Abstract
In this work, nickel/SiC foam was produced by the co-electrodeposition method. The effect of nanoparticle concentration (0, 0.1, 0.5 and 1 g/L SiC) and the current type on the nickel foam properties has been investigated. Microscopic studies showed that the pulse current significantly reduces the thickness difference between the surface and the foam core. In addition, the columnar structure converts into equiaxed due to the addition of nanoparticles and pulse current. In the pulse current state, less agglomeration occurred, and more uniform dispersion of particles was observed. According to the compression test, the foam produced in the bath contains 0.1 g/L SiC, and in the pulse state, the strength and energy absorption are 4.9 MPa and 132 MJ/m3, respectively, which are higher than those for pure nickel foam. But the other composite foams are weaker than non-composite specimens. The low-strength over-agglomerated particles are cut at low stress levels, and therefore, reduce the composite material’s strength. The results of the microhardness test showed that, on average, pulse current increased the hardness by about 84 Vickers. The fine-grained, uniform dispersion of nanoparticles and the higher deposition of nanoparticles by pulse current increase this hardness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nickel foam is one of the essential metal foams used in the industry. The unique properties of nickel have led to the high stability of these foams in the working conditions. For example, high corrosion resistance, reasonable mechanical strength, good electrical conductivity, and high-temperature resistance provide a wide range of applications for this foam. One of the most important uses of nickel foam is as an electrode in batteries such as nickel−cadmium-type (Ni−Cd) or metallic nickel−hydride (Ni−MH) batteries because of it’s high electrochemical resistance.[1,2,3,4,5] The corrosive and high tempreture operating condition caused Ni foam to be an attractive candidate for exhcust as a catalyst support.[6]
The most common and cost-effective method for the production of nickel foam is the electrochemical deposition of nickel on a polymeric foam substrate. The initial polymeric model must be conductive before immersion in the electroplating bath. Therefore, a thin conductive layer is created on the polymeric model by techniques like electroless process or graphite deposition. Electrical plating is then performed by applying certain current and voltage to this precursor. The polymer substrate is finally removed by heat treatment.[7,8,9,10]
One of the production problems by this method is the non-uniform distribution of current density in different areas of foam, which results in the heterogeneous deposition thickness at the surface and core of the foam. The formation of electromagnetic shields acting as the Faraday cage on the outermost layers prevents ions from entering the core of the foam. Therefore, a sharp concentration gradient is created from the surface to the foam core, significantly reducing the foam core access to ions and limiting the transfer of the matter to the core of the foam. Recently, using pulsed current rather than direct current has been proposed to achieve uniform thickness of deposition and significantly reduce the concentration gradient created during the deposition process.[11,12,13]
In general, many efforts have been made to improve the physical, mechanical and electrochemical properties of nickel foams. Works such as using aluminum substrates to enhance mechanical properties,[11,12,13] neutral atmosphere heat treatment to improve compressive strength and ductility,[10] aluminizing alloying and chromizing[14,15] to increase corrosion resistance have been investigated so far. One of the best ways to enhance the metallurgical properties of materials is to reinforce them with ceramic particles. In recent years, the production of nanocomposite coatings by electrochemical deposition method is one of the developing fields. In this method, the insoluble dispersed particles are added to the coating during electroplating process. This process can produce metal coatings containing particles of ceramics and organic materials.[16,17,18,19]
Various studies have already been conducted on the production of composite nickel coatings using different ceramic reinforcing particles such as Al2O3, SiC, WC, Cr2O3, TiO2.[16,20] Since SiC has properties such as hardness of about 5100 Vickers, the elastic modulus of 450 GPa, high wear and erosion resistance, excellent corrosion resistance, and is also inexpensive, so its use in composites and composite coatings has been widely developed. It is reported that the presence of SiC particles in nickel coatings has increased the hardness and improved the wear and corrosion properties.[17,18,19, 21, 22]
In this study, nickel base open-cell foam containing SiC reinforcing particles were produced. The effect of particle concentration in the deposition bath and the type of applied current (direct and pulse) on the metallurgical properties have been investigated. Uniformity in the deposition thickness and microstructure of the foams was studied and analyzed using light and electron microscopy and EDS analysis. Compressive strength testing has also been performed per ISO 13314[23] for metal foams. The microhardness of the foams produced under different conditions has also been compared to each other.
Materials and Methods
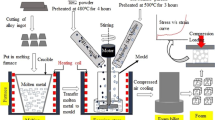
Polyurethane foam was used as the primary model to produce nickel foam by the electrodeposition method. Commercial electroless solution (Growel Company, India) was used to create the conductive polymeric substrate. The conductive specimens were electroplated in Watts solution with the composition of NiSO4.6H2O (200 g.L−1) + NiCl (10 g.L−1) + H3BO3 (30 g.L−1) + H2O, at 60 °C and pH 3.6. Electroplating bath contained a different amount of nano-SiC particles (us-nano company, 45 to 65 nm): 0, 0.1, 0.5 and, 1 g/L. The electrolyte was continuously stirred by a magnetic stirrer for 24 hours before electroplating to prevent agglomeration. Saccharin was also added as a surfactant to reduce the nanoparticle agglomeration. The maximum applied current density was 0.1 A/dm2 for both direct current (DC) and pulsed current (PC) conditions. The samples were prepared at a duty cycle of 50 pct and a frequency of 100 Hz for the pulsed current type. The thickness of the nickel layer was controlled by deposition time, and the electroplating process continued until the samples reached a density of 0.95 ± 0.02 g/cm3. To ensure the reproducibility of the results, five samples were produced in each condition.
The produced composite foams were analyzed by SEM/EDS analysis. For each condition, at least 3 samples (out of the 5 prepared samples) were analyzed by EDS. According to the standard ISO 13314,[23] the compressive load was applied with strain rate 3 × 10−3 s−1 by a universal testing equipment ZWICK Z250. Two main compressive properties including plateau stress and energy absorption are investigated in this study. According to the definition in JIS H 7902 standard, the plateau stress (σp) equals to the average stress in the strain range of 20 to 30 pct. The strain accordance with 1.3 times of the plateau stress is called densification strain (εd) as DIN 50134 standard. Energy absorption (Ea) could be calculated by Eq. [1][24,25]:
The compression test was repeated five times for each specific condition. Then the average results with standard deviation for σp and Ea are reported in the present work.
The Vickers microhardness measurements were also performed with a 25 g load and indentation time of 10 s by using Buehler hardness tester. Five samples were produced for each condition. Some pieces of these 5 samples (from the core and the surface of the foams) were randomly mounted by resin and then microhardness was measured five times on the mounted specimen. The results were reported as the average of the 5 replicates.
Results
Thickness Homogeneity
As well known, the deposition thickness at the surface is usually several times thicker than that at the core of the foam. This problem arises from mass transfer limitation and electromagnetic shielding of surface struts that act as a Faraday cage.[11,12,13] To investigate the effect of pulsed current and nano-particles on this phenomenon, the strut thickness measurements were performed for at least seven pores of the surface or the core randomly from five samples produced under the same conditions. How to measure the strut thickness for a pore is shown in Figure 1.
Figure 1 shows the cross-section of the surface and core of the pure nickel foams produced by DC and PC conditions. The thickness of the deposited nickel layer is measured by Clemex, a commercial image analysis software. The average thickness of the deposited nickel layer in the surface and core of produced foams in various baths by pulsed and direct currents is plotted in Figure 2. The thickness difference enhanced by increasing the amount of nanoparticles in the electroplating solution. These inert particles reduce bath conductivity. Therefore, the access of the foam core to the ions is significantly reduced. In contrast, the pulsed electrodeposition process has remarkably decreased the thickness difference in the surface and core of foams, even in the presence of the neutral particles.
Microstructure and EDS Analysis
Figures 3(a) and (b) are the surface morphologies of the pure nickel foams electrodeposited under DC and PC conditions, respectively. The surface in both conditions is similar to cauliflower, but it seems that a smoother surface is created under pulsed electroplating condition.
Figure 4 shows a cross-sectional image of non-composite nickel foams produced in both direct current and pulse modes etched with Aqua Regiareagent. As can be seen from Figure 4, the deposition of nickel is columnar in the direct current condition. It is reported that the shape of the grains in the standard Watts bath is columnar at the beginning of deposition and then becomes equiaxed, which is also seen in Figure 4(a).[26] Obviously, with increasing the deposition thickness, the diameter of the columns also increases (several grains were labled in the figure).[27] In Figure 4(a), the growth of the columns has occurred explosively, and this leads to an increase in internal stress. This internal stress is seen in the non-uniform colour of the etched sample. At the end of deposition, by the columnar structure growth, the etching intensity is higher, indicating the concentration of stress in these areas. Figure 4(b) shows that the pulse current has been able to change the shape of the grains from columnar to equiaxed.
In general, to reduce the grain size and change its structure from columnar to equiaxed, continuous nucleation must occur. According to literature it can be achieved by three methods: (a) adding grain refining agents to the deposition bath[28]; (b) adding a secondary reinforcing phase, and (c) applying pulsed current.[29] Figure 5 shows the SEM image of the nickel foam specimens deposited in the bath containing 0.1 g/L of SiC nanoparticles. Samples have been etched with Aqua Regiareagent. The surface roughness of the etched specimens in the Figure 4 is due to the separation of the SiC nanoparticles removed by etching from the nickel matrix. As can be seen in the figures, the presence of nanoparticles in both DC and PC conditions resulted in equiaxed grains, which causes less internal stress than condition without nanoparticles and direct current. On the other hand, the average grain size in the direct current state is 21.3 ± 7.1 microns and in the pulsed current condition is 5.8 ± 3.6 microns (The grain size measurements were performed according to ASTM E112-13[30] by Lineal Intercept method). Figure 5(a) also shows the occasional agglomeration of nanoparticles in the direct current condition in this bath, while in pulsed current mode, the uniform distribution of nanoparticles is shown.
Figure 6 shows the SEM images and EDS analysis of as-polished samples of nickel foams produced in the bath containing 1 g/L of SiC nanoparticles. As can be seen, under direct current conditions, the nanoparticles have become more agglomerated, and the pulse current has led to a uniform distribution of nanoparticles in the nickel matrix.
Compressive Strength and Energy Absorption Capability
Studied samples after the compressive tests are shown in Figure 7. The pure nickel foam can be compressed up to 70 pct compaction; while the walls of all composite foams collapsed before 70% deformation due to embrittlement.
Figure 8 shows the stress-strain curves obtained from the compressive tests for composite and non-composite specimens which behave quite differently. It is well known that the presence of fluctuations in the compressive curve of metal foams indicates embrittlement.[10,31] Among specimens, the pure nickel foams produced by both DC and PC conditions possess high ductility. Adding SiC particles to the ductile nickel matrix makes it brittle. The fluctuations have considerably increased for nanocomposite foams produced in bathes containing 0.5 and 1 g/L nanoparticles. There is the same behaviour in compressive curves of all composite samples for DC and PC conditions except that of electroplated in 0.1 g/L SiC bath and by the pulsed current. The compressive behaviour of this foam is more similar to that of foams produced in non-composite bathes. There are also much fewer fluctuations in its curve compared to the compressive curves of other composite foams in Figure 8.
Plateau strength and energy absorption as a function of electroplating conditions are shown in Figures 9 and 10. In general, the application of pulsed current has improved both mechanical properties in comparison with applying direct current for each bath conditions.
Besides, composite foams produced in baths contained 0.5 and 1 g/L nano-SiC have no positive effect on the improvement of mechanical properties. Whereas, there is an increase in plateau strength and energy absorption for the nickel foam electroplated in the presence of 0.1 g/L nano-SiC compared to the non-composite sample. Although the specimens produced in this bath have approximately the same plateau strength in both direct and pulsed current conditions, the energy absorption capability of the specimen produced by pulsed mode is much higher than that of another one.
Microhardness
Microhardness measurement of nickel foams produced in different conditions is reported in Figure 11. The hardness of nickel foams electrodeposited in the PC condition was higher than that of ones prepared under DC condition. The current type and SiC concentration in electrodeposition solution have a significant effect on microhardness. Both of them increase hardness. This implicitly reveals that as the concentration of particles in the bath increases, the more particles codeposited in the nickel matrix.
Discussion
Grain refining and dispersion of hard particles of the second phase are the two most effective methods to increase strength and hardness. Therefore, the finer structure, the higher the strength. The Hall-Petch relationship expresses the dependence of yield stress on grain size. This relationship applies when the grain size is more than a few microns.[32]
Applying a pulsed current increases the nucleation rate of nickel, thereby decreasing its grains size.[18,33,34] Anions have been reported to be cathodically absorbed during toff, and this absorption encourages further nucleation during ton.[22,35] The microstructural studies testify to an enhancement of nuclei (Figure 3), the alteration of morphology from columnar to equiaxed (Figure 4) and, the grain refinement (Figure 5) due to the application of pulse current. Therefore, in all production conditions of the studied foams, the foams produced by pulse current have higher plateau strength, higher energy absorption capability and hardness than the produced samples by direct current. In addition, in the presence of secondary phase particles in the bath, pulse current leads to more deposition of these particles and their uniform distribution in the metal matrix,[36] which can be another reason for increasing the mechanical properties of nickel foams.
In most studies on Ni-SiC composite coatings, the grain refinement (Hall-Petch relationship) and Orowan mechanism are introduced as the reinforcement mechanisms.[16,37,38,39] Comparison of Figures 4(a) and 5(a) shows the change in the grain shape from columnar to the equiaxed and the grain size reduction due to the nanoparticle addition. However, according to the results of the compression test (Figures 8, 9 and 10), the mechanical properties (σp and Ea) of composite foams have decreased compared to non-composite foams except in one case (the foam produced in 0.1 g/L SiC bath and under PC condition). At room temperature, dislocations have two ways to pass through hard particles: 1. cutting the particles, and 2- bypassing the particles (Orowan mechanism).[32] Electron microscopy images of composite foam samples produced in baths with high concentrations of nanoparticles (baths containing 0.5 and 1 g/L) show that nanoparticles have been agglomerated and produced particles with dimensions of several microns. Under pulse conditions, the particles are clustered in smaller sizes and have a more uniform distribution in the matrix. The agglomerated particles have low strength, and so easily have been cut off by dislocations, and the strength of the foam is significantly reduced.
Moreover, these phenomena make them severely brittle. However, in the case of composite foam produced in the bath with a lower concentration (contained 0.5 g/L nano-SiC) and in pulse mode, the compressive strength has increased compared to pure nickel foam. As clear from the microscopic images for this foam, the agglomerated particle size is submicron. It can be said that the Orowan mechanism also activates in particle strengthening. Nie and Korayem proved this mechanism by TEM images for SiC and Al2O3 nano-particles in Mg matrix, respectively.[40,41]
The hardness increases by adding more nano-particles to the bath since the amount of these hard particles increase in the deposition matrix. However, this increase in hardness has not resulted in an increase in strength. In fact, adding SiC nano-particles could be useful for improvement of the surface hardness of the foam but not suitable for bulk properties such as compressive strength.
Conclusion
The composite nickel foam was produced by co-electrodeposition of nickel and nano SiC particles on a polyurethane foam substrate. The effect of SiC concentration in the bath (0, 0.1, 0.5 and 1 g/L) and current type (DC and PC conditions) on the mechanical and microstructural properties were investigated. The research yielded the following results:
-
1.
Microscopic images showed that the microstructure in the common plating condition is columnar. Both pulse current and precipitation of neutral particles in the bath individually lead to equiaxed grains. In addition, these two factors are the grain refinements of the microstructure. Pulse currents lead to the more uniform dispersion of particles in the nickel matrix and, more deposited particles compared to the direct current state.
-
2.
The results of the standard compression test for the studied foams showed that the non-composite foams are much more ductile than the composite foams. The presence of SiC particles has led to severe brittleness of foam. In composite foams produced in baths of 0.5 and 1 g/L, the compressive properties of the foam are significantly reduced compared to the non-composite foam. The reason for this is the severe agglomeration of SiC particles, which form particles as large as a few microns. Because of the ease of cutting these particles by dislocations, composite foams are broken at much lower stresses than non-composite foams.
-
3.
The highest strength of the plateau and the ability to absorb energy among the studied samples attributed to the foam produced in the bath containing 0.1 g/L of nanoparticles and under pulse current. It can be said that the mechanism of grain refining and Orowan have increased the strength of this composite foam compared to pure nickel foam under similar conditions. Although this foam is more brittle than pure foam, it is much less brittle than other composite foams.
-
4.
The microhardness measurement of the studied foams indicates an increase in hardness when the particle concentration enhances in the electrolyte. This implies that the particle amount has increased in the deposition. The pulse current has also led to an increase in hardness by approximately 84 Vickers. The fine-grained, uniform dispersion of nanoparticles and the greater deposition of nanoparticles by pulse current increase this hardness.
References
1. S.-F. Fan, T. Zhang, Y. Kun, H.-J. Fang, H.-Q. Xiong, Y.-L. Dai, D.-Y. Jiang, H.-L. Zhu (2017) Trans. Nonferrous Metals Soc. China 27:117–124
L. Lin, S. Tang, S. Zhao, X. Peng and N. Hu, Electrochimica Acta, 2017, 228, 175-182.
C.-H. Liao, P.-S. Hung, Y. Cheng, S.-Y. Chang and P.-W. Wu, Materials Letters, 2018, 215, 152-156.
O. B. Olurin, D. S. Wilkinson, G. C. Weatherly, V. Paserin and J. Shu, Composites science and technology, 2003, 63, 2317-2329.
T. T. Nguyen, D. Mohapatra, D. R. Kumar, M. Baynosa, S. Sahoo, J. Lee and J.-J. Shim, Electrochimica Acta, 2021, 367, 137226.
G. Walther and and B. Kloeden, CELLMET Conference, 2008.
P. Liu and K. Liang, Materials science and technology, 2000, 16, 575-578.
P. Liu, H. Chen, K. Liang, S. Gu, Q. Yu, T. Li and C. Fu, Journal of applied electrochemistry, 2000, 30, 1183-1186.
X. Badiche, S. Forest, T. Guibert, Y. Bienvenu, J.-D. Bartout, P. Ienny, M. Croset and H. Bernet, Materials Science and Engineering: A, 2000, 289, 276-288.
F. Barzegar, A. Salehi and A. Moloodi, Metallurgical and Materials Transactions B, 2019, 50, 1988-1996.
A. Jung, H. Natter, R. Hempelmann, S. Diebels, M. R. Koblischka, U. Hartmann and E. Lach, ECS Transactions, 2010, 25, 165-172.
A. Jung, M. R. Koblischka, E. Lach, S. Diebels and H. Natter, International Journal of Material Science, 2012, 2, 97-107.
B. Bouwhuis, J. McCrea, G. Palumbo and G. Hibbard, Acta materialia, 2009, 57, 4046-4053.
H. Choe and D. C. Dunand, Materials Science and Engineering: A, 2004, 384, 184-193.
A. Hodge and D. C. Dunand, Intermetallics, 2001, 9, 581-589.
S. Dehgahi, R. Amini and M. Alizadeh, Surface and Coatings Technology, 2016, 304, 502-511.
W. Jiang, L. Shen, M. Qiu, X. Wang, M. Fan and Z. Tian, Journal of Alloys and Compounds, 2018, 762, 115-124.
H. Kazimierczak, K. Szymkiewicz, E. Gileadi and N. Eliaz, Coatings, 2019, 9, 93.
Y. Zhang, F. Chen, X. Tang, H. Huang, M. Ni and T. Chen, Journal of Composite Materials, 2018, 52, 953-962.
I. Duarte and J. M. Ferreira, Materials, 2016, 9, 79.
M. Vaezi, S. Sadrnezhaad and L. Nikzad, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 315, 176-182.
P. Gyftou, E. Pavlatou and N. Spyrellis, Applied Surface Science, 2008, 254, 5910-5916.
ISO 13314, ISO: Geneva, Switzerland, 2011.
DIN 50134, German National Standard, 2008.
J.H., 7902 standard, 2008.
T. Lampke, B. Wielage, D. Dietrich and A. Leopold, Applied Surface Science, 2006, 253, 2399-2408.
F. Ebrahimi, G. Bourne, M. S. Kelly and T. Matthews, Nanostructured materials, 1999, 11, 343-350.
J. Amblard, I. Epelboin, M. Froment and G. Maurin, Journal of applied electrochemistry, 1979, 9, 233-242.
E. Pavlatou, M. Raptakis and N. Spyrellis, Surface and coatings technology, 2007, 201, 4571-4577.
ASTM E112-13; ASTM International: West Conshohocken, PA, USA, 2013.
Y. Sun, R. Burgueño, W. Wang and I. Lee, International Journal of Solids and Structures, 2015, 54, 135-146.
P. P. Dieter (1988) Crystal Research and Technology. Mc Graw-Hill Book Co., New York
T. Frade, V. Bouzon, A. Gomes, M. da SilvaPereira (2010) Surface Coatings Technol. 204:3592-3598
P. I. Nemes, M. Lekka, L. Fedrizzi and L. M. Muresan, Surface and Coatings Technology, 2014, 252, 102-107.
C. Kollia, N. Spyrellis, J. Amblard, M. Froment and G. Maurin, Journal of applied electrochemistry, 1990, 20, 1025-1032.
36. J. Fustes, A. Gomes, M. da Silva Pereira (2008) J. Solid State Electrochem. 12:1435-1443
Y. Zhou, F. Xie, X. Wu, W. Zhao and X. Chen, Journal of Alloys and Compounds, 2017, 699, 366-377.
S. Wang, N. Zhou and F. C. Walsh, Transactions of the IMF, 2016, 94, 274-282.
S.-C. Wang and W.-C. J. Wei, Journal of materials research, 2003, 18, 1566-1574.
M. Habibnejad-Korayem, R. Mahmudi, H. Ghasemi and W. Poole, Wear, 2010, 268, 405-412.
K. Nie, K. Deng, X. Wang and K. Wu, Journal of Materials Research, 2017, 32, 2609.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted on October 24, 2020; June 28, 2021.
Rights and permissions
About this article
Cite this article
Karimi, E.Z., Barzegar, F., Moloodi, A. et al. Hardness and Compressive Properties of Open-Cell Nickel Foam Reinforced by Nano-SiC Particles. Metall Mater Trans B 52, 3439–3446 (2021). https://doi.org/10.1007/s11663-021-02273-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02273-9