Abstract
The present study deals with the thermodynamic optimization of the Ca-Fe-O system. All available phase equilibrium and thermodynamic experimental data are critically assessed to obtain a self-consistent set of model parameters for the Gibbs energies of all stoichiometric and solution phases. Model predictions of the present study are compared with previous assessments. Wüstite and lime are described as one monoxide solution with a miscibility gap, using the random mixing Bragg-Williams model. The solubility of CaO in the “Fe3O4” magnetite (spinel) phase is described using the sublattice model based on the Compound Energy Formalism. The effect of CaO on the stability of the spinel phase is evaluated. The liquid CaO-FeO-Fe2O3 slag is modeled using the Modified Quasichemical Formalism. Liquid metal phase is described as a separate solution by an associate model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The CaO-FeO-Fe2O3 portion of the Ca-Fe-O system is one of the essential subsystems for many metallurgical processes, including iron ore sintering, copper-making industry, and iron- and steel-making processes. The thermodynamic description of the solubility of oxygen in the Fe-Ca alloys is important for the deoxidation of steel.
Comprehensive assessments of the Ca-Fe-O system are available in the literature.[1,2] Very good results were obtained; however, some previously existing[3,4] and new experimental data[4–6] were not taken into account in these studies. As a result, there is a discrepancy between model predictions and these experimental data. Furthermore, the oxygen content in the Fe-Ca liquid metal was not assessed. The purpose of the present study is to obtain a thermodynamic database capable of describing all experimental data available up-to-date. In order to achieve that, a thermodynamic optimization of the system is performed. The first step is selecting the appropriate thermodynamic models for all phases in the system. Then, a critical and simultaneous evaluation/assessment of all available thermodynamic and phase diagram data is performed. Finally, the thermodynamic model parameters are optimized in order to obtain one self-consistent set of model equations for the Gibbs energies of all phases, best reproducing the experimental data as functions of temperature and composition.
The list of the stable phases, models, and notations used in the present study is given in Table I.
Thermodynamic properties and phase diagrams can be back-calculated from the model equations. Thermodynamic property data, such as enthalpies and activities, can aid in assessing phase diagrams, and phase diagram measurements can be used to derive the thermodynamic properties. The described procedure is sometimes referred to as Calphad technique.
The present thermodynamic optimization has been performed using the FactSage thermodynamic software package[7] as a part of the development of a self-consistent thermodynamic database for the Al-Ca-Cu-Fe-Mg-Si-O-S multi-components system.
Binary Subsystems
Fe-O
The assessment of the Fe-O system was reported earlier.[8,9] In the present study, the properties of solid phases were adopted from Hidayat et al.[8] and the properties of slag and liquid metal are from Shishin et al.[9] The assessed phase diagram is presented in Figure 1. It may be compared with model predictions of Selleby and Sundman.[2]
Phase diagram of the Fe-O system at total pressure of 1 atm: solid lines are calculated in this study, and dashed lines are calculated using the database of the previous study[2]
Ca-O
The available experimental data on the Ca-O system are very limited. Bevan and Richardson[10] studied the mixtures of Ca and CaO. They observed the full solubility of CaO particles in Ca at 1523 K (1250 °C) up to 20 mol pct CaO. Making a correction for the final size of lime particles, they suggested that the solubility of lime in Ca is 15 to 18 mol pct. The measured eutectic temperature between Ca and CaO was 1106 K to 1109 K (833 °C to 836 °C).[10] In the assessment of Wriedt,[11] lower solubility of CaO in Ca than reported by Bevan and Richardson[10] by was suggested (Figure 2). According to Wriedt,[11] higher solubility would result in a very low eutectic temperature. In the present study, the evaluated phase diagram is similar to the assessment of Wriedt[11] and closer to the experimental points than the model predictions of Selleby and Sundman.[2]
Ca-Fe
The phase diagram of the Ca-Fe system has been assessed by Anglezio et al.[12] Their evaluation was accepted in the present study. The solubility of Ca in the iron-rich phases, bcc, fcc, and liquid, is very small. The solubility of Fe in the Ca-rich phases, fcc, bcc, and liquid, is unknown, but should be also small. The calculated phase diagram near the Fe corner is shown in Figure 3. The model predictions of Selleby and Sundman[2] give practically the same results and are not shown in Figure 3.
Thermodynamic Models
Stoichiometric Compounds
Many solid phases in the present study are treated as stoichiometric compounds (Table I). Their Gibbs energies are independent of composition. The Gibbs energy of formation of a compound from elements in their standard state at a temperature of T (K) and a pressure of 1 atm is given by
where ΔH 298.15° is the standard enthalpy of formation at 298.15 K (25 °C), S 298.15° is the standard entropy at 298.15 K (25 °C), and C P (T) is the molar heat capacity.
Monoxide
Wüstite Fe1−x O has a range of non-stoichiometry towards oxygen[8] and a partial mutual solubility with lime (CaO). This solid solution was modeled as a random mixture of Fe2+, Fe3+, and Ca2+ ions on cation sites with simple polynomial excess Gibbs energy terms. It is assumed that cation vacancies remain associated with Fe3+ ions to comply with the local charge balance condition and thus do not contribute to the configurational entropy. This approach is discussed in detail in Hidayat et al.[8] The Gibbs energy per mole of components FeO, FeO1.5 and CaO is given by
where g ° M is the Gibbs energy of pure component M and X M is the mole fraction. The interaction energy L M,N between components M and N is expanded as a polynomial in the mole fractions of the components:
where q ij M,N are the binary model parameters. No ternary terms were used in the present study. The binary terms are extrapolated into the ternary system using the Kohler method.[13]
Spinel
Ca is soluble in magnetite, Fe3O4, which has a cubic spinel structure.
The model for spinel was developed within the framework of the Compound Energy Formalism (CEF). The structure of spinel may be derived from the fcc close packing of oxygen anions. Cations occupy half of the octahedral interstices and one-eighth of the tetrahedral interstices.[14] It is a common practice to distinguish tetrahedral and octahedral sublattices and represent spinels as \( \left( {{\text{A}}, {\text{B}}, \ldots } \right)^{\text{tetr}} \left[ {{\text{A}}, {\text{B}}, \ldots } \right]^{\text{oct}}_{ 2} {\text{O}}_{ 4}, \)where A, B, etc., are cations with charges +2 and +3, rarely +4 or +1. Oxygen has a charge of −2. The condition of electroneutrality must be always respected.
The Gibbs energy expression in the CEF per formula unit is[15,16]
where Y ′ i and \( {Y_{j}^{\prime\prime}}\) represent the site fractions of constituents i and j on the first and second sublattices, respectively, G ij is the Gibbs energy of a pseudocomponent (i)[ j ]2O4, S config is the configurational entropy,
and g E is the excess Gibbs energy, which was set to zero in the present study.
The non-stoichiometry of spinel towards excess oxygen was modeled in Reference 14 by introducing neutral vacancies (Va) on the octahedral sublattice. Taking vacancies into account, the model for spinel in the Ca-Fe-O system is based on the following formula unit:
The Ca2+ cations were introduced only on the octahedral sites for two reasons. First of all, it is well known that compounds such as CaFe2O4, CaAl2O4, and CaCr2O4 do not have the spinel crystal structures, presumably because relatively large Ca2+ cations cannot be squeezed into small tetrahedral sites. Secondly, the solubility of Ca2+ is found to be much higher in Fe3O4 than in FeAl2O4, which may be explained by the fact that Fe3O4 is an inverse spinel and divalent cations (Fe2+) on the octahedral sites can be replaced by Ca2+; the hercynite (FeAl2O4) is a normal spinel, and Ca2+ should substitute Fe2+ on tetrahedral sites. It is argued that if it was possible for calcium to replace Fe2+ on tetrahedral site, the solubility of Ca2+ in FeAl2O4 would be of the same order as that in Fe3O4.
The introduction of Ca2+ only on the octahedral sites results in only two additional end-members: \( \left[ {\left( {{\text{Fe}}^{3+} } \right)^{\text{tetr}} } \right[{\text{Ca}}^{2+} \left] {_{ 2}^{\text{oct}} {\text{O}}_{ 4}^{-2} } \right]^{-1} \) and \( \left[ {\left( {{\text{Fe}}^{2+} } \right)^{\text{tetr}} } \right[{\text{Ca}}^{2+} \left] {_{ 2}^{\text{oct}} {\text{O}}_{ 4}^{2-} } \right]^{ - 2} \) . The Gibbs energies of these end-members can be obtained from two equations:
where \( {S^{mix}} = - 2R { \ln } 0. 5 \) , and
In this study, the model parameter \( \Delta_{{{\text{Fe}}^{3+} {\text{Fe}}^{2+} : {\text{Fe}}^{2+} {\text{Ca}}^{2+} }} \)is set to zero and the solubility of Ca2+ in spinel can be modeled optimizing only the Gibbs energy of the hypothetical neutral inverse spinel: (Fe3+)tetr[Ca 2+0.5 , Fe 3+0.5 ] oct2 O −24 . The heat capacity of this spinel is approximated to be the same as for calcium ferrite, CaFe2O4, while the enthalpy of formation and entropy are variable parameters.
fcc and bcc
Both iron and calcium can exist in an fcc and bcc structures, but they are only partially miscible in each other. The Bragg–Williams model that assumes random mixing of atoms was used for fcc and bcc solid solutions. The Gibbs energy per mole of atoms is given by
where g ° M is the Gibbs energy of pure component M and X M is the mole fraction. The interaction energy L Ca,Fe between components M and N is expanded as a Redlich–Kister polynomial:
where L iCa,Fe are the binary model parameters that can be temperature-dependent. Contrary to calcium, there exist magnetic transitions in fcc and bcc. The contributions of the magnetic transitions to the thermodynamic functions of the Fe-Ca solid alloys were described using the method proposed by Hillert and Jarl.[17] Critical temperature, T C, and magnetic constant, β, were interpolated linearly between values for Fe and zero for calcium.
Liquid Oxide (Slag)
The liquid oxide phase is an ionic melt where metal cations are predominantly surrounded by oxygen anions. In other words, there is almost full first-nearest-neighbor (FNN) short-range ordering (SRO) between cations and anions. Furthermore, there is a strong tendency for second-nearest-neighbor SRO of some cations in melts. In particular, basic cations such as Ca2+ form strong second-nearest-neighbor (SNN) pairs with acidic cations such as Si+4. The effects of both FNN and SNN SRO are described by the Modified Quasichemical Model (MQM).[18,19] Even though in the CaO-FeO-Fe2O3 slag, SNN ordering is not very strong, the present model is used with a vision to develop a broader database for calculations in multicomponent systems, including SiO2.
To explain the meaning of the parameters, let us consider the binary system Fe2O3-CaO as an example. The most significant parameter of the MQM model is the Gibbs energy of the corresponding pair exchange reaction:
where (Ca2+-Fe3+) represents a second-nearest-neighbor pair. The molar Gibbs energy of the solution is given by[19]
where X i and g ° i are the mole fractions and molar Gibbs energies of the components and \( X_{{{\text{Fe}}^{3+} {\text{Ca}}^{2+} }} \) is the mole fraction of Ca2+-Fe3+ pairs at equilibrium. ΔS config is the configuration entropy, the expression for which is given elsewhere.[18,19]
Δg AB can be expanded as an empirical polynomial in terms of equivalent component fractions
where Δg °AB and q ijAB are empirical binary coefficients which may be functions of temperature and pressure. Y m are “coordination-equivalent” fractions, which are defined as
where n m and Z m are the number of moles and the coordination number of the component m, respectively. The composition of maximum short-range ordering is determined by the ratio of the coordination numbers, which was explained in detail earlier.[18]
A symmetric Kohler model was previously used[20] for the interpolation of CaO-FeO, FeO-FeO1.5, and CaO-FeO1.5 binary parameters into the ternary system. In the present study, the Kohler/Toop extrapolation[19] with FeO1.5 as an “asymmetric component” is selected to achieve a better description of the experimental data in the CaO-FeO-Fe2O3 system. This is equivalent to treating FeO1.5 as an “amphoteric” component, as opposed to CaO and FeO which are “basic” components.
Ternary parameters are also used in the present study to better describe the experimental data. The ternary parameters, q ijk A,B(C) , are the effects of the presence of the third component C upon binary interactions between components A and B. The formulae are given elsewhere.[19]
Liquid Metal
The model for the liquid metal assumes random mixing of components and associates on one sublattice:
The molar Gibbs energy is given by
where X i are the molar fractions of components and g ° i are the standard Gibbs energies of pure components. The molar excess Gibbs energy of a binary system with components A and B is expressed as
where α AB parameter may be expanded in a Redlich–Kister form:
The interpolation of binary terms into ternary subsystems is done using the Muggianu method.[13]
Assessment of Experimental Data
Thermodynamic Properties of Stoichiometric Compounds
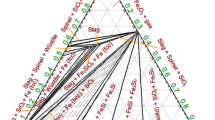
Figures 4 and 5 show the compositions of ternary compounds in the Ca-Fe-O system: Ca2Fe2O5 (C2F), CaFe2O4 (CF), CaFe3O5 (CWF), CaFe5O7 (CW3F), and Ca4Fe9O17 (C4WF4). The corresponding three-phase equilibria are calculated.
To fix the thermodynamic properties of these stoichiometric compounds, the heat capacity, C P (T), entropy, S °298.15 , and enthalpy of formation, ΔH °298.15 , must be established.
For CaFe2O4 (CF), the low temperature C P (T) was measured by King[21] from 52.3 K to 298.16 K (−220.7 °C to 25 °C) using the adiabatic calorimetry. He extrapolated the heat capacity to 0 K (−273 °C) and calculated the entropy at 298.15 K (25 °C) using the third law to be S °298.15 = 145.2 ± 0.8 J mol−1 K−1. Rajagopalan et al.[22] used a laser flash method to measure the heat capacity of CaFe2O4 from 297 K to 1091 K (24 °C to 818 °C). The obtained values were significantly higher than the sum of heat capacities of pure oxides and were not in agreement with the heat content data available from Bonnickson.[23] The latter measured the heat content of calcium ferrite samples over the temperature range from 411 K to 1805 K (138 °C to 1532 °C). His data are shown in Figure 6.
No direct measurements of the enthalpy of formation are available. The Gibbs energy of formation of CaFe2O4 from oxides was measured by Rezukhina and Bagin’ska,[24] by Jacob et al.[25] and by Forsberg et al.[26] All authors used the EMF cell:
to measure the Gibbs energy of the reaction
The results are summarized in Figure 7. The data of Rezukhina and Bagin’ska[24] and Forsberg et al.[26] are in agreement but differ from that of Jacob et al.[25]
For Ca2Fe2O5 (C2F), the low temperature C P (T) was measured by King[21] from 53.3 K to 298.16 K (−219.7 °C to 25 °C) using adiabatic calorimetry. The entropy at 298.15 K (25 °C) was calculated to be S °298.15 = 188.7 ± 1.3 J mol−1 K−1. Rajagopalan et al.[22] used a laser flash method to measure the heat capacity of Ca2Fe2O5 over the temperature range from 296 K to 1090 K (23 °C to 817 °C). The obtained results are in agreement with the sum of heat capacities of oxides and with the heat content data of Bonnickson,[23] who measured the heat content of C2F from 376.5 K to 1838 K (103.5 °C to 1565 °C). His data are shown in Figure 8.
Rezukhina and Bagin’ska,[24] Jacob et al.[25], and Forsberg et al.[26] measured the EMF of the following cell:
which corresponded to the Gibbs energy of the reaction.
The results are plotted in Figure 7. Again, there exists a discrepancy between Jacob et al.[25] and the other two studies.[24,26]
No heat capacity and entropy measurements are available for C4WF4, CWF, and CW3F.
Rosen and Saitton[4] and Li and Guo[5] determined the oxygen partial pressure for the CF + C2F + C4WF4 three-phase equilibrium using the EMF technique with calcia-stabilized zirconia as a solid electrolyte. In the former article,[4] pairs Ni-NiO or Cu-Cu2O were employed as reference electrodes, while in the latter study,[5] the flow of air was used as a reference electrode. The results of Rosen and Saitton[4] and of Li and Guo[5] are plotted in Figure 9. There is a large discrepancy between two sets of data. The probable reasons are non-achievement of the target equilibrium and difficulty in maintaining inert condition of the cell. In a view of this discrepancy, the authors repeated the experiment of Rosen and Saitton[4]. The details are given in Aspiala et al.[6] and the results are plotted in Figure 9(b).
Reeve and Gregory[27] equilibrated mixtures of CaO and Fe2O3 with the flow of CO/CO2 gas of controlled composition in Pt crucibles. After the equilibration, samples were quenched and analyzed for Fe2+, Fe3+, and Ca2+ using titration. Phases were identified by X-ray diffraction. The oxygen partial pressure over the Sp + C2F + CWF and Sp + Mono1 + CWF three-phase regions was determined as intermediate between two neighboring 2-phase regions. The experimental points are plotted in Figure 9. Reeve and Gregory[27] observed the C2F + CWF + Mono1. Later, it was confirmed by Bergman and Song[28] that CW3F is stable up to its melting point (see Section IV–G), and it is C2F + CW3F + Mono1 phase assemblage that should be stable at the temperatures of the experiment of Reeve and Gregory[27]. The reported oxygen potential over this three-phase region is shown in Figure 9.
Chung et al.[29] summarizes the results of previous measurements[30,31] on the reduction of calcium ferrite by the CO/CO2 gas mixtures. The following EMF cell was used:
The reference electrode was either air or a CO/CO2 mixture. When the CO/CO2 mixture was used, its composition was adjusted until zero EMF was reached. The studied three-phase mixtures were Mono2 + C2F + Fe, Mono1 + C2F + Fe, CWF + C2F + CW3F, and Mono1 + C2F + CW3F. The results are given in Figure 9.
Tare and Deo[32] studied mixtures of lime and wüstite of several compositions using the EMF method with calcia-stabilized zirconia as a solid electrolyte and Fe/FeO reference electrode. At low temperatures, lime and wüstite reacted to form three-phase fields: either Mono1 + CF2 + Fe or Mono1 + CW3F + Fe. Bjorkman[33] used a similar method to study the equilibrium P(O2) for three-phase equilibria Mono2 + C2F + Fe, Mono1 + Mono2 + Fe, Mono1 + C2F + Fe, and Mono1 + CW3F + Fe. The results are given in Figure 9. Two-phase equilibria Mono1 + Fe and Mono2 + Fe were also studied and are to be discussed in Section IV–C.
The experimental data of Burdese and Brisi,[34] Lykasov and Kozheurova,[35], Malquori and Cirilli[36], and Perrot[37] were assessed previously by Hillert et al.[1] They are plotted in Figure 9.
The experimental data on the oxygen potential for the three-phase equilibria fix the properties of CWF and CW3F rather well.
The properties of C4WF4 remain uncertain, even with the results of the recent experiments[4–6] (Figure 9(b)). According to the evaluation of Selleby and Sundman,[2] C4WF4 is rather stable and exists over a wide range of temperature and P(O2). However, the calculated three-phase equilibrium Sp + CWF + C4WF4 is not in agreement with the observations of Reeve and Gregory,[27] who reports an Sp + C2F + CWF equilibrium instead at 1273 K (1000 °C) and oxygen potential −265 ± 3 kJ mol−1 (Figure 9(a)). There is also an evidence of existence of C4WF4 phase in equilibrium with the Cu2O-FeO-Fe2O3-CaO slag and liquid copper at 1373 K (1100 °C), as reported by Nikolic et al.[38] This fact will be used in the present study.
Very little information is available for the other two ternary compounds, CaFe4O7 (CF2) and Ca4Fe17O29 (C4WF8). The ranges of stability of CF2 and C4WF8 will be discussed in Section IV–B–3 and Section V, respectively.
Phase Diagrams
FeO-CaO in equilibrium with iron
There are numerous articles devoted to the experimental determination of the “FeO-CaO phase diagram at iron saturation.” In order to proceed further, it is worth discussing different ways of representing the results of these experiments, since it is often a source of confusion in assessments of oxide systems. The portion of the phase diagram of the Ca-Fe-O system at 1473 K (1200 °C) is shown in Figure 10. It can be seen from the figure that the compositions of the Mono1 and liquid slag phases in equilibrium with iron are shifted from the FeO-CaO section towards higher oxygen content. In most experiments, the Ca/Fe ratio in these phases was measured, but the exact oxygen content was not.
To eliminate the uncertainty in the oxygen content, it is possible to project the compositions of these phases onto the FeO-CaO section through the oxygen corner as shown in Figure 10 by the magenta lines. This geometric operation preserves the metal ratio Ca/(Ca + Fe). For instance, if the EPMA technique is used, only the ratio of metals can be measured reliably. The precise measurements of the oxygen are very difficult due to the high absorption of its characteristic lines. Therefore, the projection through the oxygen corner is a reasonable choice to represent the results of EPMA studies. The same is true for wet chemical analytical techniques, in case when only total iron and total calcium contents are measured.
Only when special efforts are made to measure the Fe2+ and Fe3+ content of the phase after the equilibration, it is possible to represent the composition of the phase in the basis of FeO, Fe2O3, and CaO. In this case, the composition of the phase may be projected either through the oxygen corner or through the iron corner. The latter operation is shown by the green lines in Figure 10. The reason why it is desirable sometimes to make the projection through the iron corner is that it corresponds to the standard calculation procedure of phase diagrams in software packages as FactSage or Thermocalc. The example of such diagram is shown in Figure 11 using black lines. The diagram in Figure 11 represents the FeO-CaO section of the ternary system, showing what phases are stable at a particular composition on the FeO-CaO join at a given temperature. At the same time, it shows the projection of the compositions of these phases at equilibrium onto the FeO-CaO section through the iron corner. Indeed, if one refers back to Figure 10 and trace the boundary between Mono1 + fcc(Fe) + L(Slag) and L(Slag) + fcc(Fe) phase fields, he will see that it connects the composition of the slag with almost pure iron, and the intersection with the FeO-CaO section gives the signal on the diagram in Figure 11. The results of the experiment, where phase boundaries are measured as functions of composition on the FeO-CaO section, can be compared with the projection through the iron corner. This statement is true for phase diagrams at metal saturation in other chemical systems, as soon as the metal of saturation does not dissolve much of the system components. The experimental techniques such as DTA and equilibration/quenching technique produce results which should be compared with projections through the iron corner if the overall composition is on the section. To prevent the confusion, calculations are made for both types of projections, through iron corner and through oxygen corner. They are shown in Figure 11.
FeO-CaO section of the Ca-Fe-O phase diagram: experimental points[39–50] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines. Black lines show phase boundaries; red lines show the calculated compositions of the liquid slag, Mono1 and Mono2 phases projected to the FeO-CaO section through the oxygen corner
Oelsen and Maetz[40] conducted the DTA of mixtures of wüstite and lime in iron crucible. The composition of wüstite is not provided by the authors as well as procedure to recalculate the compositions to FeO-CaO section. Presumably, the correction on the non-stoichiometry of wüstite has been made. In this case, the position of the experimental points on the FeO-CaO section corresponds to the projection through the iron corner (Figure 11).
Abbattista et al.[41] prepared samples from CaCO3, Fe2O3, and excess Fe. Samples were equilibrated in Pt crucibles under the flow of helium at temperatures 1123 K to 1403 K (850 °C to 1130 °C). After the equilibration, they were quenched in water with ice, and studied by XRD. The authors observed phase transitions at 1318 ± 5 K (1042 °C) and at 1403 ± 5 K (1130 °C), which corresponded to the decomposition of C2F and to the formation of liquid, respectively. The compositions of Mono1 and Mono2 were determined from XRD using the Vegard’s law. They are plotted in Figure 11 and correspond to the projection through the iron corner.
Zhao et al.[42] equilibrated samples of lime and wüstite in iron crucibles under the flow of argon at 1173 K to 1473 K (900 °C to 1200 °C). After the equilibration, samples were quenched and analyzed using EPMA equipped with WDS detector. The resulting points are plotted in Figure 11. They were projected through the oxygen corner.
Larson and Chipman[43] equilibrated the slag samples with air or CO/CO2 mixtures in Pt crucibles at 1823 K (1550 °C). After the equilibration, samples were quenched in liquid mercury and analyzed for total Fe, Fe2+, and Fe3+. The content of CaO was determined by difference. The break in the Fe3+/(Fe3+ + Fe2+) vs CaO/(CaO + FeO + Fe2O3) line for a given P(O2) indicated the saturation with the solid phase. A single experimental point corresponding to saturation with iron and lime was projected through the oxygen corner and shown in Figure 11. The Fe3+ content in this point is plotted in Figure 12.
The Fe3+ content in slag saturated with iron and solid oxide phases at liquidus temperatures: experimental points[39,43,44,48–50] and calculated lines. The calculations of this study are shown with a solid line, and the calculations of Selleby and Sundman[2] are shown with a dashed line. X-axis represents the projection of the liquid slag composition onto the FeO-CaO section through the oxygen corner
Allen and Snow[44] prepared the samples from ferrous oxalate and calcium carbonate. The mixtures were equilibrated in iron crucibles under N2 atmosphere. After the equilibration, the samples were quenched in a water-isopropanol solution. The first portion of samples was analyzed for phase composition by optical microscopy and XRD. These results are shown with black symbols. The second portion of samples was re-equilibrated in a crucible at temperature, closer to the liquidus. After quenching and crushing, free iron particles were removed with a magnet. The remaining quenched liquid was analyzed for total iron and Fe2+ and CaO by wet chemical methods. The composition is represented in the basis of (FeO, Fe2O3, CaO). The projection of the liquid slag composition through the oxygen corner is plotted in Figure 11 using red symbols. The measured Fe3+ content as a function of slag composition at liquidus temperatures is shown in Figure 12.
Fisher and Fleischer[45] equilibrated FeO-CaO-MnO slag and liquid iron samples in lime crucible in air. Since the amount of liquid iron was large, around 500 g, it was not oxidized during the experiment. However, the fact that the reducing atmosphere was not created adds uncertainty on whether the equilibrium between slag, liquid iron, and lime was really established. After the equilibration, samples of iron and slag were taken with a quartz tube. The slag was analyzed for total iron, manganese, and calcium. The extrapolation of the compositions to manganese-free slag, projected through the oxygen corner, is plotted in Figure 11. The Fe3+ content at this point is plotted in Figure 12.
Görl et al.[46] equilibrated the mixtures of CaO-FeO-SiO2 slags and iron and small quantities of sulfur in lime crucibles at 1823 K, 1873 K, and 1923 K (1550 °C, 1600 °C, and 1650 °C). The quenched slags were analyzed for total iron, Fe3+, silica, and sulfur. The sulfur content was always <1 wt pct. For low-SiO2 slag, the Fe3+ content was not measured. Figure 11 shows the extrapolation of slag compositions to the SiO2-free system. They were projected through the oxygen corner.
Takeda et al.[39] equilibrated the mixtures of CaO, Fe2O3, and Fe in iron or Pt crucibles under a controlled atmosphere of CO/CO2 at 1473 K and 1573 K (1200 °C and 1300 °C). After the equilibration, samples were quenched and crushed. Iron particles were removed with a magnet. The top and bottom parts of the samples were analyzed individually for total iron, Fe3+, and Ca2+. The difference in the measured metal contents between the top part, the bottom part, and the original charge indicated the precipitation of the solid phase. The exact composition of liquid in equilibrium with solid was obtained by extrapolating the compositional difference towards zero. The compositions of liquid in saturation with iron and either wüstite or lime are shown in Figure 11. The experimental points were projected through the oxygen corner. The ferrous/ferric ratio in this liquid is plotted in Figure 12.
Obst et al.[47] conducted an EPMA study of CaO-FeO slags in saturation with iron and lime. Samples were equilibrated in iron crucibles below 1723 K (1450 °C) or in lime crucibles above 1723 K (1450 °C). In the latter case, an excess iron was added. The quenched samples were analyzed by optical microscopy and EPMA with WDS detector. Thus, metal ratio in slag and Mono2 phases was obtained. The experimental points are plotted in Figure 11. They correspond to the projections of liquid and Mono2 compositions projected through the oxygen corner.
Timucin and Morris[48] conducted an experiment similar to Larson and Chipman.[43] The slags were prepared from CaCO3 and Fe2O3 and equilibrated with the flow of CO/CO2 or O2/N2 gas in Pt or Pt-Rh crucibles at 1723 K and 1823 K (1450 °C and 1550 °C). After the equilibration, samples were quenched in isopropyl alcohol and studied by optical microscopy. Optical microscopy showed whether the sample composition lays in the single-phase region or liquid + solid region. A single-phase liquid was analyzed for Fe2+, total iron, and Ca2+. At 1723 K (1450 °C), experiment with iron crucible was conducted to determine the position of double saturation with iron and Mono2. The composition of liquid at double saturation, projected through the oxygen corner, is shown in Figure 11. The Fe3+ content at this point is plotted in Figure 12.
Hara et al.[49] equilibrated mixtures of hematite and iron in lime crucibles for 4 hours at temperatures from 1623 K to 1873 K (from 1350 °C to 1600 °C). Quenched samples were analyzed by EPMA with the EDS detector. There may be uncertainty in the data since EDS detector is known only suitable for qualitative measurement. Results are shown in Figure 11. The points were projected through the oxygen corner. In another series of experiments, a mixture of hematite and lime was equilibrated under the flow of CO2/H2 in Pt-Rh crucibles. The oxidation state of iron was determined from the weight loss measured by a thermobalance. The Fe3+ content of slag, saturated with lime and iron, is obtained by the extrapolation and plotted in Figure 12.
Scheel[50] heated the FeO and CaO in lime crucibles. Three different gas mixtures were used: the flow of unspecified neutral gas, the CO/CO2 mixture with P(O2) = 10−5, and the flow of air. In the first case, an excess of metallic iron was added to the crucible. After the equilibration, samples were quenched, and the slag and lime (Mono2) phases were separated using the undisclosed method. It is not reported how the excess iron was eliminated from the slag. The slag was analyzed chemically for total iron, Fe3+, and Ca2+, and the Mono2 phase was analyzed for total iron. Selected samples were studied using the EPMA. The type of detector is not reported. The results for neutral gas and iron saturation are plotted in Figures 11 and 12. The results for P(O2) = 0.21 atm are discussed in Section IV–B–3. The results for P(O2) = 10−5 atm were taken into account in the assessment, but they are not plotted.
The comparison of experimental data in Figure 11 shows that there is a significant discrepancy in the composition of liquid slag and Mono2 (lime), particularly for L(Slag) + Mono2 + Fe equilibrium.
Isothermal Sections
Figures 13 through 19 show the CaO-FeO-Fe2O3 portions of the isothermal sections of the Ca-Fe-O phase diagram at temperatures 1473 to 1873 K (1200 °C to 1600 °C). Along with the phase boundaries, the oxygen isobars are calculated and compared with the available experimental data.
The CaO-FeO-Fe2O3 portion of the isothermal section of the Ca-Fe-O phase diagram at 1473 K (1200 °C) and total pressure 1 atm: experimental points[39] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines
The CaO-FeO-Fe2O3 portion of the isothermal section of the Ca-Fe-O phase diagram at 1573 K (1300 °C) and total pressure 1 atm: experimental points[39] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines
The CaO-FeO-Fe2O3 portion of the isothermal section of the Ca-Fe-O phase diagram at 1673 K (1400 °C) and total pressure 1 atm: experimental points[51,52] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines
The CaO-FeO-Fe2O3 portion of the isothermal section of the Ca-Fe-O phase diagram at 1723 K (1450 °C) and total pressure 1 atm: experimental points[48] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines
The CaO-FeO-Fe2O3 portion of the isothermal section of the Ca-Fe-O phase diagram at 1773 K (1500 °C) and total pressure 1 atm: experimental points[49] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines
The CaO-FeO-Fe2O3 portion of the isothermal section of the Ca-Fe-O phase diagram at 1823 K (1550 °C) and total pressure 1 atm: experimental points[43,48] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines
The CaO-FeO-Fe2O3 portion of the isothermal section of the Ca-Fe-O phase diagram at 1873 K (1600 °C) and total pressure 1 atm: experimental points[52,53] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines
The experiments of Takeda et al.[39] (Figures 13, 14), Larson and Chipman[43] (Figure 18), Timucin and Morris[48] (Figures 16, 18), and Hara et al.[49] (Figure 17) were described earlier in Section IV–B–1.
Ban-Ya et al.[51] equilibrated slags with the H2O/H2 gas mixture in iron crucibles. After the equilibration, samples were quenched, and the slag was separated and analyzed for total iron, Fe2+, and Ca2+. The composition of slag is plotted in Figure 15.
White[52] measured the composition of slag in equilibrium with N2/O2 gas mixtures in Pt crucible. A high-temperature balance was used to measure the loss of oxygen from the initial Fe2O3-CaO mixture, which gave the number of moles of FeO. The results are plotted in Figures 15 and 19.
Gurry and Darken[53] equilibrated mixtures of Fe2O3 and CaO with O2 gas or CO2 gas in Pt crucibles. The quenched samples were analyzed for total iron, Fe2+, and Ca2+. The composition of slag is plotted in Figure 19.
It is not possible to directly compare the results of different authors. They obtained the isobars for different partial pressures of oxygen and at different temperatures. The indirect comparison is possible using thermodynamic calculations.
Fe2O3-CaO at High P(O2)
Figures 20 and 21 show the phase diagrams at fixed oxygen partial pressures, 0.21 and 1 atm, respectively. When the oxygen partial pressure is fixed, the projections of phase compositions through the oxygen corner onto a given section coincide with the phase boundaries on this section. Only the metal ratio in the bulk composition is important. This means that it is easier to interpret the experimental results and compare them with calculations than it was in the case of phase the diagrams at metal saturation. It is only necessary to choose a section on which the phase compositions will be projected. It is possible to use the Fe2O3-CaO section, the FeO-CaO section, or even the Fe-Ca section. Traditionally, for the phase diagrams at P(O2) = 0.21 and 1 atm, the Fe2O3-CaO section is selected. The choice of the Fe2O3-CaO section does not mean that the equilibrium amounts of FeO in the phases under these conditions are negligible. The oxygen isobars in Figures 14 through 19 show that the slag contains a substantial amount of FeO even at high P(O2).
Phillips and Muan[54] melted the mixtures of Fe2O3 and CaO in Pt crucibles in air or in oxygen at several temperatures. The samples were quenched and studied by XRD and optical microscopy. In some cases, when it was hard to distinguish the quenched crystals from primary crystals, high-temperature XRD was used. A portion of the quenched samples was crushed and analyzed for Fe2+. The total iron content was determined only for several samples to confirm that it corresponded to the initial amount of Fe2O3. Based on the quenching experiments, Phillips and Muan[54] suggested the phase boundaries which are plotted in Figures 20 and 21.
The experiments of Scheel[50] were described earlier. The data points corresponding to P(O2) = 0.21 atm are plotted in Figure 20. It should be noted that the original diagram presented by Scheel[50] is contradictory. The X-axis is reported to be the mass ratio CaO/(CaO + FeOn), but the composition of the C2F phase is plotted at 0.41, which is consistent with the projection onto the CaO-Fe2O3 section and X-axis being the mass ratio CaO/(CaO + Fe2O3). This letter interpretation was adopted in the present study and was used to plot the experimental results.
Hara et al.[49] equilibrated mixtures of hematite in lime crucibles for 4 hours at temperatures from 1623 K to 1873 K (1350 °C to 1600 °C). The quenched samples were analyzed by EPMA with an EDS detector. The results are shown in Figure 20.
The experiments of Timucin and Morris[48] were described earlier in Section IV–B–1. The optical microscopy observations of the quenched samples indicated the position of the Mono2 liquidus as shown in Figure 21.
Phase relations involving the CF2 phase are the only source of data available to fix its thermodynamic properties. Phillips and Muan[54] suggested that it is stable between 1428 K and 1499 K (1155 °C and 1226 °C) in air and between 1445 K and 1501 K (1172 °C and 1228 °C) at P(O2) = 1 atm. Edsröm[55] studied the stability region of CF2 at P(O2) = 1 atm by a sintering/quenching/XRD method and found it to be 1393 K to 1501 K (1120 °C to 1228 °C). Phillips and Muan[54] cited the results of Batti,[56] who suggested the stability range of CF2 in air to be 1403 K to 1503 K (1130 °C to 1230 °C). Liu[57] reports CF2 to be stable in air up to 1503 K (1203 °C). Although all the authors agree on the upper temperature of the stability region within 5 K (−268 °C), there is a large disagreement on the lower temperature. The decomposition of CF2 into Fe2O3 and CF is a sluggish solid-state reaction, which is difficult to study. In the present work, more weight was given to the results of Phillips and Muan,[54] who approached this reaction from both low- and high-temperature sides and equilibrated samples for up to 24 hours before quenching or used high-temperature XRD.
Monoxide
Tare and Deo[32] and Bjorkman[33] conducted an EMF study of mixtures of wüstite and lime. Both studies used calcia-stabilized zirconia as a solid electrolyte and a Fe/FeO reference electrode. Tare and Deo[32] reported the composition of wüstite to be FeO1.06. Bjorkman[33] did not specify the composition of wüstite and reported the composition of the Mono1 phase in terms of “X(FetO)”. This representation is somewhat ambiguous and does not allow to establish the precise composition of the monoxide phase. However, the introduced uncertainty is small, less than 0.005 in molar ratio Ca/(Ca + Fe). In the present study, the EMF values at 1273 K, 1353 K, and 1373 K (1000 °C, 1080 °C, and 1100 °C) were obtained by linear regression of original data. They were recalculated to P(O2) and plotted in Figure 22.
Johnson and Muan[58] equilibrated mixtures of wüstite, lime, and silica in Pt crucibles with the flow of CO/CO2 at 1353 K (1080 °C). After the equilibration, samples were quenched and analyzed by X-ray and optical microscopy. The CO/CO2 ratio was varied until the precipitation of Fe was observed by optical microscopy. The bulk composition was located in the Mono2 + Ca2SiO4 + Fe and Mono1 + Ca2SiO4 + Fe regions. The oxygen partial pressure was calculated from the equilibrium CO/CO2 ratio. In the present study, the reported activities were used to obtain P(O2) for the Fe + monoxide region. It is shown in Figure 22.
Abbattista et al.[41] reported the composition of CO/CO2 mixtures in equilibrium with Mono1 + Fe. The details of the experiment are not provided. The results are plotted in Figure 22.
Slag
The equilibrium partial pressure of oxygen for slags saturated with iron was studied by Takeda et al.[39], Ban-Ya et al.[51], and Iwase et al.[59]
The experiments of Takeda et al.[39] were described in Section IV–B–1, and the study of Ban-Ya et al.[51] was previously mentioned in Section IV–B–2. Their results are plotted in Figure 23.
Equilibrium oxygen partial pressure over L(slag) + iron: experimental points and calculated lines. Solid lines are the calculations of the present study, and dashed and dotted lines are the calculations of Selleby and Sundman[2]
Iwase et al.[59] measured the EMF of the galvanic cell:
The measurements were performed at 1673 K (1400 °C). In the present study, the EMF values were recalculated to P(O2) using the first Ca-free point as a reference value. The results are shown in Figure 23.
Ban-Ya et al.[3] measured the heat of dissolution of solid CaO in liquid Fe-O slag placed in an iron crucible at 1693 K (1420 °C). The isoperibol calorimeter of the isothermal jacket type was used. After the heat effect was measured, the slag was quenched and analyzed for the total iron and calcium content. Unfortunately, the authors do not provide the primary data. Instead, they give the enthalpy of mixing between liquid Fe-O slag and liquid CaO. In order to obtain the latter values from the primary data, it is necessary to know the enthalpy of transition of solid CaO into liquid CaO at the temperature of the experiment [1693 K (1420 °C)]. The authors cited the work of Chang and Howald,[60] who gave the enthalpy of fusion as 59.05 kJ mol−1 at 3173 K (2900 °C) and gave the heat capacity functions of liquid and solid CaO. Based on the data of Chang and Howald,[60] the primary data of Ban-Ya et al.[3] were back-calculated in the present study (see Figure 24).
Solubility of Calcium in Spinel
Berggren[61,62] equilibrated small amounts of calcium oxalate with iron oxalate (or hydroxide) in Pt crucibles with a flow of CO/CO2 gas for temperatures ranging between 1245 K and 1524 K (972 °C and 1251 °C). The Sp/Sp + CWF and Sp/Sp + slag phase boundaries were determined by selecting the oxygen partial pressure in such a way that the overall composition was located either in the single-phase region or in the corresponding two-phase region. The quenched samples were analyzed chemically for the overall CaO content and by XRD. The calcium content of spinel was obtained from the measured lattice parameters using the Vegards’s law. The experimental results are shown in Figure 25. As can be deduced from Figures 5 and 13, these points should be located below the Sp + C2F + CWF and above the Sp + CWF (or Slag) + Mono1 line in Figure 25.
Liu[57] used equilibration in Pt crucibles under the flow of Ar, followed by quenching and EPMA analysis. The experiments were conducted in the temperature range from 1323 K to 1473 K (1050 °C to 1200 °C), and the overall composition corresponded to the Sp + CF + C4WF4, Sp + C2F + C4WF4 or Sp + C2F + CWF field. The presence of the C4WF4 phase from 1323 K to 1473 K (1050 °C to 1200 °C) was confirmed. The measured calcium content is plotted in Figure 25.
Solubility of Oxygen in Liquid Metal
Ca behaves as an extremely strong deoxidant for steels. In the Ca-Fe-O system, the addition of small quantities of Ca to the Fe-O liquid metal causes, at first, formation of the slag phase (Figure 26). Adding more Ca results in precipitation of solid lime and a drop of oxygen content by several orders of magnitude. This effect was previously assessed by Jung et al.[63] The results of their evaluation along with the selected experimental data[64–69] are presented in Figure 27. Jung et al.[63] introduced CaO associates in the liquid metal phase in order to reproduce the experimental data. Even though the database of Selleby and Sundman[2] predicts the drop in the oxygen content with the addition of Ca to the liquid metal, it does not reproduce the experimental data quantitatively, as can be seen from Figure 27.
Phase diagram of the Ca-Fe-O system at 1873 K (1600 °C). Thick lines are calculated in the present study, and thin lines are calculated using the database from.[2] The formation of the gaseous Ca is suppressed
Liquidus Projection
The liquidus in the high-P(O2) region of the CaO-FeO-Fe2O3 phase diagram was studied by Phillips and Muan.[70] They used equilibration in sealed Pt-Rh crucibles followed by quenching and reflective optical microscopy analysis. Solid phases were identified by powder X-ray diffraction. Selected samples were analyzed chemically for the FeO content before and after the experiment to confirm that no change in the composition occurred. Based on the experimental results, the primary phase fields of spinel, Fe2O3, C2F, C4WF4, C4WF8, CF, and CF2 were constructed and invariant temperatures were suggested. These data are shown in Figure 28 and Table II.
Liquidus projection of the CaO-FeO-Fe2O3 portion of the Ca-Fe-O phase diagram up to 2000 K (1727 °C). The formation of gas phase is suppressed. Experimental points[54,70] and calculated lines. The calculations of the present study are shown by the solid lines, and the calculations of Selleby and Sundman[2] are shown by the dashed lines
The melting of CW3F phase was studied by Bergman and Song.[28] They sintered the mixture of CaO, Fe2O3, and Fe in sealed Pt crucible. The quenched samples were analyzed by the SEM. It was discovered that the CW3F phase does not decompose into solid phases before melting. The fine-structure regions were observed in samples prepared at 1373 K (1100 °C). These regions were attributed to the formation of the liquid phase. The corresponding invariant points involving CW3F and liquid slag are shown in Table II.
Optimization of Model Parameters
The optimization procedure was as follows. First of all, the heat capacity, entropy, and enthalpy of formation of CF and C2F were fixed using the data on heat content (Figures 6, 8), low temperature heat capacity data of King[21], and EMF data from Figure 7. In the latter case, more weight was given to the data of Jacob et al.[25]
The properties of wüstite-lime (monoxide) solid solution were established using the data in Figures 9a, 11, and 22. For the mutual solubility, the preference was given to the new data of Zhao et al.[42], which were obtained using the EPMA technique. Two excess parameters, q 11CaO,FeO and q 21CaO,FeO , were optimized.
The heat capacities of CWF and CW3F were obtained by the increment method. The experimental data in Figure 9(a) were used to fix the entropy and enthalpy of formation of these compounds.
The solubility of Ca in spinel was established from the data in Figure 25. The enthalpy of formation and entropy of the hypothetical neutral inverse spinel (Fe3+)tetr[Ca 2+0.5 , Fe 3+0.5 ] oct2 O -24 were optimized, while the heat capacity was assumed to be the same as for CaFe2O4. As can be seen from Figure 25, the optimized solubility of Ca in spinel is in agreement with the recent data of Liu[57] and the earlier data of Berggren[61,62] within the experimental accuracy. It is higher than obtained in the assessment of Selleby and Sundman.[2]
The heat capacity of C4WF4 was approximated by the increment method. The oxygen partial pressure over CF + C2F + C4WF4 data was used to set the enthalpy of formation and entropy. More weight was given to the data of Aspiala et al.[6] and Li and Guo[5] (Figure 9b). More positive enthalpy of formation could describe the data of Rosen and Saitton,[4] but as a result, C4WF4 would be unstable in equilibrium with the Cu2O-FeO-Fe2O3-CaO slag and liquid copper at 1373 K (1100 °C), which contradicts the observations of Nikolic et al.[38] More negative enthalpy of formation compromises the Sp + CF + C4WF4 equilibrium at 1323 K (1050 °C) as reported by Liu[57] (Figure 25).
Optimizing the slag parameters was a challenge. The presence of iron in two oxidation states makes it necessary to adjust the FeO-CaO, Fe2O3-CaO, and CaO-FeO-Fe2O3 interactions simultaneously. However, the phase diagrams in air and in oxygen (Figures 20 and 21) are mostly defined by the Fe2O3-CaO parameters. Thus, in the first iteration, these phase diagrams were described using the \( \Delta g_{{{\text{Ca}}^{2+}, {\text{Fe}}^{3+} }}^{\circ} \), \( g_{{{\text{Ca}}^{2+}, {\text{Fe}}^{3+} }}^{01} \) and \( g_{{{\text{Ca}}^{2+} , {\text{Fe}}^{3+} }}^{10} \) parameters. The temperature dependence in \( g_{{{\text{Ca}}^{2+} , {\text{Fe}}^{3+} }}^{01} \) was necessary to describe the enthalpy of melting of CF and C2F (Figures 6, 8). Small adjustments in the entropy and enthalpy of formation of CF and C2F were made. Then, the \( \Delta g_{{{\text{Ca}}^{2+} , {\text{Fe}}^{2+} }}^{\circ} \)and \( g_{{{\text{Ca}}^{2+}, {\text{Fe}}^{2+} }}^{01} \) parameters were introduced to fit the phase diagram at iron saturation in Figure 11. The resulting spinel and C2F liquid (Figures 13, 14) were too wide and the introduction of negative ternary parameters was necessary. After extensive trial and error attempts, parameters \( q_{{{\text{Fe}}^{3+}, {\text{Ca}}^{2+} ( {\text{Fe}}^{2+})}}^{111} \) and \(q_{{{\text{Fe}}^{3+}, {\text{Ca}}^{2+} ( {\text{Fe}}^{2+})}}^{011}\) were selected. They had effects on all the above-mentioned diagrams. Moreover, they had a major effect on the Fe3+ content of the iron-saturated slag (Figure 12). Because of the Toop-type extrapolation of the Fe2O3-CaO interactions into the ternary system, the curve in Figure 12 was also affected by the parameters on the Fe2O3-CaO side. The \( g_{{{\text{Ca}}^{2+}, {\text{Fe}}^{3+} }}^{20} \) parameter was introduced to achieve the drop in the Fe3+ content with the increase of the CaO content in accordance with the experimental data of Scheel.[48] The temperature dependence of \( q_{{{\text{Fe}}^{3+} , {\text{Ca}}^{2+} ( {\text{Fe}}^{2+} )}}^{111}\) and \( q_{{{\text{Fe}}^{3+}, {\text{Ca}}^{2+} ( {\text{Fe}}^{2+} )}}^{011} \) was necessary to describe Figure 12 and the enthalpy of dissolution of CaO in the iron-saturated slag (Figure 24). In the final cycle of optimization, all slag parameters were adjusted simultaneously to best describe all experimental data. The oxygen isobars in Figures 13 through 19 were taken into account.
The heat capacity of the CF2 and C4WF8 phases were approximated by the increment method. For CF2, the enthalpy of formation and entropy were fixed using the range of stability in air and in oxygen (Figures 20, 21). For C4WF8, the enthalpy and entropy were chosen in such a way that stability range of CF2 and C4WF8 was similar to that predicted by Selleby and Sundman[2] (Figure 9; Table II).
For the liquid metal phase, the L 0Ca,CaO parameter was introduced to describe Figure 2. All other parameters were taken from Jung et al.[63]
All the calculations in Figures 1 through 28 were made using the final set of model parameters. The list of parameters is given in Table III. The results are compared with experimental data and with the previous thermodynamic optimization of Selleby and Sundman.[2]
Conclusion
A complete critical evaluation of all available phase diagram and thermodynamic data at 1 atm total pressure for the Ca-Fe-O system has been carried out, and a database of optimized model parameters has been developed. A wide variety of available data are described by few model parameters. The most recent experimental data have been taken into account. In particular, the properties of Ca4Fe9O17 (C4WF4) phase, the solubility of Ca in spinel, the miscibility gap in the wüstite-lime (monoxide) solution, and the lime liquidus at iron saturation have been re-evaluated.
With the present optimized database, it is possible to calculate any phase diagram section for all compositions and oxygen partial pressures. The current database provides the best presently available description of the thermodynamic properties and phase equilibria in the Ca-Fe-O system.
In the present study, the thermodynamic database for the oxide phases has been combined with the physically realistic model for the liquid Fe-Ca-O alloy, which may be used for the prediction of deoxidation of steels.
The thermodynamic database reported here can be combined with the earlier optimizations of other chemical systems[9,72–76] to produce a multicomponent database, which can be used in combination with the liquid matte solution[77–79] for many applications in pyrometallurgy, steel-making, development of new ceramic materials, and refractory linings.
References
M. Hillert, M. Selleby, and B. Sundman: Metall. Trans. A, 1990. 21: pp. 2759–76.
M. Selleby, and B. Sundman: Calphad, 1996. 20: pp. 381–92.
S. Ban-Ya, Y. Iguchi, H. Ishizuka, T. Shibata, and M. Arai: in 3rd International Conference on Molten Slags and Fluxes, 27–29 June, 1988, 1989. University of Strathclyde, Glasgow.
E. Rosen, and B. Saitton: Scand. J. Metall., 1995, 24: 176–79.
H.-Y. Li, and X.-M. Guo: Metall. Mater. Trans. B, 2015. 46: 278–85.
M. Aspiala, T. Hidayat, P. Taskinen, and E. Jak: J. Alloys. Compd, 2015. doi:10.1016/j.jallcom.2015.10.280.
Bale, C.W., E. Belisle, P. Chartrand, S.A. Decterov, G. Eriksson, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melancon, A.D. Pelton, C. Robelin, and S. Petersen: Calphad, 2009. 33:295–311.
T. Hidayat, D. Shishin, E. Jak, and S. Decterov: Calphad, 2015. 48:131–44.
D. Shishin, V. Prostakova, E. Jak, and S. Decterov: Metall. Mater. Trans. B, submitted, 2015.
D.J.M. Bevan, and F.D. Richardson: Proc. Aust. Atomic Energy Symp. on the Peaceful Uses of Atomic Energy, 1958, Melbourne University Press, Melbourne, Australia, 1959, 1958, pp. 586–87.
H.A. Wriedt: (1985) Bull. Alloy Phase Diagrams. 6:337–42
Anglezio, J.C., C. Servant, and I. Ansara: Calphad, 1994. 18: 273–309.
A.D. Pelton: Calphad, 2001. 25:319–28.
Decterov, S.A., E. Jak, P.C. Hayes, and A.D. Pelton: Metall. Mater. Trans. B, 2001. 32: 643–57.
Hillert, M. and L.-I. Staffansson: Acta Chem. Scand., 1970. 24:3618–26.
16. Hillert, M., B. Jansson, and B. Sundman: Z. Metallkd., 1988. 79: 81–87.
Hillert, M. and M. Jarl: Calphad, 1978. 2: 227–38.
Pelton, A.D., S.A. Decterov, G. Eriksson, C. Robelin, and Y. Dessureault: Metall. Mater. Trans. B, 2000. 31: 651–59.
Pelton, A.D. and P. Chartrand: Metall. Mater. Trans. A, 2001. 32: 1355–60.
E. Jak: in Unpublished Work: Centre de Recherce en Calcul Thermochimique (CRCT), École Polytechnique, Montréal, Québec. 1999.
King, E.G.: J. Am. Chem. Soc., 1954. 76: 5849–50.
Rajagopalan, K.V., R. Prasad, R. Kalyanaraman, and M. Sundaresan: J. Therm. Anal., 1991. 37: 2253–58.
Bonnickson, K.R.: J. Am. Chem. Soc., 1954. 76: 1480–82.
Rezukhina, T.N. and Y. Bagin’ska: Elektrokhimiya, 1967. 3:1146–49.
Jacob, K.T., N. Dasgupta, and Y. Waseda: Z. Metallkd., 1999. 90:486–90.
Forsberg, S., P. Wikstrom, and E. Rosen: Metall. Mater. Trans. B, 2002. 33:385–92.
D.A. Reeve and A.G. Gregory: Inst. Min. Metall. Trans. Sect. C, 1967, vol. 76, pp. 268–77.
Bergman, B. and C. Song: J. Am. Ceram. Soc. 1989. 72, pp. 1364–67.
Chung, W.S., T. Murayama, and Y. Ono: Kogaku Shuho - Kyushu Daigaku, 1989. 62, pp. 133–40.
W.S. Chung, T. Murayama, and Y. Ono: Trans. ISIJ, 1988, vol. 28, p. B-4.
Chung, W.S., T. Murayama, and Y. Ono: Nippon Kinzoku Gakkaishi, 1988. 52: p. 973–80.
V.B. Tare and B. Deo: Montpellier, 1975, vol. 3, pp. 104–13.
Bjorkman, B.: J. Metall., 1984, vol. 13, pp. 193–200.
A. Burdese and C. Brisi: Ricerca Sci., 1952, vol. 22, pp. 1564–67.
Lykasov, A.A. and N.V. Kozheurova: Izv. Akad. Nauk SSSR, Neorg. Mater., 1980. 16: p. 1079-82.
Malquori, G. and V. Cirilli: 1945. 75: p. 137–56.
Perrot, P.: Rev. Chim. Miner., 1967. 4, p. 465–93.
Nikolic, S., P.C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2009. 40: p. 900-09.
Takeda, Y., S. Nakazawa, and A. Yazawa: Can. Metall. Q., 1980. 19, pp. 297–305.
W. Oelsen and H. Maetz, Mitt. Kaiser-Wilhelm Inst. Eisenforsch, 1941, vol. 23, pp. 195–245.
41. Abbattista, F., A. Burdese, and M. Maja: Rev. Int. Hautes Temp. Refract., 1975, 12, pp. 337–42.
B. Zhao, E. Jak, and P. Hayes, Experimental studies in Ca-Fe-O slag system. Unpublished Work: Pyrometallurgy Research Centre, The University of Queensland, 2003.
Larson H, Chipman J (1953) J. Met., 197:1089–96.
Allen, W.C. and R.B. Snow: J. Am. Ceram. Soc., 1955. 38, pp. 264–80.
Fischer, W.A. and H.J. Fleischer: Arch. Eisenhuettenwes., 1961. 32 p. 305–13.
Görl, E., F. Oeters, and R. Scheel: Arch. Eisenhuettenwes., 1966. 37: 441–45.
Obst KH, Horn HC, Stradtmann J (1968) Mikrochim. Acta, Suppl., 3:147–54
Timucin, M. and A.E. Morris: Metall. Trans., 1970. 1, pp. 3193–3201.
S. Hara, T. Araki, and K. Ogino: in Int. Symp. Proc. 2nd, 1984. 1984. Metall. Soc. AIME, Warrendale.
Scheel, R.: Arch. Eisenhuttenwes., 1974. 45, pp. 751–56.
Ban-Ya, S., A. Chiba, and A. Hikosaka: J. Iron Steel Inst. Jpn., 1980. 66, pp. 1484–93.
White J (1938) Iron Steel Inst. 27:1–75
Gurry, R.W. and L.S. Darken: J. Am. Chem. Soc., 1950. 72, 3906–10.
Phillips, B. and A. Muan: J. Am. Ceram. Soc., 1958. 41, 445–54.
Edstroem, J.O.: Jernkontorets Ann., 1956. 40: p. 101–15.
P. Batti: Chim. Ind. (Milan, Italy)1956, vol. 38, pp. 864–66
X.G. Liu: in School of Chemical Engineering. 2012, University of Queensland, Brisbane, pp. 108.
Johnson, R.E. and A. Muan: Trans. Met. Soc. AIME, 1967. 239: p. 1931.
Iwase, M., N. Yamada, K. Nishida, and E. Ichise: Trans. Iron Steel Soc. AIME, 1984. 4: p. 69–75.
Chang, D.R. and R.A. Howald: High Temp. Sci., 1982. 15: 209–18.
Berggren, J.: Scand. J. Metall., 1975. 4, pp. 75-80.
Berggren, J.: Scand. J. Metall., 1975. 4, pp. 140–44.
Jung, I.-H., S.A. Decterov, and A.D. Pelton: Metall. Mater. Trans. B, 2004. 35: p. 493-507.
Ototani T., Kataura Y, Degawa T (1976) Trans. Iron Steel Inst. Jpn. 16:275–82
Gustafsson, S. and P.O. Mellberg: Scand. J. Metall., 1980. 9: 111–16.
Seo, J.-D. and S.-H. Kim: Bull. Kor. Inst. Metall. Mater. (Korea), 1999. 12: p. 402-11.
Miyashita, Y. and K. Nishikawa: Tetsu to Hagane, 1971. 57: p. 1969–75.
Han, Q., X. Zhang, D. Chen, and P. Wang: Metall. Trans. B, 1988. 19: p. 617–22.
M. Ozawa: The Japan Society for the Promotion of Science, 19th Committee paper No. 9837, Iron Steel Institute of Japan, Tokyo, 1975, p. 6.
Phillips B, Muan A (1960) Trans. Am. Inst. Min. Metall. Eng. 218:1112–18
Eriksson, G. and A.D. Pelton: Metall. Trans., 1993, 24, pp. 807–16.
Jung, I.-H., S.A. Decterov, and A.D. Pelton: J. Phase Equilib., 2004. 25(4): p. 329-45.
Shishin, D., T. Hidayat, E. Jak, and S. Decterov: Calphad, 2013. 41: p. 160–79.
Jung, I.-H., Y.-B. Kang, S.A. Decterov, and A.D. Pelton: Metall. Mater. Trans. B, 2004. 35: p. 259–68.
Jung, I.-H., S.A. Decterov, and A.D. Pelton: J. Phys. Chem. Solids, 2004. 65: p. 1683–95.
Jung, I.-H., S.A. Decterov, and A.D. Pelton: J. Am. Ceram. Soc., 2005. 88:1921–28.
Shishin, D. and S.A. Decterov: Calphad, 2012. 38: p. 59-70.
D. Shishin, E. Jak, and S.A. Decterov: J. Phase Equilib. Diff. 2015.
Shishin, D., E. Jak, and S.A. Decterov: Calphad, 2015. 50: p. 144–60.
Acknowledgment
The authors would like to thank Australian Research Council Linkage Program, Rio Tinto Kennecott Utah Copper, Corp., Formerly: Xstrata Technology (Now: Glencore Technology), Formerly: Xstrata Copper (Now: Glencore), BHP Billiton Olympic Dam Operation, and Outotec Oy for their financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 14, 2013.
Rights and permissions
About this article
Cite this article
Hidayat, T., Shishin, D., Decterov, S.A. et al. Thermodynamic Optimization of the Ca-Fe-O System. Metall Mater Trans B 47, 256–281 (2016). https://doi.org/10.1007/s11663-015-0501-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0501-0