Abstract
Desulfurizing ability of the CaO-CaCl2-CaF2 slags saturated with CaO has been investigated from the viewpoint of the sulfide capacity and CaO solubility. The CaO-CaCl2-CaF2 slags containing small amounts of Cu2O and CaS were inserted in a CaO crucible with metallic copper. The CaO crucible was sealed in a nickel holder to prevent the evaporation of CaCl2, then heated up and kept at temperatures from 1573 K (1300 °C) to 1673 K (1400 °C) for 24 hours, which enabled the system inside the CaO crucible to reach the equilibrium. As expected, the sulfide capacity derived from the data obtained as well as CaO solubility of the slag increase with an increase in temperature at a constant ratio of CaCl2/CaF2. The solubility of CaO increases by the replacement of CaF2 with CaCl2, whereas the sulfide capacity slightly decreases and the activity coefficient of CaS (γ CaS) increases. This suggests that CaF2 has stronger interaction with CaS than CaCl2. The sulfur distribution ratio between carbon-saturated iron melts and the CaO-CaCl2 slag has been calculated to be about 10 000 at 1573 K (1300 °C) using the sulfide capacity obtained, which value is still large enough even with the replacement of CaF2 by CaCl2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the conventional hot metal desulfurization process, excessive solid lime is used as a desulfurizing agent and flux agent such as CaF2 is added to promote CaO dissolution. The solid CaO does not directly participate in the desulfurization reaction, which is only due to the liquid slag coexisting with solid CaO.[1,2] For the desulfurizing ability of this liquid slag, two key factors should be considered: (1) sulfide capacity from a thermodynamic point of view and (2) solubility of CaO which ensures more chances for sulfur reacting with CaO from a kinetic point of view.
Over the years, in the practical hot metal pretreatment, an excellent desulfurization has been achieved using the CaO-CaF2-based slags. However, it is strongly required to reduce the amount of CaF2 in terms of environmental concern. Taniguchi et al.[3,4] proposed to directly use the carryover of a certain amount of blast furnace slag in the hot metal ladle as a flux agent and measured the sulfide capacity of the formed CaO-Al2O3-SiO2-based slags. However, the value obtained is much lower than that of CaO-CaF2-SiO2 slags.[5] Besides that, it is well known that CaO-Al2O3-SiO2 slags have limited solubilities of CaO and CaS;[6] therefore, fluxes having higher ability are required.
As a sort of flux agent, CaCl2 is a candidate to replace CaF2. Addition of CaCl2 into CaO-based slags can effectively promote the melting of CaO, as reported elsewhere.[7] The research of Sakai et al.[8] confirmed that CaO-CaCl2 slags have high sulfide capacity in the temperature range from 1273 K to 1523 K (1000 °C to 1250 °C). However, the sulfide capacity and CaO solubility in CaO-CaCl2 based slags have not been clarified at hot metal desulfurization temperature which is usually over 1573 K (1300 °C), because the traditional experimental methods are not feasible due to the evaporation of CaCl2 at higher temperature. Therefore, the present work aims to establish a new experimental method to finally investigate the effect of replacement of CaF2 by CaCl2 on the sulfide capacity and CaO solubility of CaO-CaCl2-CaF2 slags saturated with CaO in the temperature range from 1573 K (1300 °C) to 1673 K (1400 °C).
Experimental
Experimental Principles
The sulfide capacity of molten slags was originally defined on the basis of the gas/slag reaction (Eq. [1]) as shown in Eq. [2].
where \( K_{1}, a_{{\text{O}}^{2 - }}, f_{{\text{S}}^{2 - }}, \) P i, and (pct S) are the equilibrium constant of Reaction [1], the activity of oxide ion, the Henrian activity coefficient of sulfide ion, the partial pressure of i, and the sulfur content on mass pct basis in slag, respectively. As shown in the definition, the sulfide capacity solely depends on slag composition and temperature.
For the present experiments, to suppress the loss due to the severe evaporation of CaCl2 above 1573 K (1300 °C), a gas–slag–metal equilibration technique was employed in a closed system to determine the sulfide capacity of slags. Copper was chosen as the reference metal which reflected the sulfur potential \((P_{{\text{S}}_{2}}) \) and oxygen potential \((P_{{\text{O}}_{2}}) \) derived from Eqs. [3] and [4] with the standard Gibbs energy for the dissolution reaction of gaseous sulfur and oxygen into the molten copper.[9,10]
CaS and Cu2O were added into the slags as the source of sulfur and oxygen. The final Cu2O content should be controlled as low as possible so as not to influence the property of CaO-CaCl2-CaF2 slags. A dense CaO crucible was used to contain the slag and copper and to make the slag saturated with CaO. The closed system in the experiments was supported by a nickel holder, which was carefully welded after inserting the CaO crucible. The reason for the choice of nickel holder in the present set-up instead of iron, molybdenum, or other metal holder with high melting point was the fact that nickel is relatively stable with oxygen, and thus the final oxygen potential in the closed system would not be regulated by the oxidation reaction of nickel.
Experimental Procedure
The slag samples were prepared by complete mixing of CaO calcined from CaCO3, CaCl2, CaF2, Cu2O, and CaS reagent powders. CaCl2 is hygroscopic and the water absorbed by CaCl2 would be the oxygen source at experimental temperature, which significantly increases the final equilibrium oxygen potential in the closed system. Thus, the slag powders were dehydrated at 873 K (600 °C) for 2 hours in a muffle furnace. Then, the powders were melted in a platinum crucible for 10 minutes at 1373 K (1100 °C) under argon atmosphere. After quenching the sample, 1 to 3 g of pre-melted slag and 3 g of copper were maintained in a dense CaO crucible (outer diameter: 19 mm, inner diameter: 15 mm, and height: 15 mm). After placing the CaO crucible into the nickel holder, the holder’s lid was screwed on tightly and welded. To prevent the hydration of CaCl2, the whole charging process was conducted in a glove box.
An MoSi2 electric resistance furnace equipped with a proportional–integral–differential (PID) controller with a Pt–Pt/6 pct Rh thermocouple was used for all the experiments. Inside the furnace, an alumina reaction tube (outer diameter: 100 mm, inner diameter: 85 mm, and length: 1000 mm) was positioned. The temperature was controlled within ±2 K in the temperature even zone over a length of 50 mm. After reaching the target temperature, the nickel holder was placed in the center of the reaction tube under an argon atmosphere and held for 24 hours to reach equilibrium, which has been confirmed long enough by the preliminary experiments.
Degrees of Freedom in the Closed System
For a system in an invariant equilibrium state, the degree of freedom should be zero, which can be determined by the Gibbs phase rule. In the present study, the liquid CaO-CaCl2-CaF2-Cu2O-CaS slags and copper in a CaO crucible have been equilibrated in a closed system given by a nickel holder. Accordingly, the number of the components is 8 due to the presence of calcium, copper, sulfur, chlorine, fluorine, oxygen, nitrogen, and nickel on element basis. The number of the phases is 5 due to the presence of liquid slag, molten metal, oxide and metal holder materials and gas phases. The temperature is constant. Consequently, the degree of freedom for this system is 4 and it can become zero by determining the initial content ratio of the slag components among CaCl2, CaF2, Cu2O, and CaS. That is to say, the present system can reach the equilibrium, provided that the slag composition remains in a steady state throughout the experiments.
Sample Analysis
After equilibration, the nickel holder was quickly pulled out from the furnace and quenched with ice water. Then, the holder was cut open and the slag and copper samples were subjected to chemical analysis. The oxygen content in the metal and the sulfur content in both the slag and the metal were measured with LECOFootnote 1 oxygen analyser and sulfur analyser, respectively. The contents of calcium and copper were determined by inductively coupled plasma optical emission spectrometry (ICP-OES). The CaO content of the slag was measured by neutralization titration. With the analysis results of overall calcium, CaO, CaS, and Cu2O in the slag, the contents of CaCl2 and CaF2 were derived by calculation.
Results and Discussion
Equilibrium Time
Preliminary experiments were carried out to determine the time required for equilibration. Figure 1 shows the change of CaO content and L S · [pct O], where L S is the sulfur distribution ratio between slag and metal, (pct S)/[pct S], with holding time for the system of 11.5CaO-87CaCl2-1Cu2O-0.5CaS slag (on mass pct basis) and molten copper in a CaO crucible which was placed in a closed nickel holder at 1573 K (1300 °C). The Henrian activity coefficients of oxygen and sulfur in the molten copper are both assumed to be 1, because the interaction between oxygen and sulfur is very small[9] and the content of other solute elements is extremely low. Hence, from the definition of sulfide capacity, it can be recognized that the desulfurization reaction reaches equilibrium when the value of L S · [pct O] is constant. The CaO content and L S · [pct O] become constant after about 16 hours, which confirms the attainment of equilibrium state in the closed system. For the main experiments, the holding time has been determined to be 24 hours. It is notable that for the two experiments with 32 hours of holding time, the final Cu2O contents are quite different, 1.24 and 3.77 mass pct, respectively; but the CaO content and L S · [pct O] are nearly the same, which indicates that the influence of a small amount of Cu2O addition on the property of CaO-CaCl2-CaF2 slag can be neglected.
Experimental Results
The experimental results obtained in this study are listed in Table I. The mass ratio of CaCl2/CaF2 in the final slags does not change much throughout the experiments, which suggests that the evaporation of CaCl2 had been successfully suppressed during the experiments. The oxygen content in copper after experiments confirms that the oxygen potential in the closed system was not regulated by the oxidation reaction of nickel.
Solubility of CaO
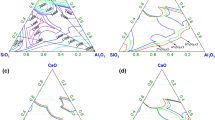
The solubility of CaO in CaO-CaCl2, CaO-CaF2, and CaO-CaCl2-CaF2 systems is shown in Figures 2 and 3, together with the results reported in the literature.[11,12,13] The CaO solubility of CaO-CaCl2 system from 1573 K to 1673 K (1300 °C to 1400 °C) is located on the line extrapolated from the results by Wenz[11] for lower temperatures. For the CaO-CaF2 system whose eutectic temperature is as high as 1633 K (1360 °C), the CaO solubility is smaller than that of CaO-CaCl2 system. For the CaO-CaCl2-CaF2 system, CaO solubility increases with the replacement of CaF2 by CaCl2. At 1673 K (1400 °C), the CaO solubility is smaller than that obtained by Sano,[12] and the discrepancy becomes larger with an increase in CaCl2 content. In Sano’s experiment, because the evaporation of CaCl2 had not been suppressed, some of the CaO once dissolved in the molten slag might have precipitated again and remained in the molten slag, which led to the final CaO content higher than the actual CaO solubility.
Sulfide Capacity for the CaO-CaCl2-CaF2 Slags Saturated with CaO
The sulfide capacity of CaO-CaCl2-CaF2 slags saturated with CaO from 1573 K to 1673 K (1300 °C to 1400 °C) is shown in Figure 4 as a function of \(X_{{\text{CaCl}}_{2}}/(X_{{\text{CaCl}}_{2}}+X_{{\text{CaF}}_{2}}) \). In the CaO-CaCl2-CaF2 slags, log \( C_{{\text{S}}^{2-}}, \) common logarithm of \( C_{{\text{S}}^{2-}}, \) increases with an increase in temperature and decreases with the replacement of CaF2 by CaCl2. An increase in the content of CaCl2 from about 25 to 75 mol pct in the slag causes a decrease in \( C_{{\text{S}}^{2-}}, \) just by 1.5 to 1.8 times, indicating that the replacement of CaF2 by CaCl2 would not significantly influence the desulfurizing ability of CaO-CaCl2-CaF2 slags saturated with CaO. Figure 5 shows the sulfide capacity as a function of reciprocal temperature at a fixed CaCl2 content in the slag. Linear relationships are observed, and the slopes of all the lines are almost the same.
The equilibrium reaction between CaO and CaS is as follows:[14]
where K 5, a CaO, γ CaS, X CaS, and P i are the equilibrium constant of Reaction [5], the activity of CaO, the activity coefficient of CaS, the mole fraction of CaS, and the partial pressure of i. In the present work, slags are saturated with CaO; thus, a CaO is unity. Considering Eq. [6], the enthalpy change for Reaction [5], \( {\Delta H_{5}^{ \circ} }\), can be expressed by Eq. [7] according to van’t Hoff equation:
Assuming that γ CaS does not change with temperature, the value of \( {\Delta H_{5}^{ \circ} }\) is given as 196 kJ/mol from the experimental data, which is larger than the reported value[14] of 92 kJ/mol. This indicates that γ CaS considerably decreases with an increase in temperature.
The activity coefficient, γ CaS, can be also calculated from Eq. [6]. Figure 6 shows the activity coefficient of CaS in the slag as a function of \(X_{{\text{CaCl}}_{2}}/(X_{{\text{CaCl}}_{2}}+X_{{\text{CaF}}_{2}}) \). γ CaS increases with the replacement CaF2 by CaCl2 at each temperature, which suggests that CaF2 has stronger interaction with CaS than CaCl2.
Thermodynamic consideration has been made for the change in CaO solubility and \( C_{{\text{S}}^{2-}} \). The \( a_{{\text{O}}^{2-}} \) in the present work is determined by the reaction of (CaO) = (Ca2+) + (O2−) and can be expressed as follows:
where \( a_{{\text{Ca}}^{2+}}, \) γ i, and X j are the activity of calcium ion, the activity coefficient of i, and the mole fraction of j in slag, respectively. Ca2+ is the only cation in the slag, and therefore \( a_{{\text{Ca}}^{2+}} \) is constant, if Temkin model[15] is applied. Consequently, \( a_{{\text{O}}^{2-}} \) is also constant and \( C_{{\text{S}}^{2-}} \) is only influenced by temperature and \( f_{{\text{S}}^{2-}} \) for the CaO-CaCl2-CaF2 slags saturated with CaO. As F− is replaced by Cl−, \( \gamma_{{\text{O}}^{2-}} \) decreases because the radius of Cl− is larger than that of F- and, accordingly, the attractive force between Ca2+ and Cl− is smaller than that between Ca2+ and F−, leading to the reduction of total attractive force between Ca2+ and halide ion, which explains why CaO solubility increases with replacing CaF2 by CaCl2. In the present work, a decrease of \( C_{{\text{S}}^{2-}} \) with replacing CaF2 by CaCl2 indicates the decrease of \( \gamma_{{\text{S}}^{2-}} \), which may be caused by the increase of O2− content because the attractive force between Ca2+ and O2− is largest in the slag.
Sulfur Distribution Ratio between Slag and Carbon-Saturated Iron Melts
The experimentally obtained sulfide capacity can predict the sulfur distribution ratio between slag and metal which is more frequently and importantly used in the practical process, such as hot metal desulfurization. Eq. [9] shows the desulfurization reaction in carbon-saturated iron melts:
The sulfur distribution ratio L S can be derived as shown in Eq. [12] by applying Eqs. [10] and [11] to the obtained sulfide capacity.[16,17]
where \( f_{{\text{S}}_{{\text{Fe - C}}_{\text{satd.}} }} \) is the activity coefficient of sulfur in carbon-saturated iron melts relative to 1 mass pct Henrian standard. At 1573 K (1300 °C), \( f_{{\text{S}}_{{\text{Fe - C}}_{\text{satd.}} }} \) is 6.25[5] and L S was calculated and is shown in Figure 7, by assuming P CO = 1 atm. Even though L S decreases with the replacement of CaF2 by CaCl2, the value of L S for the CaO-CaCl2 slag saturated with CaO is still as high as 10 000 at the temperature of 1573 K (1300 °C), which is satisfactory enough for the actual operation.
Conclusions
The sulfide capacity and CaO solubility for the CaO-CaCl2-CaF2 slags saturated with CaO were determined at temperatures from 1573 K to 1673 K (1300 °C to 1400 °C) by equilibrating molten slag and molten copper in a CaO crucible. Furthermore, the sulfur distribution ratios between slags and carbon-saturated iron melts were derived using the obtained sulfide capacity. The specific findings are summarized as follows:
-
1.
The CaO solubility and the sulfide capacity increase with an increase in temperature at a constant ratio of CaCl2/CaF2.
-
2.
The CaO solubility increases by 4.5 to 5.5 mol pct and \( C_{{\text{S}}^{2-}} \) decreases by the factor of 1.5 to 1.8 times with an increase in CaCl2 content from about 25 to 75 mol pct.
-
3.
The value of γ CaS increases with a decrease in temperature and with the replacement of CaF2 by CaCl2, which indicates that CaF2 has stronger interaction with CaS than CaCl2.
-
4.
The estimated sulfur distribution ratio between CaO-CaCl2 slag saturated with CaO and carbon-saturated iron melts is as high as 10 000 at the temperature of 1573 K (1300 °C), which suggests the feasibility of replacing CaF2 by CaCl2 in practical hot metal desulfurization.
Notes
LECO is a trademark of Leco Corporation, St. Joseph, MI.
References
K. Takahashi, K. Utagawa, H. Shibata, S. Kitamura, N. Kikuchi, and Y. Kishimoto: ISIJ Int., 2012, vol. 52, pp. 10-17.
J. C. Niedringhaus and R. J. Fruehan: Metall. Trans. B, 1988, vol. 19B, pp. 261-68.
Y. Taniguchi, L. Wang, N. Sano, and S. Seetharaman: Metall. Trans. B, 2012, vol. 43B, pp. 477-84.
Y. Taniguchi, N. Sano, and S. Seetharaman: ISIJ Int., 2009, vol. 49, pp. 156-63.
K. Susaki, M. Maeda, and N. Sano: Metall. Trans. B, 1990, vol. 21B, pp. 121-29.
M. Uo, E. Sakurai, F. Tsukihashi, and N. Sano: Steel Res., 1989, vol. 60, pp. 496-502.
T. Hamano, M. Horibe, and K. Ito: ISIJ Int., 2004, vol. 44, pp. 263-67.
T. Sakai and M. Maeda: Tetsu-to-Hagané, 1990, vol. 76, pp. 64-69.
K. Sano and H. Sakao: J. Jpn. Inst. Met., 1955, vol. 19, pp. 655-59.
M. A. El-Naggar and N. A. D. Parlee: Metall. Trans. B, 1970, vol. 1B, pp. 2975-77.
D. A. Wenz, I. Johnson, and R. D. Wolson: J. Chem. Eng. Data, 1969, vol. 14, pp. 250-52.
N. Sano, F. Tsukihashi, and A. Tagaya: ISIJ Int., 1991, vol. 31, pp. 1345-47.
R. Ries and K. Schwerdtfeger: Archiv Eisenhüttenwesen, 1980, vol. 51, pp. 123-129.
E. T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, 1980, pp. 7.
M. Temkin: Acta Physicochim URSS, 1945, vol. 20, pp. 411-20.
The 19 th Committee on Steelmaking, the Japan Society for the Promotion of Science: Steelmaking Data Source book, Gordon and Breach Science Publishers, New York, 1988, pp. 39.
M. W. Chase, C. A. Davies, J. R. Downey, D. J. Frurip, R. A. McDonald, and A. N. Syverud: J. Phys. Chem. Ref. Data, 1985, vol. 14, Suppl. 1, pp. 626.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted July 19, 2016.
Rights and permissions
About this article
Cite this article
Liu, J., Kobayashi, Y. Desulfurizing Ability of the CaOsatd.-CaCl2-CaF2 Slags. Metall Mater Trans B 48, 1108–1113 (2017). https://doi.org/10.1007/s11663-016-0890-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0890-8