Abstract
Alumina inclusions generated from aluminum deoxidation may undergo several transformations depending on steelmaking conditions. Under reducing conditions, in contact with MgO-saturated slag, alumina inclusions are known to transform to spinel. However, there are uncertainties related to CaO pick-up by alumina-based inclusions. This work aims to provide some clarification of CaO pick-up by alumina inclusions through a series of experiments. First, the role of silicon in calcium transfer from slag was examined by conducting two similar experiments: one without any silicon addition and a second with 1 wt pct electronic grade silicon addition. Second, the role of crucibles was tested by conducting experiments in ZrO2 and CaO-3 pct ZrO2 crucibles. It was found that the addition of silicon significantly enhances the rate of CaO pick-up when there was no silica in the slag at the start of reaction. The effect of the crucible on CaO pick-up was found to be weaker than that of the 1 wt pct silicon addition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In aluminum-killed steel covered with MgO-saturated slag (as is common in secondary metallurgy), alumina inclusions pick up magnesium, with simultaneous reduction of Al2O3 from the inclusions. These reactions transform the inclusions from alumina to spinel (solid solution based on MgO.Al2O3) and eventually to MgO.[1] The source of magnesium is reduction of MgO from the slag. It was shown that the rate of magnesium pick-up by the inclusions (and by the steel) can be modeled by assuming control by mass transfer in the steel to the steel–slag interface, with local equilibrium at that interface.[2,3] In principle, calcium pick-up can occur in the same way, since ladle slags are commonly at or near saturation with calcium oxide: aluminum can reduce some calcium oxide from the slag, dissolving calcium in the steel to react with inclusions. However, under carefully controlled laboratory conditions, the rate of transfer of calcium is much lower than that of magnesium.[2] Recent work indicated that this difference in rate is the result of the difference in the oxygen affinity of calcium and magnesium (CaO is much more stable than MgO) and weak interaction between dissolved calcium and dissolved oxygen in the steel (much weaker than previous solution models indicated).[4] Weak interaction between calcium and oxygen contributes to low concentrations of dissolved calcium.
However, another possible contributor to slow calcium pick-up under laboratory conditions is reaction with the crucible. If a magnesium oxide crucible is used, some dissolved aluminum is consumed by pick-up of Mg from the crucible, in parallel with slag reduction; reduction of MgO would compete with CaO reduction (by consuming dissolved aluminum), limiting Ca pick-up by the steel and inclusions. Kinetic considerations indicate that this effect would be weak: the rates of Mg and Ca pick-up (by steel and inclusions) are limited by mass transfer of dissolved Mg and Ca (at typical concentrations of a few parts per million or less) and not by mass transfer of Al (typical concentration hundreds of parts per million). However, to test for possible unanticipated effects, in this work several different crucibles were tested experimentally: MgO, ZrO2, and CaO-3 pct ZrO2.
Industrial observations indicate that silicon-rich steels pick up calcium more readily during ladle treatment, than steels with low silicon concentrations.[5] This might be caused by calcium contained in ferrosilicon (that is commonly added to steel to increase the silicon concentration of the steel). However, to test for possible additional effects of Si in solution in steel, in this work steel–slag experiments were conducted with zero and 1 pct Si in the liquid steel.
Experimental
Experiments were conducted in a laboratory induction furnace, as described elsewhere.[6,7] Changes in inclusions were tracked by taking liquid steel samples (as described previously[6,7]). Samples were mounted in resin, ground, and polished to a 1-μm finish (using diamond suspension). Automated inclusion analysis was performed with an FEI/ASPEX Explorer scanning electron microscope (SEM) at 10 kV, analyzing an area of approximately 10 mm2 on each sample. Other instrument settings were as described elsewhere[8] yielding a spatial resolution of approximately 0.3 μm.
Two experiments were conducted using MgO crucibles, testing the effect of Si alloying. Each used 600 g of low-sulfur (8 ppm) electrolytic iron and 160-170 g slag (composition in Table I) melted in an MgO crucible with the composition and size used in previous work[6] (internal diameter approximately 50 mm), in an argon atmosphere. The slag composition was chosen to be doubly saturated with the lime (CaO-based) and periclase (MgO-based) solid solutions, based on FactSage equilibrium calculations (FactSage 7.3, FToxid database). The electrolytic iron was mixed with the premelted CaO-Al2O3-MgO slag and heated to the test temperature of 1600 °C. Aluminum (0.3 wt pct of the iron mass) was added to the molten mixture approximately 15 minutes after it reached the test temperature. Aluminum was added in the form of shot wrapped in iron foil, and attached to the end of an alumina rod for immersion into the steel. The aluminum deoxidation step was defined as t = 0 minutes for the experiments. Based on previous experiments, addition of 0.3 pct Al would have resulted in approximately 0.26 pct dissolved Al. In one experiment, 1 pct silicon was added as electronics grade Si at t = 5 minutes. The addition and sampling details for the two experiments are given by Tables II and III. Samples were taken by aspirating steel into a fused-quartz tube (4 mm ID), using a pipette pump. The “SF” sample was taken from the solidified steel “puck” that remained in the crucible after the experiment.
Steel–slag reactions without any intentional MgO addition were conducted using ZrO2 crucibles of 39 mm inner diameter. Solid electrolytic iron (7 ppm S; 100 g) was melted and deoxidized by adding 0.15 pct Al; 30 g of slag (see chemistry in Table IV; near CaO saturation) was added at t = 6 minutes, see Table V. ZrO2 (2 pct of slag mass) was added in the slag to limit corrosion of the crucible.
Two experiments were conducted using Al-killed steel in a 40-mm inner diameter CaO-3 pct ZrO2 crucible (supplied by Zhengzhou Mission Ceramic Products Co, Ltd, Zhengzhou, China). One experiment was conducted using 250 g of electrolytic iron (containing 40 ppm of sulfur and approximately 360 ppm of oxygen) with 50 g of CaO-Al2O3 slag near CaO saturation (Table VI) (addition and sampling details in Table VI). The other experiment used 200 g of low-sulfur electrolytic iron (8 ppm S) with no slag addition (addition and sampling details in Table VII).
Results and discussion
Effect of Silicon Addition on Calcium Transfer
The inclusions in Al-killed steel that did not contain Si followed the familiar transformation from alumina to spinel, and finally to MgO,[9,10] with very little calcium transfer (Figure 1). Transformation was rapid, due to the relatively high Al concentration (0.3 pct) and the presence of slag at the steel–crucible interface (Figure 2); the slag avoided the formation of a spinel layer which would have slowed down magnesium pick-up from the crucible.[9]
Inclusion composition in steel samples taken at (a) 1 min, (b) 16 min, (c) 30 min, and (d) 70 min after Al addition, for reaction of Si-free Al-killed steel with CaO-Al2O3-MgO slag. (The lines in (a) and (d) indicate the boundary of inclusions that would be 50 pct liquid at 1550 °C; “a.f.” is the area fraction of inclusions.)
The observed increase of alumina in inclusions from S2 (t = 16 minutes) to S3 (31 minutes) apparently reflects minor reoxidation of the melt that generated fresh Al-Mg-O inclusions and a small increase in the area fraction of inclusions. Most inclusions in the final sample (70 minutes) were MgO with limited calcium. Figure 3 shows such a calcium-containing inclusion: the inclusion was mainly MgO with small amount of calcium sulfide.
In contrast, the steel that contained 1 pct Si did show significant calcium pick-up (Figure 4). The inclusions in the first sample were spinel and spinel-MgO combinations, with a minor concentration of CaO in a few. From the second sample, the inclusions contained more calcium, with a few falling in the calcium corner of the ternary diagram (representing CaS) (see Figure 4(b)). The third sample indicated continued pick up of calcium and a lower magnesium concentration in inclusions, indicating that calcium from steel–slag reaction may have reduced MgO at the steel–inclusion interface as proposed by Yang et al.[11] The inclusions in this sample were mixtures of liquid or partially liquid CaO-Al2O3-MgO inclusions and MgO-rich MgO-Al2O3 inclusions. Similar inclusions were found in the samples taken later (S4 and S5, Figures 4(d) and (e)). Examples of inclusion micrographs (taken with a higher-resolution field-emission gun SEM) are shown in Figure 5. The inclusions in the final sample (Figure 4(f)) were notably different: mainly MgO-rich inclusions, with some CaO-Al2O3-MgO inclusions and occasional MgO-CaS inclusions. The large change from the preceding sample likely resulted from changes during solidification: the last sample was taken from the steel puck that solidified in the crucible, with a much longer solidification time than the 4-mm-diameter samples taken during the experiment; continued supply of magnesium from the crucible and slag, and sulfur removal to the slag, may also have contributed to continued inclusion evolution.
These results do indicate that silicon can promote calcium pick-up by inclusions, perhaps because silicon is a strong deoxidizer if the slag does not contain SiO2 (as was the case at the start of the experiments). Current results are similar to those showed by Mu et al.[10] for modification of alumina/spinel inclusions by calcium transfer from CaO and MgO-saturated slag reacting with steel containing 2 wt pct Al. It should be noted that much lower calcium transfer to inclusions was observed for experiments conducted with lower Al additions (0.1, 0.5, and 1 wt pct) in that study.
To evaluate the reduction effect of added silicon, the equilibrium steel compositions were calculated for the two cases (0.3 pct Al added, to iron with zero or 1 pct Si), after reaction with slag as listed in Table I, using the steel and slag masses as stated in Tables II and III. FactSage 8.0 was used[12] (FToxid slag and solid-oxide models, and FSstel liquid steel model, with the Ca*O associate suppressed).[4] In the Si-bearing case, some reduction of Al2O3 from the slag was predicted, with the slag containing approximately 1 pct SiO2 at equilibrium. The corresponding steel compositions are listed in Table VIII, indicating that the more-reducing conditions (resulting from the addition of Si) did give higher equilibrium concentrations of Al, Ca, and Mg in the steel. However, the Ca concentration is predicted to increase by a factor of two only (comparing Si-alloyed steel with Si-free steel), which appears too small to account for the observed much more extensive calcium transfer to inclusions (by a factor of approximately thirty), in the Si-alloyed steel. The average composition of inclusions in the second sample taken at 15-20 minutes, in the two experiments are shown in Table IX showing significantly higher CaO concentration in inclusions due to silicon alloying. Rather, it is possible that the reaction between silicon (in the steel) and alumina (in the slag) may have resulted in slag emulsification. Such emulsification would increase the slag–steel contact area, and could also transfer calcium aluminate droplets as inclusions into the steel. Emulsification at the slag–steel interface during redox reactions has been documented in the past, and recently reviewed and modeled by Spooner et al.[13]
Effect of Crucible: ZrO2 and CaO-ZrO2
ZrO2 crucible
In the ZrO2-crucible test, Al-killed steel was in contact with CaO-Al2O3 slag (containing no added MgO). The slag was kept MgO-free to avoid the possible competition between Mg and Ca pick-up at the steel–slag interface. Analysis of inclusions in samples S1 (24 minutes after deoxidation) and SF (92 minutes) are shown in Figures 6(a) through (d). For each sample, compositions are plotted on two ternary diagrams: Al-Mg-Ca and Al-Mg-Zr. Two types of inclusions were found in these samples: ZrO2-rich inclusions (Type 1) and Al2O3-rich inclusions (Type 2). Examples of both types are shown in Figure 7 and their main characteristics are summarized in Table X. Note that some MgO pick-up was found; possible sources of magnesium are discussed later.
Automated inclusion analysis was affected by the presence of the ZrO2-rich phase that appeared bright (because of its high average atomic number, see Figure 7(a)). Typically, during automated analysis possible inclusions are identified by lower backscattered electron yield (appearing darker). It is likely that some (bright) ZrO2-rich inclusions were missed during automated analysis of these samples.
The measurable calcium content in the ZrO2-rich phase of some type 1 inclusions indicates that these may have resulted from slag–crucible reaction, forming calcium zirconate that may have eroded into the steel melt subsequently. As the CaO-ZrO2-AlO1.5 phase diagram in Figure 8 shows, calcium zirconate (CaZrO3) is the expected product if CaO-rich CaO-Al2O3 slag reacts with ZrO2. Whether these inclusions formed by transfer of dissolved calcium or of calcium zirconate, in all cases the extent of calcium pick-up by inclusions was very limited (Figure 6). The observed behavior is not much different than that found in similar experiments done earlier using MgO crucibles.[2] In those experiments, alumina inclusions quickly transformed to spinel inclusions picking up Mg from the crucible and the slag. There was no observable calcium pick-up.
CaO-ZrO2-AlO1.5 phase diagram at 1873 K (1600 °C) drawn using FactSage[12]
Fines from direct erosion of the ZrO2 crucible also appeared to contribute to the formation of the Type 1 inclusions. The example in Figure 9 indicates that MgO and Al2O3 precipitated on ZrO2. Equilibrium calculations (with FactSage) confirmed that formation of Al2O3 by reaction of 0.15 pct Al steel with ZrO2 is possible. Examination of the inner wall of the crucible after experiments clearly showed slag attack on the crucible (Figure 10).
Even though the slag and crucible did not contain any MgO, MgO was detected in the inclusions. Analysis of the crucible before and after the experiment found little evidence of MgO except for a Si-Ca-Mg-Al-O fiber on the crucible wall after the experiment (Figure 11). The most likely source of such fibers is paper used in wrapping the crucibles for shipping, or used during experimental setup. Because of the low total inclusion concentration in the steel for these experiments, even a small amount of contamination would have affected the measured inclusion composition: In total, the steel contained only ~0.1 ppm of MgO (in inclusions), equivalent to 10 µg of MgO (Figure 6).
CaO-3 pct ZrO2 crucible
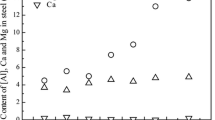
After reaction of Al-killed steel with CaO-Al2O3 slag in a CaO-3 pct ZrO2 crucible, the inclusions were alumina + spinel with limited calcium and zirconium contents (see Figure 12). Figure 13 shows a calcium-containing inclusion observed in the sample taken at 34 minutes. The extent of magnesium pick-up in these inclusions was less than in the experiments using MgO and ZrO2 crucibles. The calcium and zirconium content in inclusions increased slightly with time: The average cation mole fraction of calcium (out of the total of Ca, Mg, Al, Si, and Zr) increased from 0.90 to 3.8 pct from the sample taken at 34 minutes to that taken at 54 minutes, while the area fraction of inclusions decreased from 102 to 28 ppm due to inclusion flotation. Zirconium pick-up in these inclusions was apparently by the reduction of zirconia from the crucible by aluminum from steel.
Change in inclusion content and composition observed during reaction of Al-killed steel with CaO-Al2O3 slag in a CaO-3 pct ZrO2 crucible, for samples taken at two different times and showing two different inclusion cation distributions for each: (a) Ca-Al-Mg diagram at 34 min; (b) Zr-Al-Mg diagram at 34 min; (c) Ca-Al Mg diagram at 54 min; (d) Zr-Al-Mg diagram at 54 min
While 50 g of CaO-Al2O3 slag had been added at t=6 minutes, at the end of the experiment no slag was observed on top of the solidified steel; the slag had penetrated the crucible (Figure 14).
Slag penetration was characterized by polishing a cross-section of the CaO-ZrO2 crucible after the experiment, using acetone for cleaning during polishing to avoid attack of the crucible by water. Figure 15 confirms that slag penetrated along the grain boundaries of the crucible; aluminum is a clear indication of the presence of slag.
Images of polished cross-section through a CaO-ZrO2 crucible in the region penetrated by CaO-Al2O3 slag. (a) Region near the outside diameter, showing pores and other phases along the grain boundaries (backscattered electron image). (b) Slag penetration between grains near the inside diameter (backscattered electron image). EDS map near the inside diameter show (c) calcium (brighter areas) and (d) aluminum (darker areas)
SEM-EDS analysis of the grain boundary region of the crucible (away from the region with slag penetration) showed the presence of silicon and magnesium as shown in Figure 16. This indicates that the crucible could have been the source of magnesium observed in the inclusions (Figure 12).
More calcium pick-up was observed in the experiment performed without slag (Al-killed steel contained in a CaO-ZrO2 crucible, Figure 17): After 15 minutes, the inclusions were alumina and spinel with limited calcium and zirconium contents. The calcium and zirconium content in inclusions increased with time; partial calcium modification of spinel inclusions can be observed in the final sample. Figure 18 shows a calcium-containing inclusion observed in the sample taken at the 45th minute. The sample taken after 30 minutes showed an increase in the area fraction of inclusions (from 13 ppm to 95 ppm) and inclusions were alumina rich, indicating reoxidation during sampling; the inclusions in this sample were also higher in silicon. The increase in silicon concentration indicates likely silica contamination by the fused-quartz sampler tube. However, the significant CaO in the inclusions in the subsequent samples indicates continued calcium pick-up by steel–crucible reaction. Calcium pick-up is clearly possible, but to a limited extent; note that the total inclusion concentration (indicated by the area fraction of inclusions) was very low in these experiments.
Inclusion composition change during reaction of Al-killed steel with a CaO-3 pct ZrO2 crucible, with no added slag. Two inclusion cationic composition distributions are shown for three different times: (a) 15 min—Ca-Al-Mg diagram; (b) 15 min—Zr-Al-Mg diagram; (c) 30 min—Ca-Al-Mg diagram; (d) 30 min—Zr-Al-Mg diagram; (e) 45 min (bulk metal in crucible)—Ca-Al-Mg diagram; (f) 45 min (bulk metal in crucible)—Zr-Al-Mg diagram
Conclusions
-
1.
Induction-furnace experiments were designed to clarify the effect of silicon addition on the calcium modification of spinel inclusions by steel–slag reaction. The use of high-purity (electronic grade) silicon clarified that the source of calcium can be steel–slag reactions in addition to calcium impurities in ferrosilicon, for Al-killed steel in contact with CaO-Al2O3-MgO slag.
-
2.
There was no significant calcium transfer from CaO-Al2O3-MgO slag to alumina inclusions in Al-killed steel not containing added Si, contained in an MgO crucible. Limited calcium transfer to alumina inclusions was observed when the steel was contained in a ZrO2 crucible instead of MgO crucible. Crucible fines were observed to contribute to ZrO2-Al2O3 inclusion formation for steel contained in a ZrO2 crucible.
-
3.
For reaction of Al-killed steel not alloyed with Si, the largest extent of Ca pick-up was observed when using CaO-3 pct ZrO2 crucibles. The calcium transfer occurred due to both steel–slag and steel–crucible reactions.
References
D. Kumar and P. C. Pistorius: Advances in Molten Slags, Fluzes, and Salts: Proceedings of The 10th International Conference on Molten Slags, Fluxes and Salts (MOLTEN16), 2016, pp. 145–54.
D. Kumar and P. C. Pistorius: AISTech 2016 – Proceedings of the Iron & Steel Technology Conference, Association for Iron & Steel Technology, Warrandale, PA, 2016, pp. 1151–59
3.D. Kumar, K. C. Ahlborg, and P. C. Pistorius: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2163-2174
4.C. Liu, D. Kumar, B. A. Webler, and P. C. Pistorius: Metall. Mater. Trans. B, 2020, vol. 51, pp. 529–542
Eugene Pretorius, Nucor Steel, private communications
S.P.T. Piva, D. Kumar, and P.C. Pistorius (2017) Metall. Mater. Trans. B 48: 37-45.
7.D. Roy, P.C. Pistorius, and R.J. Fruehan: Metall. Mater. Trans. B, 2013, vol. 44, pp. 1095–1104
8.D. Tang, M. E. Ferreira, and P. C. Pistorius: Microsc. Microanal., 2017, vol. 23, no. 6, pp. 1082-1090
9.D. Kumar and P. C. Pistorius: Metall. Mater. Trans. B, 2019, vol. 50, no. 1, pp. 181–191
10.H. Mu, T. Zhang, R. J. Fruehan, and B. A. Webler: Metall. Mater. Trans. B, 2018, vol. 49, no. 4, pp. 1665–74.
11.S. Yang, Q. Wang, L. Zhang, J. Li, and K. Peaslee: Metall. Mater. Trans. B, 2012, vol. 43, no. 4, pp. 731–50.
12.C. W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, and M.-A. Van Ende: CALPHAD, 2016, vol. 54, pp. 35–53.
S. Spooner, Z. Li and S. Sridhar: Scientific Reports, 2017, vol. 7, Article Number 5450.
Acknowledgments
The authors acknowledge support from the member companies of the Center for Iron and Steelmaking Research and use of the Materials Characterization Facility at Carnegie Mellon, supported by Grant MCF-677785.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted 24 August 2020; accepted 09 October 2020.
Rights and permissions
About this article
Cite this article
Kumar, D., Pistorius, P.C. Calcium Transfer to Oxide Inclusions in Al-Killed Steel Without Calcium Treatment. Metall Mater Trans B 52, 163–177 (2021). https://doi.org/10.1007/s11663-020-02004-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-02004-6