Abstract
As part III of this series, the model is extended to iron oxide-containing melts. All available experimental data in the FeO-Fe2O3-Na2O-K2O-MgO-CaO-MnO-Al2O3-SiO2 system were critically evaluated based on the experimental condition. The variations of FeO and Fe2O3 in the melts were taken into account by using FactSage to calculate the Fe2+/Fe3+ distribution. The molar volume model with unary and binary model parameters can be used to predict the molar volume of the molten oxide of the Li2O-Na2O-K2O-MgO-CaO-MnO-PbO-FeO-Fe2O3-Al2O3-SiO2 system in the entire range of compositions, temperatures, and oxygen partial pressures from Fe saturation to 1 atm pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many slag systems in the steelmaking process and non-ferrous metallurgical process contain significant amounts of iron oxide. For example, the CaO-FeO-Fe2O3-SiO2 slag is the main slag system of both the basic oxygen furnace (steelmaking process) and converter process of non-ferrous metals such as Cu and Zn. In addition, the slags for ferro-alloy production and electric arc furnace slags for steel production contain a large amount of iron oxide. Molar volume or density is one of the most fundamental physical properties of liquid slags and is required for the design of industrial processes. However, the molar volume of Fe oxide-containing slag is complex because of the redox reaction in slag producing both FeO and Fe2O3 in liquid state. The amount of FeO and Fe2O3 changes with slag composition, temperature, and oxygen partial pressure.
Critical evaluation of all experimental molar volume data in the Li2O-Na2O-K2O-MgO-CaO-MnO-PbO-Al2O3-SiO2 system, the modeling of molar volume of unary and binary melts, and demonstration of predictive ability of the present structural molar volume model in ternary and multicomponent melts were already presented in the Parts I and II of this series of studies.[1,2]
The purpose of the present study is to critical review of the experimental molar volume data of the FeO-Fe2O3-Na2O-K2O-MgO-CaO-MnO-Al2O3-SiO2 system and modeling based on a structural molar volume model. The molar volume parameters of pure FeO and Fe2O3 and hypothetical binary FeO-SiO2 and Fe2O3-SiO2 melts were optimized in the present study based on the most reliable experimental data. Then the molar volumes of all ternary and multicomponent melts under any oxygen partial pressure can be accurately predicted from the present structure molar volume model.
Structural Molar Volume Model
A structural molar volume model for binary systems was presented in Part I of this study and its expansion to ternary and multicomponent systems was explained in Part II. A short review of the model is given below.
The total number of oxygen (n t) in one mole of a M2O-…-MO-…-M2O3-…-SiO2 melt (where M is any metallic cation such as Na, Ca, Mg, Fe(II), Al, Fe(III), etc.) is
where X is the mole fraction of each respective oxide in the melt. Then the number of bridged oxygen (\( n_{{{\text{O}}^{\text{o}} }} \)) in one mole of solution can be calculated by multiplying the mole fraction of Si–Si pairs (X Si–Si) in the solution, calculated by the Modified Quasichemical Model (MQM)[3] with an optimized thermodynamic database (FactSage FToxid database[4]): \( n_{{{\text{O}}^{\text{o}} }} = X_{{\text{Si}}{-}{\text{Si}}} n_{\text{t}} \). Strictly speaking, Al2O3 and Fe2O3 behave as amphoteric components in molten slags. However, for the sake of simplicity, Al2O3 and Fe2O3 were treated in the present model as network modifiers like Na2O, CaO, MgO, FeO, etc. The amount of different silicate tetrahedral Qn species in one mole of given melt can be calculated based on the random distribution of bridged and broken oxygen around each Si atom in silicate melt. In addition, the amount of free oxygen bond units, n M–M per mole of melt, can be calculated to be \( n_{\text{M - M}} = X_{{\text{M}}{-}{\text{M}}} n_{\text{t}} \), where M = all cationic species like Na, Ca, Mg, Fe(II), Al, Fe(III), except Si in given melt.
The molar volume of binary silicate MO-SiO2 melts is calculated from the molar volume of MO and SiO2 and Qn species using the following formula:
where molar volume of each unit in the model is expressed as a function of temperature, a + bT. The unary molar volume parameters and binary Qn species parameters were determined based on unary and binary molar volume data.
In order to predict the molar volume of ternary and multicomponent slags, the quantity of each Qn species for a given melt can be calculated from pair (bond) fraction information using the MQM model[3] with FactSage FToxid database.[4] Then, the molar volume of the melt can be calculated using the following formula based on a linear extrapolation of the unary and binary parameters weighted by the quantity of each M-Si pairs:
where \( n_{{{\text{Q}}^{n} }} \) and n t are the number of moles of Qn species and total oxygen in one mole of liquid. x Si − i and x i − i are bond fractions of broken oxygen (Si-i pairs) and free oxygen (i–j pairs where i and j are cations other than Si) in one mole of liquid. The molar volume parameters of pure SiO2 \( (a_{{{\text{SiO}}_{2} }} + b_{{{\text{SiO}}_{2} }} T) \), \( {\text{Q}}^{n} (a_{nM} + b_{nM} T), \) where n = 0 to 3, and MO \( (a_{\text{M - M}} + b_{{\text{M}}{-}{\text{M}}} T) \) are obtained by the reproduction of the molar volume change in the binary a MO-SiO2 melt. The last term in Eq. [3] incorporates the effect of free oxygen species. The molar volume of Mi-Mj (a i-j + b i–j T) is the average of the molar volume of Mi–Mi (a i–i + b i–i T) and Mj–Mj (a j–j + b j–j T). In the case of Na2O containing system, Na2 is considered as network modified cation species. That is, Na2 is M in Eqs. [2] and [3]. The same is applied for other alkali oxides. It should be noted that there is no ternary or higher order correction term in Eq. [3]. Once unary and binary Qn species parameters are determined, accurate prediction of molar volume of ternary and multicomponent systems can be done by the model.
The last term in Eq. [3] incorporates the effect of free oxygen species (e.g., Na-Na, Ca-Ca, Na-Ca, etc.). That is, if there is no SiO2 in the melt, the molar volume of the slag can be calculated only from the last term. Therefore, the molar volume of binary molten slag with no SiO2 changes linearly in the present model. For example, the molar volume of the ternary CaO-FeO-Fe2O3 slag varies linearly, from the present model, dependent on the amount of each component in slag. This will be discussed later.
The molar volume of the Na2O-K2O-MgO-CaO-MnO-Al2O3-SiO2 slag was already fully modeled in the Parts I[1] and II.[2] The model parameters can be found in Part I.[1] So, the addition of FeO and Fe2O3 to this multicomponent slag only requires the molar volume model parameters for unary FeO and Fe2O3 and binary FeO-SiO2 and Fe2O3-SiO2. The model parameters are listed in Table I. The details of modeling process are discussed below.
Critical Evaluation and Optimization of Molar Volume
In order to develop an accurate molar volume model containing iron oxide, an accurate thermodynamic database to calculate the amount of Fe2+ and Fe3+ in liquid slag under any partial pressure of oxygen is required. In FactSage FTOxid database,[4] critically evaluated and optimized model parameters for liquid slag with FeO-Fe2O3-SiO2-CaO-MgO-MnO-Al2O3-etc., components are stored. During the optimization of the thermodynamic model, the phase equilibria between slag and gas to quantify the variation of Fe2+ and Fe3+ as a function of oxygen partial pressure, composition, and temperature; the phase diagram data of Fe saturation, air, and various oxygen partial pressures; the activity data for liquid slag, etc., are all taken into account. Some of the published results for the optimization for FeO-Fe2O3,[6] FeO-Fe2O3-SiO2,[7] FeO-Fe2O3-MgO,[8] FeO-Fe2O3-CaO,[9] FeO-Fe2O3-Al2O3,[9] FeO-Fe2O3-CaO-SiO2,[9] FeO-Fe2O3-MgO-SiO2,[10] FeO-Fe2O3-MnO-Mn2O3-SiO2[11] systems are available in the literature.
The bond fractions of liquid slags containing iron oxide were calculated from the MQM[3] with optimized thermodynamic model parameters from FactSage FTOxid database.[4] The structural units for the molar volume are then calculated from the bond fraction data. Structural changes are automatically taken into account in the molar volume calculations as described in Eqs. [2] and [3]. All the volume parameters used in this work are listed in Part I[1] of this study and new parameters related to FeO and Fe2O3 are listed in Table I. The summary of experimental data for iron oxide-containing slags is presented in Table II. The present modeling results are also compared with the results of the linear model developed by Lange and Carmichael.[5]
FeO-Fe2O3 System
The density of the iron oxide system has been primarily studied in contact with solid iron under an inert atmosphere. Only Mori and Suzuki[17] and Hara et al.[23] examined the melts under controlled oxygen partial pressures.
The density measurements of iron oxide melts in contact with iron (iron saturation) are relatively scattered. It was noted in earlier works that the density of iron oxide melts (and other iron oxide-rich melts) could be varied depending on the inert gas used. Ward and Sachdev[15] demonstrated that they could obtain very different results depending on whether they used Ar or N2 atmosphere while using the MBP method. This effect was later attributed to the precipitation of iron from the melt due to the differences in thermal conductivities of the inert gases. The deviation between the results obtained using different inert gases could be eliminated with better temperature control. Gaskell and Ward[16] performed improved measurements using Ar, N2, and He gas and obtained virtually the same results. Therefore, it should be noted that in some of the earlier works there is the possibility of iron precipitation which significantly increases the apparent density of the melt (lowers the molar volume) depending on the inert gas used. Early results using Ar gas are comparable with the more recent/accurate results.
The optimized molar volume of the FeO-Fe2O3 system is shown in Figure 1 along with experimental data. Both the linear model and the present model assume a linear extrapolation across the system. A subset of the data in iron saturation has been shown, as there are too many data points to illustrate while maintaining clarity. In iron saturation, the most accurate results are believed to be those of Ogino et al.[20] using a DBA technique (tabulated) in both Ar and N2 atmospheres. Density values of between 4.58 and 4.59 g/cm3 at 1673 K (1400 °C) were obtained. These values are consistent with the more recent values by Shiraishi et al.[22] who used a SBA technique (tabulated) with correction of surface tension contributions.
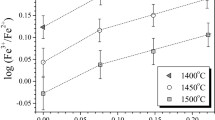
Mori and Suzuki[17] varied oxygen partial pressure by controlling the CO/CO2 ratio and used a DBA method. They reported their density measurements graphically, and only published their Fe3+/Fe ratios without oxygen partial pressure information. It was not possible to calculate the ferric to ferrous ratios using FactSage and therefore the raw data were used as reported. Hara et al.[23] measured a single composition with a CO2/H2 ratio of 22, using a DBA method. The present model and the linear model of Lange and Carmichael[5] are illustrated at two temperatures [1673 K and 1773 K (1400 °C and 1500 °C)]. The results of Lange and Carmichael may not reproduce the data exactly because all the experimental data used in this work were analyzed using FactSage. The calculate the ferric to ferrous ratios do not coincide exactly with the values used by Lange and Carmichael for the calibration of their model parameters. In addition, the model of Lange and Carmichael was calibrated primarily using the experimental data of melts studied in air and in relatively silica-rich compositions.
The experimental data of Mori and Suzuki are inconsistent with the more recent data of Hara et al. The results published by Mori and Suzuki suggest a non-linear variation in molar volume between FeO and Fe2O3. It is unknown why their results differ from Hara et al. The transcription of their results and the uncertainty of the Fe3+/Fe content does not explain the deviation. The present model is in good agreement with the most recent experimental results with iron saturation using various techniques. The molar volumes of FeO and Fe2O3 were numerically optimized by performing a multi-linear regression on the data of Hara et al.,[23] Ogino et al.,[23] and Sumita et al.[25] with the experimental data of the CaO-FeO-Fe2O3 system.
Ternary Systems
CaO-FeO-Fe2O3 system
This silica-free ternary system in iron saturation was examined by Henderson[14] (MBP), Ogino et al.[20] (DBA), and Kawai et al.[21] (MBP). Abrosimov et al.[24] (MBP), Sumita et al.[25] (DBA), Hara et al.[23] (DBA), and Vadasz et al.[43] (MBP) all examined this system in air. Hara et al.[23] (DBA) and Lee and Gaskell[19] (MBP) varied the oxygen partial pressure. Unfortunately, Lee and Gaskell did not provide the partial pressures used; therefore, their results have been omitted from this comparison.
The compositions studied by each research group along with the experimental condition and the accuracy of the present model are shown Figure 2. This system is well reproduced with a linear model, as demonstrated in the work of Hara et al.[23] at various oxygen partial pressures. The model parameters used for CaO, FeO, and Fe2O3 in this work can reproduce all the most accurate experimental data. The work by Ogino et al., Sumita et al., and Hara et al. were all performed using accurate DBA techniques and are reproduced well within 2 pct error. The data obtained from MBP measurements are less well reproduced. In iron oxide-bearing systems, the MBP method appears to provide very poor and inconsistent results compared to the DBA method.
The molar volume of this system in iron saturation is shown in Figure 3(a). Although it is with iron saturation, there are a certain amount of Fe2O3 in the melt. A significant curvature is noted in the high-FeO x region. This is due to the local maximum of the Fe3+/Fe2+ ratio at approximately 25 mol pct CaO in iron saturation. For illustrative purposes, the molar volume assuming no Fe2O3 (that is, FeO-CaO) for both the present model and the linear model is shown in Figure 3(b).
Na2O-FeO-Fe2O3 system
The Na2O-FeO-Fe2O3 system was studied in air by Sumita et al.[25] using the DBA (graphical) method. The results were published as the Fe2O3-Na2O system with the addition of Na2O in air. The measurements at 1673 K (1400 °C) are shown in Figure 4. Both the present model and the linear model fail to reproduce the experimental data. Both models predict lower molar volumes. There are several potential explanations for this failing. It is possible that the calculations by FactSage are incorrect in estimating the Fe3+ content in sodium-bearing melts; the database of the Na2O-FeO-Fe2O3 system is still under development. Another possibility is that there is an interaction between ferric iron oxide and sodium oxide which results in the formation of an associate with a larger molar volume. The linear model and the present model provide opposite trends; the linear model of Lange and Carmichael assumes a smaller volume of Na2O and a larger volume of iron oxide in air than the present model.
The molar volume of ternary Na2O-FeO-Fe2O3 system in air at 1673 K (1400 °C)[25]
FeO-Fe2O3-SiO2 system
This ternary system was primarily studied in contact with solid iron (iron saturation) or in such highly reducing conditions that they could be approximated as being in iron saturation. Only a single measurements has been performed in air, by Courtial et al.[30] using the DBA technique. Due to the quantity of the experimental data in iron saturation, only the most systematic or the most accurate data are shown in Figure 5 to maintain clarity.
The ternary FeO-Fe2O3-SiO2 system in iron saturation at 1573 K, 1673 K, and 1773 K (1300 °C, 1400 °C, and 1500 °C).[13–16,18,22,29,44] Molar volumes increase with temperature for both the present model and the linear model and this is also true for the data presented for authors at multiple temperatures
All the results obtained from DBA measurements (Adachi et al.[13] (1963), Ogino et al.,[20] Shiraishi et al.,[22] Mo et al.[29] and Thomas[44]) are self-consistent. These data were used for the optimization of the FeO-SiO2 system with more emphasis on the data of Shiraishi et al. The study by Shiraishi et al. spanned the widest composition range, provided results at multiple temperatures, used an accurate DBA technique, and tabulated their results. The results obtained from MBP methods are less consistent. For example, the results of Henderson[14] and Gaskell and Ward[16] follow the same trend as those of Shiraishi et al. but with more scatter. The results of Sokolov et al.[18] and Ward and Sachdev[15] are both believed to have precipitated iron, increasing the density and lowering the molar volume. The linear model of Lange and Carmichael does not reproduce the most accurate data in the iron oxide-rich region. Any curvature in the model of Lange and Carmichael is due to the variation of ferrous to ferric ratio with SiO2 content in the system. The FeO-SiO2 system with Fe saturation was optimized by fitting the data of Shiraishi et al. and ensuring a smooth trend from the SiO2-rich region.
The hypothetical molar volume of the FeO-SiO2 melt is calculated in Figure 6. The result of the present model is very similar to the results from the linear model by Lange and Carmichael above 33 mol pct SiO2.
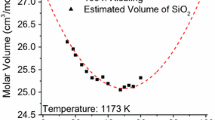
The Fe2O3-SiO2 system could not be optimized in a similar manner as the FeO-SiO2 system due to lack of experimental data. Therefore, it was optimized by assuming all the temperature dependence terms were equal to zero (as was done in the Al2O3-SiO2 system) and optimized based on experimental data in higher order systems using the DBA method. The calculated molar volume of a hypothetical Fe2O3-SiO2 system is shown in Figure 7. The variation of molar volume at 1673 K (1400 °C) is consistent with the results obtained by the linear model Lange and Carmichael in the high SiO2 region but deviates at low SiO2 region due to the restriction imposed by the molar volume of pure Fe2O3. As the binary parameters are temperature independent, the temperature dependence of molar volume in Figure 7(b) results from by those of pure Fe2O3 and SiO2.
In air, only a single composition of the FeO-Fe2O3-SiO2 system (at ~37 mol pct SiO2) was examined by Courtial et al.[30] (DBA) and their results are shown in Figure 8. The experimental molar volume of FeO-Fe2O3-37 mol pct SiO2 decreased with temperature. According to the present model, this trend is calculated to be due to the decrease in quantity of ferric to ferrous ratio with temperature in air. Both the molar volumes from the linear model and the present model are similar in this composition range and can reproduce the temperature dependence. However, both models predict higher molar volume by approximately 5 pct. It was impossible to reproduce these experimental data without significantly sacrificing the accuracy of the model in higher order systems.
The molar volume of the ternary FeO-Fe2O3-SiO2 system in air at ~37 mol pct SiO2 as a function of temperature[30]
Quaternary Systems
MgO-CaO-FeO-Fe2O3 system
This quaternary system was recently studied by Vadasz et al.[43] in air using the MBP method with nitrogen gas. The results are moderately well reproduced, as shown in Figure 9. However, it is noted that the results of Vadasz et al. are considered to be less accurate because of MBP technique. Their results in the CaO-FeO-Fe2O3 system are also less accurate compared to the data obtained by DBA technique. Part of the measurements at 1623 K (1350 °C) are potentially below the liquidus.
The quaternary MgO-CaO-FeO-Fe2O3 system in air: (a) compositions (mol pct) examined and (b) model accuracy[43]
CaO-FeO-Fe2O3-Al2O3 system
This quaternary system in Fe saturation was examined by Sikora and Zielinski[35] using the MBP method in a temperature range between 1723 K and 1923 K (1450 °C and 1650 °C) (Figure 10). They started with a CaO-Al2O3 melt and added iron oxide. Their results in the CaO-Al2O3 system suggested a very high thermal expansion and were inconsistent with other results (see Part I). Vadasz et al.[36] (MBP) examined several compositions in air at 1573 K (1300 °C). As seen in the previous CaO-MgO-FeO-Fe2O3 system, their results are much higher than the predictions from both a linear model and the present model. In addition, based on the phase diagram calculated by FactSage, the experiments were carried out at sub-liquidus temperature.
Na2O-FeO-Fe2O3-SiO2 system
Iron saturation
This quaternary system was examined by Guo[34] using a DBA technique using a 1 pct CO—99 pct Ar atmosphere. As the partial pressure of oxygen was sufficiently low, this study can be approximated as being in iron saturation. The compositions examined are along with the iso-FeO section (X FeO = 0.285) as shown in Figure 11. The experimental molar volume data show a non-linear trend (minimum at 20–30 mol pct of Na2O/(Na2O+SiO2)), which is reasonably well predicted by the present model. This non-linear behavior is due to the increasing volume trend in the binary Na2O-SiO2 system with the addition of Na2O and decreasing molar volume in the FeO-SiO2 system in iron saturation. This will be discussed in more details in Section V.
The quaternary Na2O-FeO-Fe2O3-SiO2 system in iron saturation: (a) compositions (mol pct) examined and (b) FeO = 28.5 mol pct iso-compositional section.[34] Filled symbols are at 1573 K (1300 °C) and opened symbols are at 1473 K (1200 °C)
Air (Na2O-Fe2O3-SiO2)
This quaternary system in air was examined in four publications,[29,31–33] all of which used the DBA method. All the results are moderately well predicted with the present model leaning toward a slight under-prediction, as shown in Figure 12.
K2O-FeO-Fe2O3-SiO2 System
This system was only examined in air by Liu and Lange[33] using the DBA method at temperatures between 1273 K and 1723 K (1000 °C and 1450°C). The results are well predicted as shown in Figure 13. No research groups have examined this system in iron saturation.
The quaternary K2O-FeO-Fe2O3-SiO2 system in air: (a) compositions (mol pct) examined and (b) model accuracy[33]
MgO-FeO-Fe2O3-SiO2 System
This quaternary system was examined by Bryanstev[37] using the MBP method in iron saturation (Figure 14). All measurements were performed at 1723 K (1450 °C). All the experimental results are systematically over predicted by approximately 2 pct, but the general trend is well predicted by the present model.
The quaternary MgO-FeO-Fe2O3-SiO2 system in iron saturation: (a) compositions (mol pct) examined and (b) model accuracy[37]
CaO-FeO-Fe2O3-SiO2 System
Iron saturation
The CaO-FeO-Fe2O3-SiO2 system in iron saturation, approximated as the CaO-FeO-SiO2 system, was studied across wide compositions ranges as shown in Figure 15. The experimental data were measured using DBA[20,34] and MBP[14,18,19,21,24,26,27,38,39] techniques. In order to provide further insight into this system, binary sections are shown in Figure 16 across the 30 and 50 mol pct SiO2 as well as the 40 mol pct FeO joins. The iso-SiO2 joins provide insight into the effects of CaO and FeO substitution.
The molar volume in the quaternary CaO-FeO-Fe2O3-SiO2 system in iron saturation across the (a) SiO2 = 30 mol pct,[14,21,26,27,38,39] (b) SiO2 = 50 mol pct,[14,21,26,34] and (c) FeO = 40 mol pct[14,19,21,26,34,38,39] joins. Filled symbols are at 1673 K (1400 °C) and opened symbols are at 1573 K (1300 °C)
Along the 30 and 50 mol pct SiO2 sections, the results of Gaskell et al.[27] (MBP) and Guo[34] (DBA) are well predicted. The remaining experimental data all obtained by MBP method suggest lower molar volumes and are very scattered.
The 40 mol pct FeO section was examined at multiple compositions by Guo[34] and their data are well predicted by the present model. The previous linear model also reasonably reproduces the molar volumes in this system. The large difference between two models in low SiO2 is mainly due to the differences in molar volume for CaO.
Air and other conditions
This system was examined by several research groups in air; Mo et al.[29] (DBA), Licko et al.[40] (SBA), Dingwell and Brearley[41] (DBA), Hara et al.[23] (DBA), and Vadasz et al.[36] (MBP). In addition, Hara et al.[23] (DBA) also examined several compositions under CO2 and CO2/H2=50 atmospheres. All the compositions studied in air are shown in Figure 17(a) in addition to the accuracy of the model for predicting the experimental data in Figure 17(b) (including measurements not in air). The results of Mo et al. (DBA), Licko et al. (SBA), and Hara et al. (DBA) are all well predicted. Like other systems (CaO-FeO-Fe2O3-Al2O3 and CaO-FeO-Fe2O3-MgO), the results of Vadasz et al. (MBP) are poorly reproduced. The results of Dingwell and Brearley are quite scattered, and some of their results are well reproduced while other are not. The composition measured by Dingwell and Brearly is located in the middle of the composition range studied by Vadasz et al. and their data are well predicted within 0.5 to 1 pct error.
MnO-FeO-Fe2O3-SiO2 System
The manganese-containing iron silicate system has only been studied in iron saturation. The compositions measured are shown in Figure 18 along with the accuracy of the model. Sokolov et al.[18] and Gaskell et al.[27] performed their experiments using the MBP method, while Ogino et al.[20] used the DBA method. To better illustrate some of the experimental data, the 28 and 38 mol pct SiO2 sections are shown in Figure 19. The results of Gaskell et al. and Ogino et al. are reasonably well predicted. However, the results of Sokolov et al. are very scattered.
FeO-Fe2O3-Al2O3-SiO2 system
A single composition of the FeO-Fe2O3-Al2O3-SiO2 system with Fe saturation was examined by Abrosimov et al.[24] using the MBP method. The molar volumes of a 47 SiO2-8 Al2O3-45 FeO melt (in mol pct) at multiple temperatures are shown in Figure 20. Tomasek et al.[45] and Fadeev et al.[46] also examined the system using the MBP method, but it was not possible to obtain either of these results. They were referred in the review performed by Mills and Keene.[47]
Molar volume of a 45 FeO—47 SiO2—8 Al2O3 melt (in mol pct) with iron saturation as a function of temperature[24]
Quinary and Higher Order Systems
Several higher order systems have been examined. However, it is difficult to illustrate the compositions studied graphically. The prediction accuracy of the model for the experimental data in higher order systems is shown in Figure 21. The most accurate data available are from of Courtial et al.[30] (DBA in the MgO-CaO-Al2O3-FeOx-SiO2 system in air) and Guo et al.[34] (DBA in various systems under a 99 pct Ar—1 pct CO atmosphere) and they were well predicted by the present model. The data of Bryanstev[37] (MBP in the MgO-FeOx-SiO2 system with Na2O, CaO, or Al2O3 additions in iron saturation) and Sokolov et al.[18] (MBP in the CaO-MnO-FeO x -SiO2 system in iron saturation) are less accurate. The data of Nelson and Carmichael[42] (DBA with a single composition in the CaO-Al2O3-FeO x -SiO2 system in iron saturation) is uncertain as some of their results have been questioned in other systems.
Discussions
Model and Experimental Data for Fe Oxide-Containing System
The present model for iron-containing systems provides a reasonable reproduction (typically within 2 pct error) of available reliable experimental data. Iron oxide-containing systems are less reproducible by linear models. Although the accurate data of Hara et al.[23] in the CaO-FeO-Fe2O3 system are reproducible with a linear model, these parameters are not compatible with the parameters required to reproduce the molar volumes of silica-bearing melts. This suggests that a linear model cannot reproduce all the experimental data across the entire composition ranges and that non-linear terms are required. The non-linear behavior of molar volume with SiO2 can be seen in the FeO x -SiO2 system in iron saturation. The linear model of Lange and Carmichael[5] can reproduce the experimental data of geological melts containing a small amount of iron oxide, but its extrapolation to low silica region is poor.
There are various systems which require more information in order to better constrain molar volumes and thermal expansions. In particular, even simple systems under oxidizing conditions have rarely been studied. In particular, the FeO x -SiO2 system under oxidizing conditions (Fe2O3-SiO2) would aid greatly in optimizing the molar volume of Fe2O3 and Fe2O3-SiO2 system. In addition, the temperature dependence (thermal expansion) of most iron-bearing melts is relatively poorly studied.
Molar Volume of Alkali Oxide-FeO-SiO2 Melts
As can be seen in Figure 11, the molar volume of Na2O-FeO-SiO2 melt in iron saturation (reducing atmosphere) clearly shows a non-linear behavior with the variation of Na2O/(Na2O + SiO2). In particular, a minimum in the molar volume is observed experimentally, which is well predicted by the present model. This behavior seems to result from the competitive contribution of Na and Fe to the molar volume of Q species.
Figure 22(a) shows the predicted molar volume of alkali oxide-SiO2 with 28.5 mol pct FeO in iron saturation at 1573 K (1300 °C). The predicted molar volume for K2O- and Li2O-containing melts shows increasing and decreasing trend with A2O/(A2O + SiO2), respectively, where A = K or Li. On the other hand, the molar volume of Na2O-containing melt shows the minimum as discussed above. In order to examine the different trends in molar volume more clearly, the change in bond fraction in the alkali oxide-FeO-SiO2 is calculated in Figure 22(b). Because the trend of the bond fraction was similar for all alkali oxide cases, only the results of Na2O-FeO-SiO2 melt are plotted. At constant FeO concentrations, in general, the amount of broken oxygen bond increases with increasing amount of Na2O. In the low-Na2O region, the broken oxygen bonds are mostly caused by Fe, O-(Fe). Increasing Na2O in melt gradually increases the broken oxygen bonds, O-(Na). When the molar ratio of Na2O/(Na2O + SiO2) in the melt is higher than 0.27, the contribution of Na to the broken oxygen bond becomes larger than Fe. Similar trends in bond fractions are also calculated for the melts containing K2O and Li2O.
In the presented molar volume model, molar volume is calculated based on the volume of individual Qn species. For ternary and multicomponent melt, the molar volume of Qn species is calculated considering the linear volume change between binary Qn species:
where \( a_{ni} + b_{ni} T \) is binary molar volume parameter of Qn and \( x_{{{\text{Si}} - i}} \) is bond fraction of broken oxygen by i cation (for example, O−(Na) and O−(Fe) calculated in Figure 22). Therefore, the molar volume of binary Qn species and the amount of broken oxygen by each cation determine the molar volume in ternary and multicomponent systems.
In the case of Na2O-FeO-SiO2 melt, the molar volume of ternary melt in Figure 22 is mainly calculated from the binary Na2O-SiO2 and FeO-SiO2. At low Na2O concentration, the molar volume is primarily a function of the FeO-SiO2 melt. As can be seen in Figures 5 and 6, the molar volume of FeO-SiO2 melt decreases with decreasing SiO2 content. The molar volume of Na2O-SiO2 melt is almost constant between 0.0 and 0.2 \( X_{{{\text{Na}}_{2} {\text{O}}}} \) and increases with increasing Na2O content. These two competitive contributions of Na2O and FeO result in the minimum in the molar volume of Na2O-FeO-SiO2 melt as shown in Figure 22. However, in the case of K2O-FeO-SiO2 melt, the molar volume increases continuously with increasing K2O because binary molar volume of K2O-SiO2 increases sharply with increasing SiO2 and the increasing rate of molar volume by the addition of K2O is higher than decreasing rate by FeO. The molar volume of Li2O-SiO2 decreases with increasing Li2O content which results in a decreasing molar volume in the Li2O-FeO-SiO2 melt.
A linear molar volume model cannot reproduce such non-linear molar volume behavior in the Na2O-FeO-SiO2 melt. Similar non-linear molar volume behavior can be expected in many ternary and multicomponent melts.
General Discussion on Molar Volume Model and Future experimental Studies
The goal of this work was to fully examine the available literature on the molar volume of metallurgical slags and to obtain a non-linear melt structure-based model which can accurately reproduce molar volume in experimentally explored compositions and temperatures regimes, and to provide predictions in the unexplored regions. The molar volume of molten oxides should be related to their structure at a given composition and temperature. In the present work, the structural information was calculated from the Modified Quasichemical Model with optimized thermodynamic parameters. The model may be improved as more information on melt structure is made available to verify the Q-species amounts.
Based on the examination of the literature, there are wide composition ranges which have only been studied using relatively inadequate experimental techniques (e.g., systematic errors in SBA measurements, large errors in MBP measurements, and questionable imaging technique results) which promote the importance of modeling work based on the most accurate experimental data and systematic analysis. Previous modeling attempts of molar volume with linear models have focused primarily on silicate-rich melts. In the silica-rich region (>50 mol pct SiO2), a linear model is shown to provide accurate reproduction of the available experimental data and give reasonable predictions. A linear model is also mathematically simple to optimize and perform calculations. However, a growing quantity of experimental data in the low-SiO2 region where most metallurgical slags are located suggests non-linear behavior of molar volume. This has been shown in various silicate systems such as the alkali binary silicates,[48,49] PbO-SiO2,[50,51] FeO x -SiO2, CaO-Al2O3-SiO2,[52] and TiO2-bearing melts[53] (not examined in this work). The thorough examinations by Bottinga et al.[54–56] also suggests that linear models should only be applied between 0.35 and 0.85 mol fraction silica and non-linear terms should be introduced into the alumina-bearing systems. The present structure-based model can provide more accurate calculation of molar volume in the entire range of compositions.
Summary
A structural molar volume model was developed to accurately reproduce the molar volume of molten slags containing iron oxides under the oxygen partial pressures from metallic saturation to air. The experimental data for FeO-Fe2O3-Na2O-K2O-MgO-CaO-MnO-Al2O3-SiO2 systems were critically reviewed and the unary and binary model parameters were optimized to reproduce the experimental data. The variation of ferric and ferrous iron oxides was taken carefully into account during the review of the experimental data and molar volume modeling.
The present structural molar volume model can be used to calculate the molar volume of molten oxide in the Li2O-Na2O-K2O-MgO-CaO-MnO-PbO-FeO-Fe2O3-Al2O3-SiO2 system across all ranges of compositions, temperature, and oxygen partial pressures under 1 atm total pressure. In future, the model and database can be further expanded to the systems containing Ti oxides, B2O3, and fluoride for a wider range of applications.
References
E. Thibodeau, A.E. Gheribi, and I.-H. Jung: “Part I,” accepted.
E. Thibodeau, A.E. Gheribi, and I.-H. Jung: “Part II,” accepted.
A. D. Pelton and M. Blander, “Thermodynamic analysis of ordered liquid solutions by a modified quasichemical approach - application to silicate slags,” Metallurgical and Materials Transactions B, vol. 17, pp. 805-815 (1986).
C. W. Bale, E. Bélisle, P. Chartrand, S. A. Decterov, G. Eriksson, K. Hack, I. H. Jung, Y. B. Kang, J. Melançon, A. D. Pelton, C. Robelin, and S. Petersen, “FactSage thermochemical software and databases— recent developments,” Calphad, vol. 33, pp. 295-311 (2009).
R. L. Lange and I. S. E. Carmichael, “Thermodynamic properties of silicate liquids with emphasis on density, thermal expansion and compressibility,” Reviews in Mineralogy and Geochemistry, vol. 24, pp. 25-64 (1990).
S. A. Decterov, E. Jak, P. C. Hayes, and A. D. Pelton, “Experimental study of phase equilibria and thermodynamic optimization of the Fe-Zn-O system,” Metallurgical and Materials Transactions B, vol. 32B, pp. 643-657 (2001).
E. Jak, P. Hayes, A. Pelton, and S. Decterov, “Thermodynamic optimisation of the FeO-Fe2O3-SiO2 (Fe-O-Si) system with FactSage,” International Journal of Materials Research, vol. 98, pp. 847-854 (2007).
I.-H. Jung, S. A. Decterov, and A. D. Pelton, “Critical thermodynamic evaluation and optimization of the Fe-Mg-O system,” Journal of Physics and Chemistry of Solids, vol. 65, pp. 1683-1695 (2004).
S. Decterov, I. H. Jung, E. Jak, Y. B. Kang, P. Hayes, and A. Pelton: VII International Conference on Molten Slags, Fluxes and Salts, 2004, pp. 839–49.
I.-H. Jung, S. A. Decterov, and A. D. Pelton, “Critical thermodynamic evaluation and optimization of the FeO-Fe2O3-MgO-SiO2 system,” Metallurgical and Materials Transactions B, vol. 35B, pp. 877-889 (2004).
Y. B. Kang and I. H. Jung: VIII International Conference on Molten Slags, Fluxes and Salt, 2009, pp. 459–71.
12. J. Henderson, R. G. Hudson, R. G. Ward, and G. Derge, “Density of liquid iron silicates,” Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers, vol. 221, pp. 807-811 (1961).
A. Adachi, K. Ogino, and S. Kawasaki, “Measurement of the density of molten FeO-SiO2 slags by the archimedean method,” Technology Reports of the Osaka University, vol. 13, pp. 411-415 (1963).
J. Henderson, “Density of lime-iron oxide-silica melts,” Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers, vol. 230, pp. 501-504 (1964).
R. G. Ward and P. L. Sachdev, “Density of iron oxide-silica melts,” Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers, vol. 233, pp. 1496-1499 (1965).
D. R. Gaskell and R. G. Ward, “Density of iron oxide-silica melts,” Transactions of the Metallurgical Society of AIME, vol. 239, pp. 249-252 (1967).
K. Mori and K. Suzuki, “Density of iron oxide melts in equilibrium with carbon dioxide-carbon monoxide gas mixtures,” Tetsu to Hagane, vol. 54, pp. 1123-1127 (1968).
V. I. Sokolov, S. I. Popel, and O. A. Esin, “Density and molar volume of slags,” Izvestiya Vysshikh Uchebnykh Zavedenii, Chernaya Metallurgiya, vol. 13, pp. 10-15 (1970).
D. R. Gaskell and Y. E. Lee, “Densities and structures of melts in the system calcium oxide-iron(II, III) oxide-silicon dioxide,” Metallurgical Transactions, vol. 5, pp. 853-860 (1974).
K. Ogino, M. Hirano, and A. Adachi, “Density of iron silicate slags,” Technology Reports of the Osaka University, vol. 24, pp. 49-55 (1974).
Y. Kawai, K. Mori, H. Shiraishi, and N. Yamada, “Surface tension and density of FeO-CaO-SiO2 melts,” Tetsu to Hagane, vol. 62, pp. 53-61 (1976).
Y. Shiraishi, K. Ikeda, A. Tamura, and T. Saito, “On the viscosity and density of the molten FeO-SiO2 system,” Transactions of the Japan Institute of Metals, vol. 19, pp. 264-274 (1978).
S. Hara, K. Irie, D. R. Gaskell, and K. Ogino, “Densities of melts in the iron(II) oxide-iron(III) oxide-calcium oxide and iron(II) oxide-iron(III) oxide-dicalcium silicate (2CaO.SiO2) systems,” Transactions of the Japan Institute of Metals, vol. 29, pp. 977-989 (1988).
A. S. Abrosimov, E. G. Gavrin, and V. I. Eremeichenkov, Izvestiya Vysshikh Uchebnykh Zavedenii, Chernaya Metallurgiya, 1975, pp. 14–17.
S. Sumita, K. Morinaga, and T. Yanagase, “Density and surface tension of binary ferrite melts,” Journal of the Japan Institute of Metals, vol. 47, pp. 127-131 (1983).
A. Adachi and K. Ogino, “The density of molten slags containing ferrous oxide,” Technology Reports of the Osaka University, vol. 12, pp. 147-152 (1962).
D. R. Gaskell, A. McLean, and R. G. Ward, “Density and structure of ternary silicate melts,” Transactions of the Faraday Society, vol. 65, pp. 1498-1508 (1969).
J. Holeczy, L. Bodnar, and K. Tomasek, “Density of an iron-oxygen-silica slag system,” Hutnicke Listy, vol. 27, pp. 364-368 (1972).
X. Mo, I. S. E. Carmichael, M. Rivers, and J. Stebbins, “The partial molar volume of iron oxide in multicomponent silicate liquids and the pressure dependence of oxygen fugacity in magmas,” Mineralogical Magazine, vol. 45, pp. 237-245 (1982).
P. Courtial, E. Ohtani, and D. B. Dingwell, “High-temperature densities of some mantle melts,” Geochimica et Cosmochimica Acta, vol. 61, pp. 3111-3119 (1997).
R. A. Lange and I. S. E. Carmichael, “Densities of Na2O-K2O-MgO-MgO-FeO-Fe2O3-Al3O3-TiO2-SiO2 liquids: New measurements and derived partial molar properties,” Geochimica et Cosmochimica Acta, vol. 51, pp. 2931-2946 (1987).
D. B. Dingwell, M. Brearley, and J. E. Dickinson Jr, “Melt densities in the Na2O-FeO-Fe2O3-SiO2 system and the partial molar volume of tetrahedrally-coordinated ferric iron in silicate melts,” Geochimica et Cosmochimica Acta, vol. 52, pp. 2467-2475 (1988).
Q. Liu and R. A. Lange, “The partial molar volume of Fe2O3 in alkali silicate melts: Evidence for an average Fe3+ coordination number near five,” American Mineralogist, vol. 91, pp. 385-393 (2006).
X. Guo: Ph.D. Thesis, University of Michigan, 2013.
M. Zielinski and B. Sikora, “Surface tension of the calcium oxide-alumina system with additions of silica, iron(III) oxide, calcium fluoride, sodium fluoride, and magnesia,” Prace Instytutu Metalurgii Zelaza im. Stanislawa Staszica, vol. 29, pp. 157-165 (1977).
P. Vadász, M. Havlík, and V. Danêk, “Density and surface tension of calcium-ferritic slags I. The systems CaO-FeO-Fe2O3-SiO2 and CaO-FeO-Fe2O3-Al2O3,” Canadian Metallurgical Quarterly, vol. 39, pp. 143-152 (2000).
B. A. Bryantsev, Zhurnal Prikladnoi Khimii, vol. 41, pp. 2156-2159 (1968).
A. Adachi, K. Ogino, and S. Kuno, “The density of molten slags containing ferrous oxide,” Tetsu to Hagane, vol. 46, pp. 1243-1244 (1960).
T.-F. Wu, A. F. Vishkarev, and V. I. Yavoiskii, “Density of molten steel slags,” Izvestiya Vysshikh Uchebnykh Zavedenii, Chernaya Metallurgiya, vol. 5, pp. 66-75 (1962).
T. Licko, V. Danek, and Z. Panek, “Densities of melts in the system calcium oxide-iron(II) oxide-iron(III) oxide-silicon dioxide,” Chemical Papers, vol. 39, pp. 599-605 (1985).
D. B. Dingwell and M. Brearley, “Melt densities in the CaO-FeO-Fe2O3-SiO2 system and the compositional dependence of the partial molar volume of ferric iron in silicate melts,” Geochimica et Cosmochimica Acta, vol. 52, pp. 2815-2825 (1988).
S. A. Nelson and I. S. E. Carmichael, “Partial molar volumes of oxide components in silicate liquids,” Contributions to Mineralogy and Petrology, vol. 71, pp. 117-124 (1979).
P. Vadász, M. Havlík, and V. Daněk, “Density and surface tension of the systems CaO-FeO-Fe2O3-MgO, CaO-FeO-Fe2O3-ZnO and CaO-Fe2O3-Cu2O,” Central European Journal of Chemistry, vol. 4, pp. 174-193 (2006).
C. W. Thomas: Ph.D. Thesis, California Institute of Technology, 2013.
K. Tomasek, L. Bodnar, and J. Holeczy, “Density of iron-oxygen-silicon dioxide slag systems with lime, alumina, magnesia, or zinc oxide and its relation to surface tension,” Hutnicke Listy, vol. 30, pp. 136-138 (1975).
O. N. Fadeev, I. F. Khudyakov, I. D. Kashcheev, and G. P. Kharitidi, “Study of the density and surface tension of ferrous oxide-ferric oxide-silicon dioxide-aluminum oxide system melts,” Izvestiya Vysshikh Uchebnykh Zavedenii, Tsvetnaya Metallurgiya, vol. 1, pp. 17-20 (1980).
K. C. Mills and B. J. Keene, “Physical properties of BOS slags,” International Materials Reviews, vol. 32, pp. 1-120 (1987).
J. O. M. Bockris, J. W. Tomlinson, and J. L. White, “The structure of the liquid silicates: Partial molar volumes and expansivities,” Transactions of the Faraday Society, vol. 52, pp. 299-310 (1956).
J. W. Tomlinson, M. S. R. Heynes, and J. O. M. Bockris, “The structure of liquid silicates. Part 2.-Molar volumes and expansivities,” Transactions of the Faraday Society, vol. 54, pp. 1822-1833 (1958).
M. Hino, T. Ejima, and M. Kameda, “Surface tension and density of liquid lead silicate,” Journal of the Japan Institute of Metals, vol. 31, pp. 113-119 (1967).
51. H. Ito and T. Yanagase, “Studies on lead silicate melts,” Transactions of the Japan Insitute of Melts, vol. 1, pp. 115-120 (1960).
P. Courtial and D. B. Dingwell, “Nonlinear composition dependence of molar volume of melts in the CaO-Al2O3-SiO2 system,” Geochimica et Cosmochimica Acta, vol. 59, pp. 3685-3695 (1995).
Q. Liu and R. A. Lange, “The partial molar volume and thermal expansivity of TiO2 in alkali silicate melts: Systematic variation with Ti coordination,” Geochimica et Cosmochimica Acta, vol. 65, pp. 2379-2393 (2001).
Y. Bottinga and D. F. Weill, “Densities of liquid silicate systems calculated from partial molar volumes of oxide components,” American Journal of Science, vol. 269, pp. 169-182 (1970).
Y. Bottinga, D. Weill, and P. Richet, “Density calculations for silicate liquids. I. Revised method for aluminosilicate compositions,” Geochimica et Cosmochimica Acta, vol. 46, pp. 909-919 (1982).
Y. Bottinga, P. Richet, and D. F. Weill, “Calculation of the density and thermal expansion coefficient of silicate liquids,” Bulletin de Mineralogie, vol. 106, pp. 129-138 (1983).
Acknowledgments
Financial support from Hyundai Steel, JFE Steel Corporation, Nippon Steel & Sumitomo Metal, Nucor Steel, Posco, RioTinto, RHI, RIST, Tata Steel Europe, Voestalpine, and the Natural Science and Engineering Research Council of Canada (NSERC) is gratefully acknowledged. One of the authors (E.T.) also acknowledges the scholarship from the Fonds de Recherche du Quebec - Nature et Technologies (FRQNT) supporting his master study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 27, 2014.
Rights and permissions
About this article
Cite this article
Thibodeau, E., Gheribi, A.E. & Jung, IH. A Structural Molar Volume Model for Oxide Melts Part III: Fe Oxide-Containing Melts. Metall Mater Trans B 47, 1187–1202 (2016). https://doi.org/10.1007/s11663-015-0549-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0549-x