, magnesium, aluminum, and titanium.
By analyzing Table 1, it can be concluded that any effort to reduce the titanium price must be focused on two different directions [3,4,5]. They are either (1) achieving a process cheaper than the currently employed one and/or (2) the development or improvement of inexpensive techniques for the fabrication of final components [8, 9]. On the other hand, the industry must find a more economical way to extract the metal or produce components [4, 5]. Nowadays, more than 10 different new methods to produce titanium metals or its powder are under research (Table 2).
Finally, Near-Net Shape (NNS) processes could be the way to avoid huge scrap production due to the fact that they use much less material for manufacturing components. NNS includes casting and powder metallurgy (PM). Powder metallurgy (PM) has been explored in different parts of the world, applying techniques such as metal powder injection molding (PIM or MIM), conventional steps of Press-Die-Sinter, elastomeric bag, Cold Isostatic Pressing (CIP), Hot Isostatic Pressing (HIP), Additive Manufacturing (AM), and/or Direct Powder Rolling (DPR) which use either Blended (BE) or Prealloyed Powder (PA/PREP). PM seems to be an affordable and economical route to produce low-cost components but cheap titanium powder must be available [10,11,12,13,14,15,16].
Worldwide utilization of titanium products in the industrial market was approximately 26,000 metric tons (mt) in 2016 [17]. Figure 1 shows an industrial titanium demand forecast projected until the year 2020 [18], according to which the consumption of this metal is likely to increase by about 5.8% in the next 5 years. Oil & gas industry could promote the highest consumption increase (about 57%), followed by the chemical processing sector, with 10.3%; contrastingly, consumption in the desalination sector might undergo a drop of about 57%.
Worldwide titanium mill products shipments [18] (Color figure online)
Titanium powders are readily formed and sintered into components that possess exceptional corrosion resistance, biological inertness, high strength at room and high temperatures, and relative lightness. They are used to produce refractory compounds, particulate-reinforced titanium matrix composites, metal–polymer anticorrosive compositions, mechanical components, ceramic–metal porous filters, gas absorbers of electronic vacuum devices, surgical tools, prosthesis, frames for lenses, weapon details, and different components in the automobile, aerospace, and chemical industries. Titanium P/M components can be welded using argon-arc, contacts, diffusion welding, and processed using metal-cutting machine tools [19]. Table 3 estimates the penetration of titanium-powder-made components by the year 2020, when the demand is projected to come close to 18.944 metric tons (mt) yearly. AM exhibiting the largest projected consumption counts [20].
In the last two decades, the research race began again to obtain low-cost titanium powder directly from its titanium dioxide (TiO2) or tetrachloride (TiCl4). Electrochemical processes to obtain titanium powder from TiO2 by reduction through melting calcium or its salts had been researched at the same time by Suzuki [21, 22] and Fray (FFC process) [23, 24]. The Kroll and Hunter processes have improved to produce continuous titanium powder by the Australians (TiRo™ process) [25] and the American (Armstrong ITP Process) [26], respectively. Okabe [27] researched the direct production of titanium through calciothermic reduction of TiO2. Unfortunately, nowadays, there is no industrial scale up for these processes to assure a massive production of titanium powder at low cost. A lot of information about the new developments on this subject is not publicly available because of requirements from various government agencies, which have declared titanium as strategic material. Also, companies keep the secret of production and their developed technologies altogether for themselves [28]. Furthermore, the necessary steps to produce titanium powder must be reduced in comparison to commercial processes that use metallic titanium. During the last years, some researchers have investigated the magnesiothermic reduction of titanium dioxide with the addition of magnesium dichloride (MgCl2), magnesium chloride hexahydrate (MgCl2·6H2O), sodium chloride (NaCl), calcium hydroxide Ca(OH)2 in argon, hydrogen, or nitrogen atmospheres to obtain titanium, titanium hydrogen, or titanium nitride [29,30,31,32]. The initiative to use H2 as deoxidant during the magnesiothermic reduction in trial of 50 g of reactants [29] is an attractive process and the content of oxygen reached in comparison to this research is better. However, magnesiothermic reductions carried out in two different unsealed and sealed reactors without movement with a mixture of approximately 160 g of TiO2 and Mg [33, 34] produce heterogeneous and no replicable products. The amount of the reactants changes the reaction’s yield dramatically and sometimes reactions become explosive. As results, some particles of TiO2 are not reduced or the titanium that has already been produced is reoxidized due to the high temperature generated by the violent exothermic reaction. The sealed rotary reactor designed for this research promoted a solid–gas reaction to obtain reproducible and heterogeneous products, using an amount of reactants of approximately 423 g, weighing higher than other researches. Compounds containing chlorine were not used to avoid negative effects on mechanical properties of titanium [19, 28].
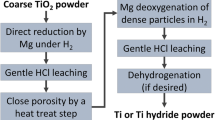
As magnesium exhibits a boiling point 192 °C lower than calcium, it is used as reducer that could save energy during the reduction process in comparison to calcium. Magnesium cannot deoxidize titanium to the required levels, but calcium does. The use of calcium as dioxider is necessary. With this aim, a four-step process to obtain titanium powder is proposed, it consists in a first reduction of titanium dioxide by magnesium to low titanium oxygen content, followed by an acid leaching step to recover the powder which is used as raw material in a subsequent calcium deoxidation process with its leaching process. Finally, another acid leaching step would obtain the low oxygen content titanium powder. The first and second steps, the dotted area shown in Fig. 2, are precisely what this research has investigated in order to define the suitability of other steps.
Materials and Methods
Raw Materials
The reactants used for the reduction process were titanium dioxide powder “Kronos 3000” with the following properties: TiO2 purity ≥ 99 wt%, size distribution 86 wt% of particles > 100 µm and 0.2 wt% of particles < 800 µm, X50-Value (media size particle) of 200 µm, and granulated magnesium of commercial grade purity. The leaching step was carried out with analytical grade HCl, HNO3, and H2SO4 acids.
Experimental Methods
Magnesiothermic Reduction of TiO2
Reduction was carried out in a sealed rotary tube reactor formed by two different compartments joined by a joint flange in-between. Each compartment had a capacity of 350 ml. It was made from high-elevated temperature strength stainless steel DIN 1.4841. The reactor was sealed on its ends with blind flanges and screws made of the same material. Graphite gaskets of “Sigraflex® Economy” were used on the joint flanges. The sealed reactor was designed to support pressure of 9.8 bars at 1000 °C. The trials were heated on an electrical atmosphere controlling rotating furnace (2 rpm) with facilities to inject argon (2 l/min) as protecting gas to ensure a complete mixture of the reagents during the reaction and free oxygen atmosphere outside the reactor, respectively. The heating program was fixed with preheating at 400 °C and 640 °C for 30 min and then up to the set temperature. Sponge titanium was placed into the sealed reactor in each compartment to remove the enclosed air by reaction at low temperature. The reactor allowed three different configurations as follows:
On the first configuration (1C, Table 4), the compartments were used as separated forms, each one worked as a single reactor at the same time (Fig. 3(1)). In each compartment, the reactants were put into contact with each other (TiO2 200 g). The aim of this configuration was to determine the influence of rotary movement on the yield of reduction. A factorial experimental design 22 with three repetitions was implemented. Variables were (1) the molar composition ratio of Mg/TiO2 mol (φ) of 2.5 (152.2 g) or 4 (243 g) and (2) holding reaction temperature of 850 °C or 1100 °C. The size of titanium dioxide was the powder size as received and the holding time was 240 min.
For the second one (2C, Table 4), the reagents were placed separately in each compartment. The joint flange’s hole allowed free flow of Mg vapor from the magnesium compartment to the TiO2 compartment during the entire heating time (Fig. 3(2)). The following variables were maintained constant: set-up temperature of 1100 °C, holding time of 240 min, and molar composition ratio of Mg/TiO2 mol (φ) of 4 (200 g TiO2 and 243.5 g Mg). Four repetitions were carried out.
For the third configuration (3C, Table 4), four trials were carried out throughout 240 min at 1100 °C and a molar composition ratio of Mg/TiO2 mol (φ) of 4 (200 g TiO2 and 243.5 g Mg). In this configuration (Fig. 3(3)), the compartments were isolated and the TiO2 and Mg were placed separately for each one. A special design of graphite gaskets made from Sigraflex® Economy was placed in-between the joint flanges. They allowed arranging a special nickel blind plug, which blocked up the flow of magnesium vapor between both compartments until it had melted due to a reaction with the vapor magnesium. Nickel and magnesium vapor evolved an alloy of a melting point close to the trial’s set temperature. Blank trials set the melting point of this alloy at approximately 1030 °C ± 10 after 30 min. Since the evolved nickel–magnesium alloy was melted, the magnesium vapor at approximately 0.58 bar abruptly flowed into the TiO2 compartment and began the reaction at 1030 °C. Four trials were carried out at the set temperature of 1100 °C with a holding time of 240 min.
Leaching of the Reduction Products
The goal of this experimental part was the selection of an acid solution which achieved the highest efficiency for the removal of magnesium, magnesium oxide, and magnesium titanates; and, at the same time, the minimal oxidation of titanium in the best product obtained from the magnesiothermic reduction. The efficiency of four different acid solutions (6M HCl, 3M HCl, 0.55M HCl plus a mixture of 8 HCl% + 3 HNO3%) was tested during 1 or 24 h (Table 5).
An amount of 10 ± 0.003 g of each sample was slowly added to the respective acid solution. An ice bath was used as an external cooling system to avoid the hydrogen pick up during the reaction. It maintained as solution at temperatures below 20 °C. Magnetic stirring at 180 rpm was applied. Temperature and pH values were controlled during the leaching process. The final pH after the reaction time was always 1. Once the sample was completely charged into the beaker, the set time began to be counted. The final liquor was recuperated by filtration and diluted with distilled water to a certain volume in a volumetric flask to quantify the dissolved titanium, magnesium, nickel, and iron by ICP. Sometimes, white and/or black particles were observed in the liquor, in that case, it was centrifuged and filtered again. The remaining powder was washed with distilled water, alcohol and acetone, and dried under protective gas at 120 °C for 24 h. Some powder presented a pyrophoric behavior in contact with the air during the drying process. The pyrophoric behavior is a characteristic of fine titanium particles smaller than 10 μm [21]. Content of oxygen into the powder was analyzed by EDX.
Results and Discussion
Yield, Distribution, and Amount of Products
The first configuration (1C) where the reactants were used separately obtained a mixture of gray silver (GS) (Ti, Ti2O, MgO, and Mg), black metallic (BM) (Ti, MgO, Mg1.5Ti1.5O, Ti2O, and Ti2O3), and black no metallic (BnM) (Ti2O and Mg) products in different proportions is shown in Table 6. Mentioned products’ characterization is explained in the next part.
The analysis of variance (ANOVA) or the 22 factorial design of the first configuration showed that the molar ratio has a significance of 0.006, that is, 99.4% of probability molar ratio influences the yield of production of titanium. Holding temperature showed no influence in the yield of production with a significance of 0.202 that implies a probability of 79.8%. Statistically, only a probability higher than 95% is acceptable to consider an influence of the variables over the measured result.
On the second configuration where TiO2 and Mg were placed separately, the obtained products were gray silver (GS) (Ti, Ti2O, MgO and Mg) and black metallic (BM) (Ti, MgO, Mg1.5Ti1.5O, Ti2O and Ti2O3). In this case, any black no metallic (BnM) product (Ti2O and Mg) was not obtained (Table 7).
On the third configuration, the only product obtained was black no metallic (BnM) particles (Ti2O and Mg) (Table 8).
The product distributions in the three different reactor configurations were always the same: some reduced particles formed a sinter cake, another particles detached and free, magnesium condensed on the internal walls of the reactor and fine white MgO particles detached and homogeneously distributed. The reductions carried out on the first configuration when TiO2 and Mg were in contact with each other obtained a heterogeneous no replicable mixture of titanium product, titanium oxides, magnesium titanates, and magnesium. The second configuration where the reactants were placed separately reached the best yield of production of gray silver products of 98.7 wt%. Finally, the reduction evolved in the third configuration, starting abruptly at 1030 °C, conducted at a heterogeneous product of titanium hemoxide (Ti2O) due to reoxidation of the titanium. From the results of different configurations, it can be summarized that a high yield of titanium is produced when TiO2 and Mg are placed in a separate form and a solid–gas reaction is used at lower temperature.
Obtained Products by Magnesiothermic Reduction
Three kinds of different grades of reduction grouped products were obtained during the reduction from different conditions study. Those commonly grouped products were founded in a sinter cake form, detached or placed on a specific place of the reactor. The products could be easily separated by hand due to their different colors and textures. The products were named as gray silver (GS), black metallic (BM), and black no metallic (BnM) particles in order to be analyzed by ICP, XRD, SEM, and EDX.
Product Form of Ti, Ti2O, MgO, and Mg: Gray Silver (GS)
The gray silver product (GS) (Fig. 4a) was obtained during trials where reagents were placed in contact with each other or separately in the first and second configurations (Tables 6 and 7). XRD pattern from a mixture of GS products of samples 2C-1, -2, -3, and -4 (Fig. 5) shows metallic titanium (Ti), a low proportion of hemioxide (Ti2O), magnesium oxide (MgO), and metallic magnesium (Mg).
Product Forms of Ti, MgO, Mg1.5Ti1.5O, Ti2O, and Ti2O3: Black Metallic (BM)
The black metallic (BM) products (Fig. 4b) were produced during second configuration trials where the reagents were placed separately where only a solid gas reaction could take place (Table 7). BM product mixtures from trials 2C-1, -2, -3, and -4 were composed of titanium metal (Ti) and magnesium oxide (MgO) as main components, together with low peaks of quandilite (Mg1.5Ti1.5O4), titanium sesquioxide (Ti2O3), and titanium hemioxide (Ti2O) as the XRD patterns show (Fig. 6). Although the peaks of Ti and MgO phases show higher intensity in comparison to the Mg1.5Ti1.5O, Ti2O, and Ti2O3 ones, the presence of those products suggests a partial reduction in some places of the particles, in other words, the reduction grade in the particles was heterogeneous.
Product Forms of Ti2O and Mg: Black No Metallic (BnM)
This product was obtained (Fig. 7) during the first configuration together with the previous ones (Table 6) and as a single product in the third configuration (Table 8) when a nickel blind plug was used. The XRD (Fig. 8) pattern from BnM products mixture from samples 3C-1, -2, -3, and -4 shows that titanium hemioxide (Ti2O) and magnesium oxide (MgO) are the phases present in those product.
Leaching Process
The products obtained during second configuration trials (2C-1, -2, -3, and -4 trials) were mixed to be used as raw material. The total weight of mixed products was 1701.2 g and its chemical composition can be seen in Table 9. Contamination of Ni and Fe in the product is due to the reaction of the magnesium with the high-temperature special steel reactor’s wall during the reduction process.
Samples of 10 ± 0.003 g were extracted from the bulk of mixed products and they were leached in different acid mixtures as show in Table 5. The efficiencies of dissolved metals for products are shown in Table 10. The trials carried out with 6M HCl (0s24, 0s24h) were not analyzed because of the products’ dissolution rate.
In almost all cases, the magnesium content was almost completely removed in the final product. The dissolution of magnesium was higher than 92% with a maximal efficiency for the sample No. 3s24. It may be that at the high HCl concentration, the time does not influence the dissolution percentage. This behavior could be the result of a surface passivity that inhibits any further dissolution. For these trials, some particles remained without any reaction. Other trials (2s1, 2s24, 3s1, and 3s24) exhibited an obvious behavior, the longer the time for reaction, the higher the dissolution of magnesium. No passivity was developed with the additional presence of the stronger oxidant agent (HNO3). The acid mixture 8 HCl% + 3 HNO3% during 24 h resulted in being the best leaching efficiency with both the lowest titanium dissolution (0.03%) and total dissolution of magnesium.
Yield of titanium from the best configuration of reduction process and the more efficient acid mixture resulted on 98.22%wt. It was calculated using the following data: amount of Ti (479.58 g) contains on the TiO2 used as raw material of all trials of the second configuration reactor; weight of mixed products (1701.2 g) from the magnesiothermic reduction; and the chemical composition of the powder produced from the leaching process 3s24 (Table 10). Finally, trials with the best yield of gray silver product (Ti, Ti2O, MgO, and Mg) from the first configuration, also 1C-4A, -4B, and -4B, were leached using the acid mixture (8 HCl% + 3 HNO3%) for 24 h.
Obtained Powder from the Leaching Process
Three different morphologies were observed by SEM in the particles obtained from the leaching process, as described in the following:
Equiaxed Particles from First Configuration
The particles obtained from the first configuration's leaching process (1C-4A, -4B, -4C) exhibited an equiaxed form with holes on their surface (Fig. 9). Particle size ranges from 0.5 to 3.5 μm. The SEM micrograph renders a facetted growth of the titanium particle from the prior magnesium titanates particle. The prior ones are decomposed on both MgO and titanium particles. The SEM micrograph renders a facetted titanium particle growth from the prior magnesium titanates particle. The prior ones were decomposed on both MgO and titanium particles. The MgO particles were released leaving behind a high porosity of octahedral holes on the surface which was then transformed into titanium metal by the removal of magnesium and oxygen. This micrograph shows larger particles than other samples; it could be that the smaller particles have been adsorbed by the bigger ones in a ripening process promoted by the high temperature and longer time. The titanium particle shapes are semi-spherical.
The EDX chemical analysis shows the presence of oxygen and magnesium (Table 11). The oxygen content is approximately 4.38 wt% (89.46% degree of reduction) and magnesium is only on point 1 with 2.79 wt%.
Spherical and Semi-spherical Particles
Two different morphologies (Fig. 10) were observed in the leached products (3s24) from the second configuration (2C-1, -2, -3, and -4). The first one of these morphologies corresponded to spherical and semi-spherical titanium particles with a 0.53 μm to 1.93 μm size. They were detached from the continuous matrix structure under a “free flow” behavior mode. The latter morphologie consists in an angular monolithic material, containing an elevated amount of nickel (circa 52 wt%) that suggests chemical reactions between some of the reactants and the nickel contained in the reactor’s wall of the high-temperature-resistant steel alloy. The EDX chemical analysis (Table 12) shows that the oxygen content is approximately 3.25 wt%, leading to a degree of reduction of 92.29%.
Continuous Matrix Structure
The second morphology corresponds to a continuous titanium matrix spotted by traces of small, detached titanium particles (Fig. 11), that had been absorbed by the matrix. Small cavities on its surface, left by leached MgO were observed as well. This confirms the formation of a continuous matrix resulting from facetted growth by a sinter/ripening process. The EDX shows that the oxygen content is approximately 5.22 wt%, leading to a degree of reduction of 87.35% (Table 13).
Morphology Analysis
As it can be determined by the morphological analysis, the metallic titanium powder obtained under the best of the tested conditions (separated reactants, rotational reactor, free flow of magnesium in the reactor’s compartment, 1100 °C set temperature, and 4-h holding time) exhibits free flowing particles with semi-spherical shape, sizing between 0.5 and 1.4 µm. The degree of reduction achieved was 92.3%, corresponding to 3.25% oxygen content. As it can be observed in some of the trials, it is possible to obtain spherical particles, but for the goal established, the process window is quite narrow. Owing to its elevated oxygen content, the titanium metal obtained in the present research could be used in new processes such as PIM or PMV, wherein oxygen content has been determined to be no more than 10%. This is so because the components' mechanical requirements do not require a very high performance. In addition, and as it was initially envisioned, the magnesiothermic reduction process may be an intermediate step when it comes to obtaining high-performance titanium powder. The next step could use calcium as oxygen cleaner in a lower content than the other investigated processes.
Conclusion
High yield of titanium and a homogeneous product mainly conformed by Ti, Mg, and MgO with low proportion of Ti2O and Ti2O3 was obtained when Ti2O and Mg were placed separately in the sealed rotary reactor and a solid–gas reaction lead the process of reduction. On the other hand, when TiO2 and Mg were placed with each other, a heterogeneous product conformed of Ti, Mg, MgO, Mg1.5Ti1.5O, Ti2O, and Ti2O3 was obtained. Finally, when magnesium gas was allowed to react at 1030 °C (0.68 bar) with titanium, the homogeneous product obtained was titanium hemioxide (Ti2O) and magnesium oxide (MgO).
The best leaching condition found was a mixture of 8 HCl% + 3 HNO3% for 24 h at maximum 20 °C, which results in the highest dissolution of both the magnesium excess and magnesium oxide, very close to 100% and the lowest dissolution of the titanium about of 0.03 wt%.
The semi-spherical titanium particles obtained were approximately of 0.5 μm size with a content of oxygen on an average of 3.25 wt% on average, corresponding to a degree of reduction of 92.3% referring to TiO2. Lower oxygen concentration was reached in some trials to about 2.9 wt%, and degree of reduction of about 93.17%. No chlorine contamination was detected.
The shape of the titanium powder was not homogeneous, and it depended on conditions of the trial. Generally, small and detached equiaxed particles were obtained when reactants were in contact, but when they were placed separately, a continuously sinter structure or semi-spherical particles of titanium was obtained.
References
Prasad S, Ehrensberger M, Gibson MP et al (2015) Biomaterial properties of titanium in dentistry. J Oral Biosci 57:192–199
Yang Y, Zhang C, Dai Y, Luo J (2017) Tribological properties of titanium alloys under lubrication of SEE oil and aqueous solutions. Tribol Int 109:40–47. https://doi.org/10.1016/j.triboint.2016.11.040
Hurless BE, Froes FH (2002) Lowering the cost of titanium. AMPTIAC Q. 6:3–9
Norgate TE, Wellwood G (2006) The potential applications for titanium metal powder and their life cycle impacts. JOM 58:58–63. https://doi.org/10.1007/s11837-006-0084-y
Kraft EH (2004) Summary of emerging titanium cost reduction technologies. Report by EHK Technology Vancouver, WA Study for US DofE and ORNL/Subcontract 4000023694
Barnes J, Kingsbury A, Bono E (2016) Does "low cost" titanium powder yield low cost titanium parts? In: PowderMet 2016 international conference on powder metallurgy. Boston
Faller K, Froes FH (2001) The use of titanium in family automobiles: current trends. JOM 53:27–28. https://doi.org/10.1007/s11837-001-0143-3
Ashraf Imam M, Froes FHS (2010) Low cost titanium and developing applications. JOM 62:17–20. https://doi.org/10.1007/s11837-010-0069-8
Froes FHS, Gungor MN, Ashraf Imam M (2007) Cost-affordable titanium: The component fabrication perspective. JOM 59:28–31. https://doi.org/10.1007/s11837-007-0074-8
German R (2009) Titanium powder injection moulding: a review of the current status of materials, processing, properties and applications. Powder Inject Mould Int 3:21–37. https://doi.org/10.1093/hmg/ddr404
Dutta B, Froes F (2015) The additive manufacturing (AM) of titanium alloys. In: Advanced materials research, pp 447–468
Froes FHS (2012) Titanium powder metallurgy: a review—part 1. Adv Mater Process. 170:16–22
Bolzoni L, Ruiz-Navas EM, Neubauer E, Gordo E (2012) Inductive hot-pressing of titanium and titanium alloy powders. Mater Chem Phys 131:672–679. https://doi.org/10.1016/j.matchemphys.2011.10.034
Cui C, Hu B, Zhao L, Liu S (2011) Titanium alloy production technology, market prospects and industry development. Mater Des - MATER Des 32:1684–1691. https://doi.org/10.1016/j.matdes.2010.09.011
Vlad AIO (2008) Innovative powder metallurgy process for producing low cost titanium alloy component. In: Titanium 2008, Las Vegas
Capus J (2017) Titanium powder developments for AM: a round-up. Met Powder Rep 72:384–388. https://doi.org/10.1016/j.mprp.2017.11.001
Neotiss AB (2017) Global trends in industrial market. In: Titanium Europe 20, Amsterdam
Cain KJ (2016) Industrial titanium demand forecast 2016. In: Titanium USA 2016, Boston
Gopienko VG, Neikov OD (2009) Chapter 14: production of titanium and titanium alloy powders. Handbook of non-ferrous metal powders: technologies and applications. Elsevier, Oxford, pp 314–323
Whittaker D, Froes FH (2015) 30—future prospects for titanium powder metallurgy markets BT—titanium powder metallurgy. Titanium Powder Metallurgy. Butterworth-Heinemann, Boston, pp 579–600
Suzuki RO, Ono K, Teranuma K (2003) Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2. Metall Mater Trans B 34:287–295. https://doi.org/10.1007/s11663-003-0074-1
Suzuki RO, Inoue S (2003) Calciothermic reduction of titanium oxide in molten CaCl2. Metall Mater Trans B 34:277–285. https://doi.org/10.1007/s11663-003-0073-2
Fray DJ (2001) Emerging molten salt technologies for metals production. JOM 53:27–31. https://doi.org/10.1007/s11837-001-0052-5
Mohandas KS, Fray D (2004) FFC Cambridge process and removal of oxygen from metal-oxygen systems by molten salt electrolysis: an overview. Trans Indian Inst Met 57:579–592
Hogan L, McGinn E, Kendall R (2008) Research and development in titanium - implications for a titanium metal industry in Australia. ABARE, Canberra
Borys S, Anderson RP, Benish A, Jacobsen L, Ernst W, Kogut D, Lyssenko T (2005) Development status of the armstrong process for production of low cost titanium powder. In: Aeromat 2005, Orlando
Okabe TH, Oda T, Mitsuda Y (2004) Titanium powder production by preform reduction process (PRP). J Alloys Compd 364:156–163. https://doi.org/10.1016/S0925-8388(03)00610-8
Trzcinski M (2006) The race is on for commercialization of titanium powder. Light Met Age 6:30–33
Zhang Y, Fang ZZ, Xia Y et al (2017) Hydrogen assisted magnesiothermic reduction of TiO2. Chem Eng J 308:299–310. https://doi.org/10.1016/j.cej.2016.09.066
Nersisyan HH, Won HI, Won CW et al (2014) Direct magnesiothermic reduction of titanium dioxide to titanium powder through combustion synthesis. Chem Eng J 235:67–74. https://doi.org/10.1016/j.cej.2013.08.104
Kan X, Ding J, Zhu H et al (2017) Low temperature synthesis of nanoscale titanium nitride via molten-salt-mediated magnesiothermic reduction. Powder Technol 315:81–86. https://doi.org/10.1016/j.powtec.2017.03.042
Nersisyan HH, Won HI, Won CW et al (2013) Combustion synthesis of porous titanium microspheres. Mater Chem Phys 141:283–288. https://doi.org/10.1016/j.matchemphys.2013.05.012
Bolívar R, Friedrich B (2009) Synthesis of titanium via magnesiothermic reduction of TiO2 (pigment). Proc Eur Metall Conf EMC 2009:1235–1254
Oosterhof C, Reitz J, Bolivar RBF (2010) Potentiale alternativer Herstellungskonzepte für Titanmetall und Titanlegierungen. In: 44. Metallurgische Seminar des Fachausschusses für Metallurgische, pp 131–162
Acknowledgements
The research was financially supported by the DAAD-scholarship Ph.D. Program, Universidad de Pamplona-Ph.D. Program, and by IME-RWTH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Julie M. Schoenung.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bolivar, R., Friedrich, B. Magnesiothermic Reduction from Titanium Dioxide to Produce Titanium Powder. J. Sustain. Metall. 5, 219–229 (2019). https://doi.org/10.1007/s40831-019-00215-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00215-z