Abstract
A vanadium nitride alloy can be used as an alloying source of nitrogen and vanadium elements in high-strength steel. In this study, comprehensive and systematic experiments were conducted to optimize the process conditions for producing a vanadium nitride alloy from the starting materials (V2O5 and Fe2O3). The reduction and nitridation behaviors of the starting materials were investigated using thermogravimetry-differential scanning calorimetry (TG-DSC) and horizontal tube furnace experiments under various gas conditions. The phase and chemical compositions of the final alloy products were analyzed using X-ray diffraction (XRD), scanning electron microscope-electron dispersive spectroscopy (SEM-EDS), and the inert gas fusion (IGF) technique. In addition, thermodynamic calculations were performed to help in the understanding of the Nitrovan production process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-strength low-alloy (HSLA) steels are classified as special steels, which are strong, plastic in nature, and exhibit excellent weldability and corrosion resistance compared to conventional low-carbon steels. In addition, HSLA steels are most suitable and retain their operational characteristics even at extremely low temperatures.[1,2,3]
The unique mechanical properties of HSLA steel result from microalloying with carbide- and nitride-forming elements. Nitrides aid in effective strengthening of steel; therefore, nitrogen-bearing gases or alloys are used for microintroduction of nitrogen during the secondary steelmaking process. Vanadium in the presence of nitrogen and carbon in steel can form vanadium carbonitride phases, which can improve the overall mechanical properties. Vanadium nitrides are uniformly dispersed in the final microstructure and are more stable than vanadium carbides. Therefore, vanadium strengthening is more efficient with nitrogen compared to carbon.[4]
The addition of nitrogen into steel through master alloys is an emerging technology. New alloys are actively being sought for effective nitrogen recovery in liquid steel. Ferrovanadium nitrides and vanadium nitrides are a few of the recognized nitrogen-containing master alloys. Wu et al.[5] synthesized high-quality ferrovanadium nitride (FeV65N) by roasting the mixtures of V2O5, Fe2O3, and graphite powder in N2 atmosphere between 1400 °C and 1600 °C. Samples with varying C/O molar ratios were kept at 650 °C for 2 hours and heated to 1500 °C to be held isothermally for 3 hours. Then, the samples were cooled to 1100 °C and held again for 3 hours. All the experiments were performed under N2 atmosphere. It was observed that the maximum nitrogen content was found in the samples with C/O molar ratio of 0.825. In a separate study, Wu et al.[6] also synthesized ferrovanadium nitride alloy (FeV55N) by carbonitrothermic reduction of starting materials containing V2O5 and Fe2O3 powder. Samples with varying C/O ratio (molar ratio between 0.75 and 0.90) were held isothermally at 650 °C in N2 for 2 hours, and then heated to the final target temperatures of 1350 °C, 1450 °C, and 1550 °C and held for 3 hours under N2 atmosphere. Maximum nitrogen solubility of 12.99 wt pct was obtained in the case of the samples with C/O molar ratio of 0.8 held at 1450 °C. In a follow-up study, Zhou et al.[7] synthesized high-quality ferrovanadium nitride (FeV45N, FeV55N, and FeV65N) by the carbothermal nitridation method. V2O5, Fe3O4, and graphite powders were mixed in varying C/O molar ratios. The V2O5 loss was controlled by an initial reduction of samples at 650 °C for 2 hours in N2 atmosphere. Next, the temperature was increased to 1500 °C and 1550 °C, respectively, and samples were held for 3 hours in N2 atmosphere. XRD analysis confirmed the presence of VN and Fe phases for all compositions and temperatures. The study suggested that the C/O molar ratio affects the N and C content in the final product. It was seen that when graphite was insufficient, V2O3 was present in the final product, and when graphite was in excess, there was high residual carbon content and low nitrogen content in the final product. It was noted that high temperature contributes to the increase in nitrogen content in the final product. Ziatdinov and Shatokhin[8] prepared ferrovanadium nitride using self-propagating high-temperature synthesis. They nitrided different grades of commercially available ferrovanadium alloys. The chemical analysis of ferrovanadium nitride produced using this technique constituted 44 to 48 wt pct vanadium, 9 to 11 wt pct nitrogen, and less than 0.5 wt pct carbon (remaining is Fe). Liu et al.[9] synthesized ferrovanadium nitride (FeV45N) by direct reduction and nitriding of VO2 and Fe2O3. Ammonia (NH3) was used as a reductant as well as a nitriding source. Oxide mixtures were reduced and nitrided in different temperatures ranging from 600 °C to 1000 °C and isothermally held between 1 and 6 hours. The XRD analysis of reduced and nitrided samples at lower temperatures, such as 600 °C, 700 °C, and 800 °C for 4 hours, respectively, showed the presence of VN, Fe, and Fe4N phases. However, samples reduced and nitrided at higher temperatures, such as 900 °C and 1000 °C for 4 hours, respectively, showed the presence of VN and Fe phases only. The samples held isothermally at 1000 °C for 1 hour showed the presence of VN, Fe, and V2O3. The final product constituted N2 content of approximately 11.7 wt pct and low oxygen content of 0.25 to 1.16 wt pct. Wu et al.[10] prepared high-quality FeV55N alloy using ammonia as a reductant and nitrogen source. Initial raw materials, such as ammonium vanadate (NH4VO3), Fe2O3, NH4VO3, and Fe2O3, were mixed uniformly according to the stoichiometric ratio and heated to 800 °C, 900 °C, 1000 °C, and 1100 °C, respectively, followed by isothermal holding. XRD analysis revealed that samples held at 1000 °C for 3 and 5 hours contained VN and Fe2N along with unreduced V2O3 oxides. At higher holding times of 8 and 10 hours, V2O3 reduction is complete and VN and Fe2N phases were reported.
Vanadium nitride is also used as a master alloy to introduce nitrogen in liquid steel. There are various studies[11,12,13,14,15,16,17,18] and patents[19,20] that deal with the synthesis of this alloy. Dong et al.[11] synthesized vanadium nitride in a one-step method by direction reduction of V2O5 using graphite powder. The samples were held isothermally at 650 °C for 4 hours to suppress the volatilization loss of V2O5. Next, the samples were heated to 1000 °C, 1200 °C, and 1300 °C, respectively, and held isothermally for 4 hours in N2 atmosphere. The XRD analysis revealed the presence of VC and VN in the samples. Similarly, Xinhui et al.[12] performed the direct reduction of V2O5 using graphite powder at 1400 °C in the presence of N2. The nitrogen content and vanadium content were reported to be 15 to 16 and 79 to 81 wt pct, respectively. A similar study was performed by Tripathy et al.[13] and the nitrided sample at 1500 °C constituted 20.98 wt pct N with minimal residual carbon. Chen et al.[14] developed a one-step method of carbothermal reduction and nitriding to produce vanadium nitride alloy. V2O5 extracted from low vanadium shale and carbon black was used as the initial raw material. The ample mixtures with C/O molar ratio of 1.05 were prereduced isothermally at 650 °C for 2 to 4 hours and at 700 °C for 2 hours. The XRD analysis revealed that reduction at 650 °C for 2 hours contained unreduced V2O5 along with oxides such as V6O13 and VO2. The reduced sample at 650 °C for 4 hours constituted higher melting oxides, such as V6O13 and VO2, and no V2O5 was reported. The reduction of sample at 700 °C for 2 hours had only VO2. The prereduced sample at 650 °C for 4 hours was nitrided up to 1200 °C and 1300 °C, respectively. XRD analysis of final nitrided sample showed the presence of VN and VC along with vanadium oxides. Huang et al.[15] studied the influence of the N2 flow rate during the reduction of V2O5 in the temperature range of 1420 °C to 1500 °C. The maximum nitrogen content in the final nitrided alloy was found to be 12 to 15 wt pct for the flow of 120 L/h. Wu et al.[16] synthesized vanadium nitride alloy by magnesiothermic reduction of V2O3 in nitrogen atmosphere with the assistance of magnesium chloride (MgCl2). By this route, carbon was avoided in the final product and oxygen content was reduced to a low level. A sample mixture was held isothermally for 3 hours in the temperature range of 675 °C to 800 °C. The final sample prepared at 750 °C showed the presence of 23.58 and 76.42 wt pct of N and V, respectively. A very similar study was conducted by Rui et al.[17] in which they used Mg powder instead of MgCl2. Zhao et al.[18] synthesized VN nanopowders by thermal nitridation. NH4VO3 and nanosize carbon black were used as the starting raw materials and mixed uniformly according to stoichiometric proportions using deionized water. Sample mixtures were held isothermally at 1100 °C for 0.5, 1, 1.5, and 2 hours, respectively. XRD analysis of the sample nitrided at 1100 °C for 0.5 hours confirmed the presence of VN and carbon, whereas some amount of V2O3 still remained in the final product. Samples were nitrided at 1100 °C for 1 hour and had the presence of VN and V8C7 phases. In addition, there are a few patents that describe the preparation of vanadium nitride master alloy.[19,20,21,22] According to the patents, V2O5, along with varying amounts of carbonaceous material (coke or graphite), is heated in N2 atmosphere up to 1400 °C to 1500 °C to produce Nitrovan product constituting 76 to 80 wt pct vanadium, 10 to 12 wt pct nitrogen, and 8 to 10 wt pct carbon. It is important to note that there is no Fe in the vanadium nitride alloy.
Although ferrovanadium nitrides are commercially used in secondary steelmaking to introduce nitrogen in the liquid steel, nitrogen recovery is still a concern (around 50 pct). As discussed earlier, vanadium nitride is also a nitrogen-bearing master alloy. Nitrovan (a type of vanadium nitride) is a widely used commercial product of Evraz Stratcor with nitrogen recovery of around 65 pct. In addition, vanadium nitride is more effective in the final strengthening of steel as less vanadium is required in the form of vanadium nitride compared to ferrovanadium nitride in order to achieve the same yield strength.[23] Further, it is known that the presence of Fe in the vanadium nitride master alloy increases the nitrogen content without comprising on the recovery during liquid steelmaking.[24,25] As seen, there are several studies on the production of ferrovanadium nitrides and vanadium nitride master alloys. However, there is no investigation on the synthesis of vanadium nitride with 2 to 3 wt pct Fe.
In this study, we propose a methodology to produce vanadium nitride master alloy through reduction and nitridation of V2O5 in the presence of a small amount of Fe2O3 and graphite powder under different reducing atmospheres such as N2, CO, and CO2. Both thermogravimetry-differential scanning calorimetry (TG-DSC) and tube furnace experiments were performed under controlled atmosphere in order to optimize the reduction and nitridation process. Thermodynamic calculations were also performed to assist the understanding of the process. The final target product composition is 70 to 75 wt pct vanadium, 10 to 12 wt pct nitrogen, 8 to 10 wt pct carbon, and 2 to 3 wt pct iron. It should be noted that 2 to 3 wt pct Fe is desired in the Nitrovan to increase its density, which can improve the nitrogen recovery during the injection into liquid steel in secondary steelmaking. In addition, the presence of Fe in the vanadium nitride alloy is also expected to improve the N solubility in the final product.
Experiments
Sample Preparation
For sample preparation, high-purity V2O5 (98 pct), Fe2O3 (98 pct), and graphite powder were mixed in appropriate proportions to achieve a C/O molar ratio of 0.9, 1, and 1.1 in the starting materials. Impurity in V2O5 constitutes elements such as Si, Fe, P, S, Na, and K. However, the individual concentrations of these elements are unknown. Na and K have a tendency to lower the melting point, thereby allowing for the formation of liquid phase (albeit in a low volume), which impacts the reaction rates. Conversely, impurities in Fe2O3 constitute MgO, Al2O3, and SiO2 (around 1 pct) and the remaining are trace elements (1 pct). MgO is a very stable oxide and it is well known that MgO is used as a catalyst for iron ore reduction. Given that all the experiments are performed with the same batch with reasonably close Fe/V ratios, any side effects (due to impurities in the starting material) are likely to be similar in all the experiments.
The compositions of starting materials used for the experiments are listed in Table I. A total of 5 g of each starting material was prepared by mixing V2O5, Fe2O3, and graphite powders using mortar and pestle. Ethanol was added every 15 minutes for a total of 1 hour of mixing. Then, sample mixtures were left for natural drying for 8 hours. Briquettes of 1 mm × 2 mm were made by compacting the powder mixture in the stainless steel mold and were used for DSC-TG analysis. Briquettes of 20-mm × 10-mm dimensions were used for the tube furnace experiments. The sample preparation procedure is outlined in Figure 1.
TG-DSC Experiments
TG-DSC experiments were performed in a Netzsch STA 449 F3 using alumina crucibles. As a preliminary study to understand the overall reduction and nitridation behavior of starting materials composed of V2O5, Fe2O3, and graphite, nonisothermal heating of the samples to 1400 °C was conducted under N2 atmosphere. Based on the results, the reduction processes of samples at 600 °C under different gas atmospheres were comprehensively analyzed. The temperature of 600 °C was chosen to prevent the possible sudden volatilization loss of V2O5, which was reported at about 650 °C.[5] It is noted that all the gases used during the experiments are 4N pure supplied by Praxair Gas Company Pvt. Ltd. Gases used in TG-DSC and tube furnace experiments (discussed Section C) are passed through a silica gel column to remove inherent moisture followed by a magnesium turning furnace to remove the traces of oxygen in the gas mixture. The final purified gas after the silica gel column and Mg turning furnace is analyzed for O2 content using a gas analyzer. The O2 content is found to be within the range of 0 to 10 ppm for an accuracy level of 0.01 pct. Gas mixing is electronically controlled using IDE “FlowVision” supplied by Alicat Scientific. The gas mixture after passing through MFCs is circulated in a recycled loop connected to a gas analyzer to check for O2 content.
Nonisothermal nitriding up to 1400 °C
The sample with a C/O molar ratio of 0.9 was initially heated to 1400 °C in N2 atmosphere under nonisothermal condition. The samples were heated from 30 °C to 1400 °C with a heating rate of 10 K/min under a constant 60 mL/min N2 flow rate.
Reduction of briquettes in N2 atmosphere at 600 °C
The samples with C/O molar ratio of 0.9, 1, and 1.1 were heated from 30 °C to 600 °C with a heating rate of 10 K/min under a 240 mL/min N2 flow rate. The samples were then isothermally held at 600 °C for 4 hours under a 240 mL/min N2 flow rate (maximum flow rate in STA 449 equipment) to ascertain the influence of N2 upon the reduction process.
Reduction of briquettes in CO and CO2 atmosphere at 600 °C
Three different sets of experiments were performed to evaluate the effect of CO and CO2 on the reduction behavior of V2O5 and Fe2O3 in the presence of graphite. In the first set, samples with C/O molar ratios of 0.9, 1, and 1.1 were heated from 30 °C to 600 °C at 10 K/min in CO atmosphere. CO flow was initialized at 200 °C and set at 100 mL/min. After reduction of the sample for 4 hours at 600 °C under 100 mL/min CO atmosphere, the samples were cooled to 20 °C at a cooling rate of 40 K/min. In the second set, CO2 atmosphere was used with the same experimental conditions as outlined previously. In the third set of experiments, reduction was performed under the CO and CO2 mixture with the flow rate of 50 mL/min for each gas.
Experiments with Tube Furnace
After the initial reduction experiments in TG-DSC, briquettes of varying C/O molar ratios were reduced and nitrided in the horizontal tube furnace. Five grams of briquette samples were placed in an alumina crucible inside the horizontal tube furnace. The outline of the temperature cycle for the tube furnace experiment is shown in Figure 2. The samples were heated from 30 °C to 600 °C with a heating rate of 10 K/min under 500 mL/min CO atmosphere. Then, the samples were isothermally held at 600 °C for 4 hours, subsequently heated to 1400 °C at a heating rate of 10 K/min, and held for 15 minutes in CO and N2 atmosphere with flow rates of 500 mL/min for each gas. Then, the samples were cooled to room temperature in N2 gas atmosphere at the cooling rate of 40 K/min.
Characterization
The phase analysis for the reduced briquettes after the DSC-TG experiments and tube furnace experiments was performed using X-ray diffraction (XRD) analysis. Measurements of the samples were performed using a scanning rate of 0.02 deg/s with Cu Kα source in a X-ray diffractometer supplied by PANalytical (model X’Pert3 Powder). The samples after tube furnace experiments were cold mounted using a slow curing epoxy resin, and scanning electron microscope-electron dispersive spectroscopy (SEM-EDS) analysis was also conducted for composition analysis. The N and C compositions in the tube furnace samples were determined using the inert gas fusion (IGF) method. In the ferroalloy industry, especially with Nitrovan/vanadium nitrogen alloys, N in the alloy is determined by the IGF technique, which is regarded as a fingerprint technique. The N content in the samples is analyzed using IGF against a certified reference material (CRM) with a nitrogen value of 15 wt pct. During the process, a blank run of CRM is performed to estimate the N value. Next, the samples are run against this standard CRM sample. Two runs of each sample have been carried out to record any deviation in the N value and accordingly reported in this work. In a similar way, C was also determined and reported for all the samples. During the analysis of samples, a variation of 0.5 pct was observed and the average of the two runs is reported.
As mentioned earlier, SEM-EDS is also used for composition analysis including C and N. A total of 15 data points were selected in the microstructure for composition analysis, and the average of 15 points is reported as the final composition. Per industrial practice for analysis of N and C, IGF is a standard technique per ASTM E-1409. Since IGF is a bulk technique, it is more reliable compared to SEM-EDS-based composition analysis.
Thermodynamic Analysis
In order to understand the reduction and nitridation process in the experiments, thermodynamic analysis was performed. Thermodynamic calculations were performed using FactSage 7.3 software[26] with the FACTPS database, FSStel database, and new Fe-V-O oxide database.[27] The gas phases were taken from the FACTPS database. All metallic and carbide/nitride phases in the Fe-V-C-N system were taken from the FSStel database, and in particular, the fcc vanadium carbonitride phase of (Fe, V)[C, N, Va], where Va represents vacancy in the interstitial site, is an important phase in the present thermodynamic analysis. As the complete oxide database of the Fe-V-O system is not available in the current commercial FactSage 7.3 software, a recently optimized database for the Fe-V-O system by the present authors[27] was used in the current study. Figure 3 shows the phase diagram of the Fe-V-O system at 600 °C calculated from the recent thermodynamic database.[27] As can be seen, there are several stoichiometric Fe-V-O compounds, a complete corundum solid solution (M2O3) between Fe2O3 and V2O3, and an extensive spinel solution of Fe3O4-FeV2O4. Complex phase evolutions of the Fe-V-O oxide system can be expected during the reduction process of the V2O5 and Fe2O3 mixture.
Phase diagram of the Fe-V-O system at 600 °C calculated from the thermodynamic database.[27]
Results and Discussion
Nonisothermal Nitriding Up to 1400 °C Under N2 Atmosphere
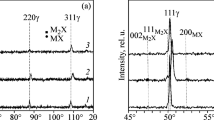
The TG-DSC curves for the sample (C/O = 0.9) heated to 1400 °C under N2 atmosphere are shown in Figure 4. As seen, a steep decrease in the TG curve is observed around 600 °C, suggesting the beginning of the reduction process. In this case, graphite alone acts as a reducing agent. Two exothermic DSC peaks are observed at 655 °C and 688 °C, suggesting the formation of reduction products. The TG data indicate a second steep weight loss around 1128 °C, and a significant peak is also recorded in the DSC curve. The final residual mass remaining in the system at 1400 °C is around 57 pct of the original sample weight. The phases in the sample after TG-DSC experiments were analyzed using the XRD technique. As shown in Figure 5, the XRD analysis confirms the presence of VN along with residual Fe and C in the sample.
XRD analysis result for the sample with 0.9 C/O molar ratio after the TG-DSC experiment (Fig. 4)
Reduction of Briquettes in N2, CO, and CO2 Atmosphere at 600 °C
The TG results of the sample mixtures with varying C/O ratios held at 600 °C for 4 hours under different gas atmospheres are summarized in Figures 6(a) through (c). As seen, TG curves for the samples in N2 atmosphere show a steady decrease with time. A very similar trend is seen for the samples in CO2 gas atmosphere. In both cases, the weight changes of samples recorded in the TG curve are not saturated with time, indicating incomplete reduction of the starting oxides within the given experimental duration. In CO atmosphere, on the other hand, the weight reductions of samples are accelerated compared to N2 or CO2 cases, which is manifested as the maximum weight loss observed around 80 minutes of isothermal holding. Interestingly, for the experiments under CO atmosphere, weight gain is observed in all the samples between 100 minutes and 4 hours of holding time. Under the combined flow of CO and CO2 gases, the maximum weight loss is observed around 120 minutes of holding time and then the sample weight is not changed. This trend is different from the case of CO gas atmosphere where mass gain is observed after maximum weight loss. The effect of varying C/O ratio is clearly observed in the TG data. Under CO atmosphere, the sample with C/O ratio 1 exhibits maximum weight loss around 11 pct, whereas it is 10 and 8 pct for samples with C/O ratios of 0.9 and 1.1, respectively. In combined CO and CO2 gas flow, the maximum weight loss increases with increasing C/O ratio. The sample with C/O ratio 0.9 shows a peak mass loss of 11 pct, whereas samples with C/O ratios 1 and 1.1 exhibit a maximum weight loss of about 12 pct.
The summary of the residual masses after isothermal reduction at 600 °C for 4 hours under different reducing atmospheres is presented in Table II. The results indicate that N2 and CO2 are least effective in reducing the starting oxides, whereas the mixture of CO and CO2 is most effective in achieving the maximum reduction. However, in the case of CO, maximum reduction is achieved within 80 minutes, whereas the CO and CO2 gas mixture requires around 120 minutes at 600 °C. Clearly, the reduction of the starting oxides is faster and more effective in the presence of CO. Hence, CO gas was selected as a reducing gas atmosphere for further experiments in the tube furnace.
The XRD analyses of the samples with varying C/O ratios reduced at 600 °C for 4 hours under different gas flow conditions are summarized in Figure 7. As seen in Figure 7(a), in N2 atmosphere, V2O5 is partially reduced to VO2 and V2O5 still remains in the system even after 4 hours. The FeVO4 spinel phase is also observed in the analysis, which melts around 850 °C.[28] If these samples are further treated at higher temperatures, the melting of V2O5 and FeVO4 is likely to occur, which is undesirable for a solid-state reduction process. In the case of isothermal holding in CO (Figure 7(b)) atmosphere, it is clearly seen that V2O5 is reduced to V2O3, VO2, and V13O16. In addition, Fe2O3 is reduced to FeO and Fe. As seen in XRD data, no intermediate phases, such as FeVO4, are formed. Vanadium oxides (V2O3, VO2, and V13O16) formed after the isothermal treatment have melting points higher than 1500 °C.[27] Hence, the samples after reduction under CO atmosphere can be further nitrided at higher temperatures. The XRD analysis of the samples reduced under CO2 atmosphere at 600 °C for 4 hours are shown in Figure 7(c). XRD analysis suggests that the reduced oxides (V2O3, VO2, V13O16, and FeO) are exactly the same as those observed under reduction with CO. However, TG data indicate that the reduction under CO2 flow is incomplete even after 4 hours of isothermal holding at 600 °C (Figure 6). From the residual mass comparison and XRD analysis results, it is confirmed that the extent of reduction is better in CO atmosphere than CO2 atmosphere. As seen in Figure 7(d), in the case of CO and CO2 combined gas flows, the reaction products obtained after isothermal holding are the same as those obtained after reduction in CO or CO2 atmosphere. The experimental results at 600 °C certainly indicate that the reduction process of the V2O5 and Fe2O3 mixture can be accelerated by CO gas and solid carbon. For example, the degrees of reduction under N2 and CO2 are much lower than those under CO and CO + CO2 gases. This proves that CO gas accelerates the reduction process of the V2O5 and Fe2O3 mixture.
Reduction of Briquette in the Course of Heating to 1400 °C in a Tube Furnace
The samples with varying C/O molar ratio were subjected to tube furnace experiments. The details of the experimental conditions are outlined in Figure 2. Basically, the samples were isothermally held at 600 °C under CO atmosphere for 4 hours followed by heating in combined CO and N2 flow until 1400 °C.
The SEM-EDS mapping analysis of a sample with C/O ratio 1 obtained after the tube furnace experiment is presented in Figure 8. The gray matrix phase in the EDS maps corresponds to Nitrovan (VN), and the light-colored phase is the Fe-rich phase. EDS composition analysis was conducted for more than 20 points of the VN phase to analyze carbon and nitrogen contents, and the results are summarized in Table III. According to SEM-EDS analysis, N and C contents in the sample of 0.9 C/O molar ratio are 7.92 and 12.69 wt pct, respectively. In the case of the sample of 1 C/O molar ratio, they are 9.43 and 18.34 wt pct, respectively, and in the case of the sample of 1.1 C/O molar ratio, they are 8.70 and 16.54 wt pct, respectively. The N and C analyses in final samples were also obtained through the IGF technique for comparison purposes. The results of IGF are similar to those of EDS analysis, as summarized in Table III. Wu et al.[6] reported N solubility of 12.99 wt pct in the ferrovanadium nitride (FeV55N) synthesized at 1450 °C under isothermal flow of N2 for 3 hours. Similarly, Xinhui et al.[12] and Chen et al.[14] found N solubility in the range of 12 to 15 wt pct in the vanadium nitride master alloy obtained during the reduction of V2O5 in the temperature range of 1200 °C to 1400 °C and isothermal holding between 4 and 6 hours. Moreover, the commercial product Nitrovan 12 constitutes N and C to be 10 to 14 wt pct and 10 wt pct maximum, respectively, in the final product. The N and C solubility observed in the present study (Table III) is in reasonable comparison with the previous studies.
SEM-EDS results for a sample with 1 C/O ratio obtained after the tube furnace experiment. See Fig. 2 for the experimental conditions
Thermodynamic Analysis
As seen in Figure 4, nonisothermal reduction behavior of the sample with 0.9 C/O ratio under N2 gas shows negligible weight loss until 600 °C, and then significant weight loss at about 650 °C and 1125 °C. The total residual mass at the end of the process at 1400 °C is about 57 pct of the original sample. In order to understand this reduction behavior with temperature, thermodynamic calculations were performed. In the calculation, 100 g of sample with 0.9 C/O ratio (71.87 g V2O5 + 5.71 g Fe2O3 + 22.42 g C) was reacted with 10 g N2 gas. The calculated equilibrium amounts of solid, liquid, and gas phases with temperature are plotted in Figure 9(a). The overall amount of all solid and liquid phases is plotted in Figure 9(b). According to the calculated results, the total residual mass is about 55 pct at 1400 °C, which is in a good agreement with the experimental data (57 pct). The sudden changes in TG slopes and DSC peaks at about 650 °C and 1125 °C seem to result from the formation of spinel solution (FeV2O4-rich solution) and V(C, N)-rich phase, respectively. The formation of liquid metallic phase is calculated at 1280 °C, and the final products at 1400 °C are calculated to be VO, V(C, N), and Fe-rich liquid phase. This is somewhat different from the experimental XRD results in Figure 5 indicating VN, Fe, and C in the final product. This difference can be explained if carbon in the sample was consumed to reduce VO instead of forming V(C, N) in real experimental conditions.
Thermodynamic calculations for the sample with C/O ratio of 0.9. (a) Variation of phase amounts with temperature and (b) calculated TG curve as a function of temperature. The inputs for the calculations are 71.87 g V2O5 + 5.71 g Fe2O3 + 22.42 g C + 10 g N2. Fe-rich, Corundum, and Spinel mean Fe-rich fcc phase, (Fe, V)2O3 phase, and FeV2O4-Fe3O4 phase, respectively
For successful production of VN through a nonisothermal process in the tube furnace (Section C), an isothermal reduction at 600 °C for 4 hours is an important step. As can be seen in Figure 6, the degree of initial reduction at 600 °C varies significantly depending on the gas atmosphere. However, the products are similar, as seen in the XRD results in Figure 7. That is, the amount of the products is different depending on the gas atmosphere, but the products that are formed are similar. In order to understand this initial reduction process and investigate the role of different gases in the reduction process, thermodynamic calculations were performed.
Figure 10 shows the thermodynamic calculation results for the reduction process at 600 °C under different gas atmospheres. In the calculations, the starting material representing 1 C/O ratio (69.99 g V2O5 + 5.71 g Fe2O3 + 24.29x g C, where x is varied from 0 to 1) is reacted with 10 g gas (CO, CO2, CO + CO2 (50 pct:50 pct), or N2) at 600 °C to simulate the isothermal process. Figures 10(a) through (c) show the result with CO gas, N2 gas, and no gas conditions, respectively. The reductions in different gas conditions are summarized and plotted in Figure 10(d). The y-axis of Figure 10(d) represents the mass of the remaining sample (all solid oxides and carbon; this is the same as mass percent in the TGA graph) calculated after the reduction process. According to the calculations, the sample can be reduced by 7.5 and 4 pct by CO gas and CO + CO2 gas, respectively, even without any carbon (Figure 10(d) at x = 0). However, when N2 gas is used, no reduction is calculated by gas itself and the overall reduction process in Figure 10(b) is the same as the result without any gas (Figure 10(c)). That is, N2 gas does not directly contribute to the reduction process. The result with CO2 gas is similar to that with N2 gas. According to Figure 10(d), the reduction with assistance of carbon is the fastest in CO atmosphere followed by the CO + CO2 gas mixture condition. However, thermodynamically, the maximum reduction can be higher in the case of N2, CO2, or no gas condition compared to the CO or CO + CO2 condition. Upon comparing the experimental TG results in Figure 6 and the thermodynamic calculation in Figure 10, we can derive the following conclusions for the isothermal reduction process at 600 °C
Thermodynamic calculations for the reduction process of the sample with C/O = 1 at 600 °C under various gas atmospheres. Reduction under (a) CO gas, (b) N2, and (c) no gas conditions and (d) the summary of mass changes (TGA) under various gas conditions. Inputs for the calculation are 69.99 g V2O5 + 5.71 g Fe2O3 + 24.29x g C + 10 g gas phase, where x is varied from 0 to 1. The x-axis of the diagrams corresponds to the x for the graphite amount in the inputs
-
(1)
The reduction process in N2 atmosphere is only driven by carbon in the samples. The main reaction can be, for example, V2O5 (s) + C (s) = 2VO2 (s) + CO (g), V2O5 (s) + 2C (s) = V2O3 (s) + 2CO (g), or 1/2Fe2O3 (s) + V2O5 (s) + 5/2 C (s) = FeV2O4 (s) + 5/2 CO (g).
-
(2)
The reduction process in CO2 atmosphere is slower compared to that in N2 atmosphere. That is, although the main reduction process in CO2 atmosphere still occurs by the reaction with carbon (as previously discussed), CO2 gas consumes part of carbon in the sample by C (s) + CO2 (g) = 2 CO (g), which slows the reduction process.
-
(3)
According to the experimental results in Figure 6, the reduction process in CO gas is much faster than that in N2 gas. Reduction of samples in CO gas occurs by both carbon and CO gas, while the reductions in N2 gas happen only by carbon. Therefore, the steep reduction in the CO gas environment is mainly due to CO gas rather than carbon. That is, the reduction kinetics by CO gas is much faster than that by solid carbon at 600 °C. The reduction kinetics by the CO + CO2 gas mixture is as fast as that in CO gas.
-
(4)
As seen in Figure 6, the recorded TG data in the CO condition shows an increase in the weight after attaining the maximum reduction, whereas in the case of CO + CO2 gas, there is no weight gain. The mass increase in TG data in the case of CO atmosphere could be attributed to either reoxidation of the sample by CO gas upon the completion of the reduction reaction or the carbon deposition by CO gas. It is noted that no weight gain is observed in the CO + CO2 condition; therefore, reoxidation as a probable reason can be ruled out. Hence, carbon deposition seems to cause the weight gain in CO atmosphere, as seen in Figure 6. Moreover, carbon deposition can also occur in the CO + CO2 case, but owing to the reaction C (s) + CO2 (g) = 2 CO (g), the deposited carbon can react with CO2 to produce CO while keeping the weight constant during the TG experiment.
It is worthwhile to note that mass-transfer coefficients will play a crucial role in determining the reduction kinetics in different gas-solid environments. The solids here refer to the starting oxides and the gas refers to the different environments such as CO, CO2, CO + CO2, and N2. In order to ascertain the mass-transfer coefficients, systematic experiments along with fluid dynamic simulations are required while considering the starting particle size, gas flow, gas bubble size, and distribution. In addition, a core-shrinking model is best suited to understand the effect of different reducing environments. In this model, it is assumed that the gas phase reacts with the starting oxides and the gas/solid interface moves continuously inward, reducing the oxides and leaving behind the reaction products. However, to apply this model, extensive reduction experiments need to be performed at varying temperatures and times. This, unfortunately, was beyond the scope of this study but needs careful future investigation.
Summary
In the present study, the production route of a vanadium nitride master alloy was studied using TG-DSC and furnace heating experiments under controlled gas atmosphere. The starting materials were composed of V2O5, Fe2O3, and graphite (molar C/O ratio = 0.9, 1, and 1.1). Initial reduction experiments were conducted isothermally at 600 °C in N2, CO, and CO + CO2 atmospheres to determine the best reducing condition at low temperature. The experiments indicated that the isothermal reduction kinetics is fastest under CO or CO + CO2 atmosphere. The maximum degree of reduction is about 10 pct at 600 °C in CO or CO + CO2 gas. The samples were held at 600 °C under CO gas for 4 hours, followed by heating to 1400 °C under a N2 + CO gas mixture. As a result, the vanadium nitride with 2 to 3 wt pct Fe and 8 to 10 wt pct carbon was produced as the final product. This is the first comprehensive and systematic study to produce Nitrovan alloy with controlled Fe and carbon compositions. The reducing and nitriding conditions for producing a vanadium nitride alloy with 2 to 3 wt pct Fe are reported for the first time.
References
M.K.G. Vermaak and P.C. Pistorius: Metall. Mater. Trans. B, 2000, vol. 31B, pp. 1091–97.
M.I. Gol’dshtein and V.M. Farber: Dispersion Hardening of Steel, Metallurgiya, Moscow, 1979 (in Russian).
K.R. Carpenter, C.R. Killmore, and R. Dippenaar: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 372–80.
H. Osuzu, T. Shiraga, Y. Shiroi, Y. Taniguchi, K. Tsujimura, and H. Kido: SAE Trans., 1986, vol. 95, pp. 701–11.
Y.D. Wu, G.H. Zhang, and K.C. Chou: Metall. Mater. Trans. B, 2016, vol. 47B, pp. 3405–12.
Y.D. Wu, G.H. Zhang, and K.C. Chou: JOM, 2017, vol. 69 (9), pp. 1676–81.
Y.C. Zhou, Y. Wang, K.C. Chou, and G.H. Zhang: J. Iron Steel Res. Int. 2020.
M.K. Ziatdinov and I.M. Shatokhin: Steel Transl., 2009, vol. 39 (11), pp. 1005–11.
Y. Liu, Y. Wang, Z. You, and X. Lv: Metals, 2020, vol. 10 (3), p. 356.
Y.D. Wu, G.H. Zhang, and K.C. Chou: JOM, 2018, vol. 70 (11), pp. 2493–98.
J. Dong, Y. Yu, and Z.L. Xue: Rare Met., 2015, vol. 34 (10), pp. 738–43.
D. Xinhui, C. Srinivasakannan, Z. Hong, and Z. Yuedan: Arab. J. Sci. Eng., 2015, vol. 40 (8), pp. 2133–39.
P.K. Tripathy, J.C. Sehra, and A.V. Kulkarni: J. Mater. Chem., 2001, vol. 11 (2), pp. 691–95.
Z.C. Chen, Z.L. Xue, W. Wang, Y. Yu, Q. Liu, and P. Li: Adv. Mater. Res., 2012, vol. 476, pp. 194–98.
J.W. Huang, H. Peng, and G.B. Xia: Ironmak. Steelmak., 2009, vol. 36 (2), pp. 110–14.
Y.D. Wu, G.H. Zhang, and K.C. Chou: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 3570–79.
X.U. Rui, Y.D. Wu, and G.H. Zhang: Trans. Nonferr. Met. Soc. China, 2019, vol. 29 (8), pp. 1776–83.
Z. Zhao, Y. Liu, H. Cao, J. Ye, S. Gao, and M. Tu: J. Alloys Compds., 2008, 464 (1–2), 75–80.
R.R. Hannum: Method of Producing a Vanadium and Nitrogen Containing Material for Use as an Addition to Steel, U.S. Patent No. 4,394,161, 1983.
J.B. Goddard and R.F. Merkert: Preparation of Low-Carbon Vanadium Nitride, U.S. Patent No. 4,562,057, 1985.
Preparation Method of High-Nitrogen Vanadium Nitride, Patent No. CN105838970B, 2018.
Production Method for Vanadium Nitride Ferroalloy, Patent No. CN102644015A, 2012.
High Strengths at Lower Costs with Nitrovan Vanadium, Bushveld Minerals, Brits, South Africa, http://www.nitrovan.co.za/documents/Vametco%20Alloys%20Brochure%20(Digital).pdf.
S.S. Yu, W.X. Li, Z.L. Ji, N.X. Fu, and Z.T. Sui: Adv. Mater. Res., 2011, vol. 150, pp. 480–83.
J.H. Downing and R.F. Merkert: Vanadium Containing Addition Agent and Process for Producing Same, U.S. Patent No. 3,334,992, 1967.
C.W. Bale, E. Belisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, and M.-A. Van Ende: CALPHAD, 2016, vol. 55, pp. 1–19.
W.-T. Du and I-H Jung: CALPHAD, 2019, vol. 67, pp. 1–13.
T. Behofsits, G. Pani, M. Gross, G. Fafilek, and M. Malys: ECS Trans., 2014, vol. 63 (1), pp. 137–44.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted May 9, 2020; accepted December 24, 2020.
Rights and permissions
About this article
Cite this article
Biswas, A., Sahoo, C., Du, WT. et al. New Production Route for Vanadium Nitride Master Alloy: Experimental and Thermodynamic Analysis. Metall Mater Trans B 52, 956–967 (2021). https://doi.org/10.1007/s11663-021-02068-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02068-y