Abstract
The paper reports successful smelting reduction of iron ore (hematite) in thermal hydrogen plasma. A specially designed reactor with water cooled copper crucible and a plasma torch was used to demonstrate the process in 1-kg scale. The number of stoichiometric requirement of hydrogen is a better parameter, instead of time, for determining the rate of the process. This parameter, along with the degree of reduction, is also helpful to determine the degree of hydrogen utilization. The ratio of the height of the molten bath to the diameter of the reactor is found to be an important parameter for effective hydrodynamics and the resultant degree of reduction. This is also an important parameter for scaling up of the process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the year 2017, 1691.2 million tons of crude steel were produced in the world.[1] The major production routes are (i) blast furnace-basic oxygen furnace (BF-BOF), (ii) direct reduced iron-electric arc furnace (DRI-EAF), and (iii) smelting reduction-basic oxygen furnace/electric arc furnace (SR-BOF/EAF); the decrease in quantity of production through these routes follow the same order. All these routes involve carbon in some form which has limitations of (future) supply and environmental pollution. Carbon dioxide (CO2) is the main greenhouse gas emitted from the steel industry; BF alone emits 1.4 tons of CO2 for every ton of hot metal produced which constitutes 65 pct of plant emissions.[2] Even though the above-mentioned process technologies are efficient, economical, and ever-evolving, for the sake of green gas concerns a lot of attempts are being made worldwide to look for alternate process routes where green gas emissions are either eliminated altogether or drastically reduced. Some such efforts include the ultra low CO2 steelmaking (ULCOS) programs running in European Union (EU) since 2004 covering areas of research in (a) top gas recycling blast furnace (TGR-BF or ULCOS-BF) incorporating carbon dioxide capture and storage (CCS)[2]; (b) HIsarna, a technology based on bath smelting which combines three reactors, the first one for preheating and partial pyrolysis of coal, the second one is a melting cyclone for ore melting followed by the smelter vessel for final ore reduction and metal production (the special aspects include reduced CO2 emission which is almost CCS ready and a process flexible to substitute coal with either biomass, natural gas or hydrogen)[3]; (c) ULCORED, a solid DRI making process using natural gas followed by melting in EAF[4]; (d) ULCOWIN, a direct electro-winning process operating slightly above 100 °C in an aqueous (slurry of) alkaline solution containing fine grains of iron ore[5]; (e) ULCOLYSIS, a molten salt electrolysis (pyro-electrolysis) process operating at steelmaking temperature using a fused slag electrolyte[6]; and use of hydrogen as reductant alternate to carbon.[7]
Besides EU, there are efforts elsewhere in the world with similar objectives. Japanese Iron and Steel Federation (JISF) runs its own program, COURSE 50, which includes two developments such as amine scrubbing of BF gas and hydrogen recovery from coke oven gas for injection into BF.[8] The Korean company POSCO is also working on its own program that includes adaptation of CCS to the COREX and FINEX processes as well as hydrogen steelmaking in FINEX process.[9] The approach in USA involves suspension hydrogen reduction of iron oxide in a flash reactor at Utah University[10] and molten oxide electrolysis (MOE) at MIT.[11] The Canadian Steel Producers Association (CSPA),[12] Arcelor Brazil[13] and Bao Steel, China[14] also have their research activities in similar directions.
All of the above developments center around three reductants: carbon, hydrogen, and electron. The processes involving the first one are well established and the effort is to reduce the emission or CCS. The processes in case of the other two are yet to be developed into commercial propositions; with another condition that these two reductants should be available in plenty at a cheaper cost and have to be from green sources. Though each of them i.e., electron (electricity) and hydrogen has their own advantages and shortcomings, hydrogen seems to be a potential candidate for reduction of iron oxides, because the combustion product of hydrogen is H2O which is non-pollutant and then, its tonnage consumption is less as compared to carbon. The main problem with hydrogen is its short supply and high cost. However, it is apparent that technologies would be developed in future to produce H2 cheaply and conveniently from water and hence use of hydrogen in future may be realistic. Keeping this in view research is continuing[9,10,15,16,17,18,19,20,21,22,23] to use hydrogen as a reductant for iron and steel making. Though molecular hydrogen is a good reductant to reduce iron oxide ores, its potential increases many fold when this gas is used in plasma medium. The reduction potential of atomic and ionic hydrogen which are present in hydrogen plasma is, respectively, 3 and 15 times higher than that of the molecular hydrogen. Besides, various other excited hydrogen molecules and atoms are also available in hydrogen plasma to aid the reduction process. Further, plasma processes are kinetically stimulating. Plasma processing also provides two options like (i) applying thermal plasma in smelting reduction systems and (ii) using non-thermal plasma to effect reduction in solid oxide-plasma interfaces.[22,23] The first one will generate molten metal whereas the second one would produce solid metal (DRI). The present paper is an attempt to reduce hematite ore with thermal hydrogen plasma.
Literature reports indicate that, though limited in number, investigations on reduction of molten iron oxides through thermal hydrogen plasma dates back to 1970s.[17,18,19,20,21,24,25,26,27,28,29] Two excellent review papers have highlighted all the significant issues relating to plasma processing of oxide minerals.[30,31] In plasma condition, higher temperatures prevail and the structure of molten iron ore consists of cations, anions, anion-complexes in a particular short-range order where the simple Fe2+ and O2− ions account for approximately 95 pct. Further, atomic (H) and ionic (H+) species of hydrogen are likely to be present and the following reactions are expected to take place during the reduction process:
Earlier reports[24,25,26,27,28,29] indicated that iron oxide could be successfully reduced to metallic iron by thermal hydrogen plasma with a degree of hydrogen utilization of around 44 pct which increased to 60 to 70 pct when a lower concentration of H2 (less than 20 pct) in the H2-Ar mixture was used. The higher hydrogen utilization was interpreted to be due to the reduction of iron oxide by atomic and ionic hydrogen. These species get a scope to recombine easily in presence of higher hydrogen concentration, thereby, leaving only the molecular hydrogen as the reductant for which the equilibrium hydrogen utilization is 40 pct. More detail research in the area of hydrogen reduction of iron oxide using plasma metallurgy has been reported from Montan University, Leoben, Austria.[15,16,17,18,19,20,21] Using a plasma reactor to treat 100-g iron ore samples, various parameters have been examined. At the interface between the plasma arc and the oxide melt, the melt temperature reached as high as 2000 °C to 2600 °C. Successful reduction of iron oxide to metallic iron took place with around 40 pct hydrogen utilization. They also deduced the following rate equation with an activation energy of 23 kJ/mol.
Here r is the specific reaction rate and T is temperature in K.
Based on the results obtained in their experiments, a conceptual design for an up-scaled steel production process for a 1 million ton/year has been conducted[19] with material and heat balance. Though a number of advantages in favor of hydrogen plasma smelting of iron ore could emerge from these studies, more areas pertaining to process development remain to be sorted out. Therefore, the present investigations have been undertaken to have a better understanding of taking the process route further ahead.
Experimental Details
The Plasma Reactor
The reduction studies were carried out in a specially designed plasma reactor that encompasses a power source, electrodes, water cooled copper crucible, and electromagnetic coil as shown in Figure 1. The main specialty in design concerns with the electrode. Usually, graphite is chosen as electrode in case of thermal arc plasma units. In the present case, carbon in graphite will act as a reductant in the reaction process, competing with hydrogen and hence, graphite cannot be used. Therefore, in the present case, the electrode-based non-transferred torch is stainless steel housed copper block, having tungsten at its tip which is of 25 mm long and 10 mm outer diameter at the tip. Argon and Hydrogen are passed through the central orifice of this torch with a nozzle diameter of 2 mm. The water cooled copper crucible has a diameter of 100 mm which can support 1 kg scale experiment successfully. An electromagnetic coil is attached at the bottom of this crucible which helps in agitation of the molten bath.
Sample Materials Used
Two hematite samples were used in the study. The first one was obtained from iron ore reserves at Joda located in Barbil, Keonjhar, Odisha, India. The same sample was upgraded by beneficiation and was used as the second sample. The chemical analysis of both the samples is listed in Table I. The major phases present in the second sample are also shown by the XRD graphs in Figure 2. The iron ore fines were granulated in a disc pelletizer unit to a size of 1- to 6-mm granules with addition of 0.5 pct Bentonite as a binder and 10 pct moisture before using in the experiments.
Experimental Procedure
Experiments were carried out as follows: 50 g of iron ore oxide granules of 1 to 6 mm size is placed in the crucible before melting them with non-transferred Ar plasma. It is targeted to maintain a power of at least 10 to 12 kW to facilitate the melting and the reduction process simultaneously. From practice, it was observed that an initial current setting of 125 A fulfils this condition, and hence, each of the experiments has been initiated at a current setting of 125 A. Once arcing is effected and the plasma strikes, followed by melting of the charge in about 2 minutes and thereafter, H2 is passed through the torch, changing the plasma gas to a H2-Ar mixture to commence the reduction reaction. The current and voltage values get set to slightly changed values with minor fluctuations, during the course of the reaction. These values are recorded at fixed time intervals. A feed rate of 50 g at specific time intervals, which is calculated based on the total targeted hydrogen supply, is maintained. The feeding system, which is placed by the side of the torch in an inclined manner, retains a part of the feed; therefore, the actual feed is determined by deducting the retained mass from the total charge. The reduced sample was cooled in Ar atmosphere and hammered to separate metal and slag. The slag is then crushed and divided into magnetic and non-magnetic portions by hand with a magnet. The degree of reduction was calculated based on the weight of the pure metal obtained and the chemical analysis done on slag.
Material Characterization and Calculation of Degree of Reduction
The hematite samples and the three product samples such as the metal nugget, the magnetic and non-magnetic fractions of the slag were subjected to chemical as well as XRD analysis. An X’Pert PRO-PANalytical model no. 3040160 was used for X-ray diffraction (XRD) studies of the phases present. The quantitative estimation of the phases was done by using standard wet chemical analysis procedure for the total iron, metallic iron, ferrous iron, phosphorous, silica, alumina, CaO, and MgO.[32,33,34] The ferric iron in the sample was estimated by deducting metallic iron and ferrous iron from total iron. Carbon and sulfur in the metal samples were determined using a carbon and sulfur analyzer of HORIBA make of EMIA-V2 series and hydrogen in the metal was determined by employing an oxygen, nitrogen, and hydrogen analyzer of HORIBA make of EMGA-900 series.
Degree of reduction was evaluated from the loss of oxygen which was estimated from the analysis data of various iron species and the degree of reduction was calculated (in pct), using the original oxygen present in the charged sample.
Results and Discussion
Initially, some trial runs were made each with 200 g of hematite sample-1; the hydrogen and argon flow rates were maintained at 5 and 10 lpm, respectively. The aim was to work out the mode of charging, time vis-à-vis hydrogen utility for effective reduction of iron oxide charge. It was observed that time dependence of degree of reduction had no significance since hydrogen (one of the reactants) flow was continuous and hence degree of reduction versus amount of hydrogen passed (which also includes time) was rather a more significant parameter. Then, it was further observed that the reduction was optimum at a hydrogen passage of about 2.5 times that of the stoichiometric amount according to Reaction [1]. Passage of more hydrogen had no significant improvement in degree of reduction which changed very little remaining just around 60 to 62 pct level. The ore charging was then made in batches of 50 g at specific time intervals instead of charging the entire lot in the beginning so as to have some sort of a continuous feeding commensurate to availability of hydrogen. The time interval was calculated based on the hydrogen gas flow rate and the total hydrogen to be passed and reported in Tables II, III, and V.
Effect of Hydrodynamics
It was thought that the degree of reduction was probably limited due to inadequate hydrodynamics, because 200 g of sample must be making a height of 5 mm with a reactor of 100 mm diameter. The plasmogen gas impinging into the bath at a rate of 15 lpm will need a reasonable height of the bath for an effective gas-liquid mixing. In case of inadequate height of the bath, when the gas will strike the bath, there will be splash and eventually part of the gas will strike the solid reactor bottom and bounce out. Therefore, the first set of experiments was made with increasing feed; time of reaction was also suitably adjusted to result in required hydrogen supply. The results are presented in Table II which shows that the degree of reduction remains nearly same at around 59 to 65 pct until 337 g feed even with hydrogen supplied is between 2.70 and 5.08 times of the stoichiometric amount. The degree of reduction improved to 86.8 pct with increase in feed to above 500 g which was 98.1 pct when 849 g sample was charged. The height of the bath calculated from the density of the material is also reported in the table. The bath height with 176 g feed is 4.5 mm which increases to 21.9 mm with 849 g sample charged into the reactor. As will be shown later, nearly complete reaction (~ 100 pct) is achieved at a bath height of around 19 mm which can be attained by a feed of about 737 g. It is to be noted that the bed height is dependent on amount of charge which is made at specific time intervals. Therefore, the height of 19 mm in case of 737 g of charge is attained at the end of charging. It can be assumed that the experiments with charges higher than 737 g encounter the favorable hydrodynamic situation for longer periods. It may also be noted that voltage shows an increasing trend with the increase in amount of feed.
Effect of Hydrogen Flow Rate
Another set of experiments was conducted to observe the effect of hydrogen flow rate on degree of reduction; the time was adjusted to supply nearly equal stoichiometric hydrogen in all the experiments. The results presented in Table III show more than 98 pct reduction in all the experiments. The bed height is above 19 mm and the total hydrogen passed was above 3 times that of the stoichiometric amount according to Reaction [1] in all the experiments. It is established that the total hydrogen supply, not the hydrogen flow rate, is the parameter which is related to degree of reduction. It is also noted that, though the voltage remains at a lower range when hydrogen flow rate is lower, the total energy consumption is lower at higher flow rate of hydrogen due to decrease in reaction time at increasing hydrogen flow rate.
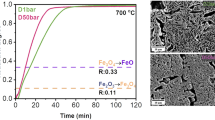
The product in all the experiments, as mentioned in the experimental section, was a fused mass having metal in the bottom and slag at the top which could be easily separated by slight hammering. The slag portion was then ground and subjected to a dry magnetic separation into two fractions, one magnetic and the other non-magnetic. All three fractions were analyzed chemically and by XRD. The detail analysis of the three products for a specific sample (Table III, serial 3) is presented in Table IV as well as Figures 3 and 4 (Figure 3 is a picture of the metal nugget, and Figure 4 shows the XRD graph of the metal nugget). The metal nugget is high purity iron, containing low amount of C, S, P, and H. The magnetic fraction of the slag contains FeO with around 9.25 pct of metallic iron, whereas the non-magnetic fraction of the slag contains mostly FeO.
Metal nugget obtained from the experiment reported in Table III, serial 3
XRD plot of the metal nugget obtained from the experiment reported in Table III, serial 3
Effect of Total Hydrogen Flow
The results reported in Tables II and III used total hydrogen above 2.7 times of that required according to Reaction [1]. The current and the voltage readings along with the time periods used in each experiment correspond to electric energy consumption between 5 and 52 kWh. The next set of experiments was conducted with 1000 g of hematite sample 2 at 10 lpm of hydrogen flow rate varying the time towards the lower range. Variation of time changes total hydrogen consumed, rate of hematite feeding, and the total electrical power consumption. The results are shown in Table V. Even though, in the five experiments, the total feed, voltage, and current were within close ranges of 906 to 920 g, 101 to 122 V, and 110 to 123 A, respectively, due to reduction in time, total hydrogen flow and power decreased, so also the degree of reduction. When the time went down to 53 and 42 minutes as shown in the two bottom rows of Table V, the energy went down to 10.98 and 8.70 kWh, respectively. This resulted in production of fused mass without clear metal slag separation. It implies that for effective reduction and proper melting of about a kilogram of the ore requires around 22 kWh of energy in this particular setup.
Reaction Rate
As mentioned in the previous sections, the reaction rate does not depend on time alone, rather on time and amount of hydrogen flow together. Therefore, for determining the rate, a simple dimension less number (total hydrogen flow/hydrogen required stoichiometrically to reduce the amount of iron oxide present as per Reaction [1]) is chosen as the parameter against which the degree of reduction was plotted in Figure 5. There appear three sets of data in Figure 5, one for total ore feed of 200 to 400 g (bath height of 5 to 10 mm), another for a total feed of 600 to 800 g (bath height of 10 to 15 mm), and the other for 1000 g of total feed (bath height above 20 mm). In case of the first two sets of experiments, the degree of reduction is apparently limited to a level even after prolonged reduction time/hydrogen flow. The bath height in these two sets was less than 15 mm. In case of the third set, where the total charge was more than 800 g and the bath height was above 20 mm, more than 98 pct of degree of reduction could be achieved. Further, from this plot the degree of hydrogen utilization can also be determined as follows: the degree of reduction as fraction/the number used in X axis. This plot is also shown in Figure 5. The hydrogen utilization is higher at lower hydrogen flow which has been reported by previous authors.[19,27,28,29,30]
Scaling Up
Results presented under Section III, A to C and Tables II, III, and V also give a direction for scaling up. While realizing near 100 pct reduction by increasing the total feed, another simple dimension less number (height of the molten bath/diameter of the reactor) needs examination. Figure 6 presents a plot of degree of reduction versus this number. There are two segments in this plot. The first one is a linear portion {y (recovery) = 299 × (bath height/diameter) + 43.43} from 0.05 to 0.16 of (height/diameter) which can be extended up to 0.19 (bath height/diameter), corresponding to 100 pct recovery. This value of (bath height/diameter) also corresponds to a bath height of 19 mm which is attained by a feed of 737 g of the feed. The second segment is a short near horizontal line just below the 100 pct recovery mark which extends beyond 0.19 (height/diameter). This means that there is a hydrodynamic limitation on completion of the reaction below a certain bath height above which near 100 pct recovery is achieved. As explained earlier in Section III–A, it may be assumed that a circular hydrodynamic disturbed region exists at the center of the bath at lower bath heights which hinders mass transport with the liquid concentrated at the periphery of the bath. This situation gradually vanishes with rise in bath height. Two experiments, one using 5000 g and the other 7000 g ore feed, were conducted in another reactor with 200 mm diameter which resulted in degree of reduction of 83.5 and 95.5 pct, respectively. The ore feed of the same density is used in these two experiments and the calculated bath heights were 32 and 44 mm, and hence, the value of the parameter (bath height/diameter) is 0.16 and 0.22, respectively. These two results are shown in both Figures 5 and 6 (as 6b1 and b2). It is interesting to note that, even though the bath heights in these two experiments are much higher than 20 mm, the metal recovery is considerably less than 100 pct (Figure 5). However, the situation in Figure 6, where the parameter (bath height/reactor diameter) is incorporated, the results of both the reactors follow the same trend, thereby supporting the direct dependence of recovery on the ratio of bath height and reactor diameter. Bath height is significant for a specific reactor where its diameter is constant; the ratio of the bath height and the reactor diameter becomes the universal parameter of significance. This parameter (bath height/diameter of the reactor) which, in other words, is a relation between the reactor size (diameter) and the amount of feed to be treated, would be helpful in further scaling up of the process. However, a detail theoretical explanation of this relationship needs more studies which can be taken up in future.
Degree of reduction vs the dimensionless number, (bath height/diameter of the reactor): (a) experiments in the reactor having 100 mm diameter—(a1) up to 600 g of actual feed and (a2) above 600 g of actual feed; (b) experiments in the reactor having 200 mm diameter—(b1) 5000 g feed and (b2) 7000 g feed
Conclusions
The following conclusions may be drawn from the present investigation:
-
(i)
Hydrogen plasma, as an effective reductant to reduce iron oxide to its metal, is demonstrated in 1 to 7 kg scale.
-
(ii)
Rate of the process is better represented through a dimension less number that represents the number of stoichiometric amount of hydrogen (according to the reduction reaction) fed during the process. This number, along with the degree of reduction, can give the degree of hydrogen utilization.
-
(iii)
Close to 100 pct reduction is possible by maintaining a specific ratio of bath height and reactor diameter. This ratio, another dimension less number which relates the reactor diameter to the amount of feed it can treat, is a useful parameter for scaling up of the process. The scaling up of the process could be successfully demonstrated from 1 kg in a reactor of 100 mm diameter to 7 kg in a reactor of 200 mm diameter.
References
Worldsteel Association: Press Releases. https://www.worldsteel.org/media-centre/press-releases/2018/World-crude-steel-output-increases-by-5.3–in-2017.html. Accessed 06 June 2018.
J.V.D. Stel: Development of ULCOS-blast furnace: working toward technology demonstration. IEAGHG/IETS Iron and Steel Industry CCUS and Process Integration Workshop, Tokyo, Japan, 4–7 November 2013. http://www.ieaghg.org/docs/General_Docs/Iron%20and%20Steel%202%20Secured%20presentations/1050%20Jan%20van%20der%20Stel.pdf. Accessed 06 June 2018.
ULCOS: HIsarna Smelter Technology. http://www.ulcos.org/en/research/isarna.php. Accessed 14 Dec 2017.
ULCORED: Industrial Efficiency Technology Database. http://ietd.iipnetwork.org/content/ulcored-0. Accessed 14 Dec 2017.
Satyendra: Ispat Guru. http://ispatguru.com/ultra-low-carbon-dioxide-steelmaking-ulcos/, 2015. Accessed 14 Dec 2017.
A. Hasanbeigi, L. Price, and M. Arens: Berkeley National Lab. https://china.lbl.gov/sites/all/files/6106e-steel-tech.pdf, 2013. Accessed 14 Dec 2017.
A. Extance: Chemistry World. http://www.rsc.org/chemistryworld/2013/04/green-energy-cheaper-hydrogen-separate-water-splitting, 2013. Accessed 14 Dec 2017.
H. Hiebler, J.F. Plaul: Metalurgija, 2004, vol. 43, pp. 155–162.
Lee, J. Jung, K. Kim, and S. Kim: The 157th ISIJ meeting, International Organized Sessions, Environment and Energy Technology/High Temperature Processes, Tokyo, 2009.
H.Y. Sohn: American Iron and Steel Institute. https://www.steel.org/~/media/Files/AISI/Public%20Policy/9953factsheet.ashx. Accessed 14 Dec 2017.
A. Allanore, L. Yin, and D.R. Sadoway: Nature, 2013, vol. 497, pp. 353–356.
Canadian Steel Producers Association. http://canadiansteel.ca/. Accessed 14 Dec 2017; ArcelorMittal Brasil. http://brasil.arcelormittal.com/. Accessed 14 Dec 2017.
BAOSTEEL: http://www.baosteel.com/group_en/. Accessed 14 Dec 2017.
A. Züttel, A.A. Remhof, A. Borgschulte, and O. Friedrichs: Philos. Trans. R. Soc., 2010, vol. 368, pp. 3329–3342.
A. Sorman: Doctoral thesis, Montanuniversitaet, Leoben, 1992.
A. Sorman, H. Hiebler, and H. Presslinger: International Conference on “New Smelting Reduction and Near Net Shape Casting Technologies for Steel”, 1990, vol. 1, pp. 57–74.
J.F. Plaul: Doctoral thesis, Montanuniversitaet, Leoben, 2005.
J.F. Plaul, W. Krieger, E. Baeck: Steel Res. Int., 2005, vol. 76, pp. 548–554.
K. Badr: Doctoral thesis, Montanuniversitaet, Leoben, 2007.
K. Badr, E. Baeck, and W. Krieger: 18th International Symposium on Plasma Chemistry, August 26–31, Kyoto, Japan, 2007.
K. Badr, E. Baeck, W. Krieger: Steel Res. Int., 2007, vol. 78, pp. 275–280.
P. Rajput, B. Bhoi, S. Sahoo, R.K. Paramguru, B.K. Mishra: Ironmak. Steelmak., 2013, vol. 40, pp. 61–68.
P. Rajput, K.C. Sabat, R.K. Paramguru, B. Bhoi, B.K. Mishra: Ironmak. Steelmak., 2014, vol. 41, pp. 721–731.
H.L. Gilles, C.W. Clump: Ind. Eng. Chem. Process Des. Dev., 1970, vol. 9, pp. 194–207.
R.G. Gold, W.R. Sandall, P.G. Cheplick: Ironmak. Steelmak., 1977, vol. 1, pp. 10–14.
K. Akashi, R. Ishizuka, and T. Mutobe: Proceedings of the Fourth International Conference on Vacuum Metallurgy, 1974, vol. 3, pp. 165–169.
T. Nakamura, K. Shibata, and K. Takeda: Plasma Chem. Plasma Process., 1981, vol. 1, pp. 149–160.
K. Kamiya and N. Kitahara: Trans. ISIJ, 1984, vol. 24, pp. 7–16.
M. Lemperle and A. Weigel: Steel Res. Int., 1985, vol. 56, pp. 465–469.
K.C. Sabat, P. Rajput, R.K. Paramguru, B. Bhoi, and B.K. Mishra: Plasma Chem. Plasma Process., 2014, vol. 34, pp. 1–23.
K.C. Sabat, A. Murphy: Metall. Mater. Trans. B, 2017, vol. 48, pp. 1561–1594.
B. R. Sant, T.P. Prasad: Talanta, 1968, vol. 15, pp. 1483-1486.
A.I. Vogel: A Text Book of Quantitative Inorganic Analysis Theory and Practice, Longmans, Green and Co., London, 1951.
BS ISO 5416: Direct Reduced Iron. Determination of Metallic Iron. Bromine-Methanol Titrimetric Method, 2006.
Acknowledgments
The authors are thankful to the Ministry of Steel, Govt. of India to fund the research studies behind this innovative work and also express gratitude to the Director, CSIR-IMMT, Bhubaneswar for his kind and extended support and active participation throughout the research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 14, 2017.
Rights and permissions
About this article
Cite this article
Behera, P.R., Bhoi, B., Paramguru, R.K. et al. Hydrogen Plasma Smelting Reduction of Fe2O3. Metall Mater Trans B 50, 262–270 (2019). https://doi.org/10.1007/s11663-018-1464-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1464-8