Abstract
Gold solubility in the CaO-SiO2-Al2O3-MgOsat slag system was measured at 1773 K (1500 °C) under a CO2–CO atmosphere over a wide range of compositions, i.e., 8 to 40 mass pct CaO, 26 to 50 mass pct SiO2, and 0 to 36 mass pct Al2O3, to determine the dissolution mechanism of gold in the CaO-based metallurgical slags. Gold solubility in the present slag system increased with increasing oxygen partial pressure and increasing activity of CaO. From the thermodynamic analysis, the dissolution mechanism of gold into the (alumino-)silicate melts is proposed as follows according to the activity of basic oxide, which indicates that the predominant species of gold is dependent on slag basicity.
The enthalpy change for the dissolution of gold into the CaO-SiO2-Al2O3-MgOsat slag system was measured to be about −80 kJ/mol, indicating that the gold dissolution is exothermic. From the iso-Au solubility contours, the dominant factor affecting the gold dissolution behavior is the (CaO + MgO)/SiO2 ratio, whereas the influence of Al2O3 was negligible. Consequently, less basic slags and higher processing temperatures, in conjunction with a strongly reducing atmosphere, are recommended to increase gold recovery during pyro-processing of Au-containing e-wastes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gold is a bright yellow, dense, soft, malleable, and ductile metal and has been a valuable and highly sought-after precious metal for coinage jewelry and other arts since long before the beginning of recorded history.[1] Physically, gold is the most ductile of all metals, is a good conductor of heat and electricity, and reflects infrared radiation strongly.[1,2] Chemically, it does not react with water, dry or humid air, and most corrosive reagents, so these are the reasons why it is well suited for use in coins and jewelry and as a protective coating on other more reactive metals.[2]
Because gold has good physicochemical properties, it plays an increasingly important role in industrial applications. For example, over 300 tons of gold are used annually in electronic components such as electroplated coatings on connectors and contacts in mobile phones, computers, and other electrical devices.[3] However, 75 pct of all gold ever produced has been extracted since 1910, and gold reserves in the world are becoming rapidly depleted.[4] The cost of gold production and its price in the market have increased rapidly, and interest in the recycling of gold-containing materials has naturally increased.
The wastes of electronic equipments (e-wastes) contain large amounts of precious metals compared to their own respective ores, and therefore such wastes may be considered as a secondary source of valuable metals. For instance, the concentration of gold in natural ore is commonly between 0.5 and 15 g/ton-ore (0.5 to 15 ppm), while its concentration in electronic circuit boards is over 10 times higher, typically being about 150 ppm in expansion cards and over 10,000 ppm in central processing units, CPUs.[5] This is the reason why Au-containing materials such as e-wastes are highly important sources in the concept of ‘Urban Mining Technology.’[6]
From the viewpoint of pyrometallurgical processing, the distribution ratio of gold between metal and slag and the solubility of gold in slags have been scarcely reported. Richardson and Billington reported that the solubility of gold in lead glass was 220 ppm but only 30 ppm in plate glass at 1673 K (1400 °C).[7,8] Toguri and Santander measured the distribution ratio of copper between Cu-Au alloy and silica-saturated fayalite slags at temperatures ranging from 1523 K to 1623 K (1250 °C to 1350 °C) as a function of the oxygen partial pressure from \( p_{{{\text{O}}_{2} }} \) = 10−8 atm to \( p_{{{\text{O}}_{2} }} \) = 10−7 atm.[9] Although the distribution of gold was plotted against the alloy composition for various conditions of temperature and oxygen partial pressure, there was a high degree of scatter. However, the solubility of gold in slag was found to be lower than that of copper by a factor of approximately 100. Also, the solubility of Au in fayalite slag was reported to be approximately 80 ppm at 1473 K (1200 °C) by Altman and Kellogg.[10] Nagamori and Mackey measured the distribution ratio of gold between copper/slag and matte/slag.[11] However, the experimental results were scattered due to the poor reproducibility of gold analysis in the slag, segregation and sampling difficulty, and the very low level of Au in the slag.
Recently, the dissolution mechanism of gold into metallurgical slags was systematically investigated by Swinbourne et al.[8] at extensive temperatures and oxygen partial pressures, viz. from 1373 K to 1573 K (1100 °C to 1300 °C), and from \( p_{{{\text{O}}_{2} }} \) = 10−10 atm to \( p_{{{\text{O}}_{2} }} \) = 1.0 atm. The solubility of gold in iron silicate (32SiO2-10Fe2O3-58FeO, mass pct throughout the paper), calcium ferrite (20CaO-80Fe2O3), and lead oxide slags increased with increasing oxygen partial pressure (\( p_{{{\text{O}}_{2} }} \)), from which gold was found to be present probably as Au+ ions in molten slags. Gold solubility also increased by increasing the fraction of oxygen ion (\( N_{{{\text{O}}^{2 - } }} \)), which was calculated using the Kapoor–Frohberg structure model, in lead silicate slag. Not only from the \( p_{{{\text{O}}_{2} }} \) dependence but also from the \( N_{{{\text{O}}^{2 - } }} \) dependence of gold solubility in slag, the dissolution mechanism of gold in lead silicate slag under an oxidizing atmosphere, viz. air, was proposed as follows:[8]
Based on the above background, not only the temperature dependency of gold solubility in slags but also the gold solubility in the calcium silicate-based slags, which are more easily reused from iron- and steelmaking companies, has not been investigated yet. The operation temperature and slag composition are very important factors affecting the recovery of gold from Waste Electrical and Electronic Equipment to design the cost-effective pyrometallurgical processing routes.
Consequently, in the present study, gold solubility in the CaO-SiO2-Al2O3-MgOsat slag system was measured at 1773 K (1500 °C) at \( p_{{{\text{O}}_{2} }} \) = 10−10 atm to \( p_{{{\text{O}}_{2} }} \) = 10−8 atm using a CO–CO2 gas mixture over a wide range of compositions, i.e., 8 to 40 mass pct CaO, 26 to 50 mass pct SiO2, and 0 to 36 mass pct Al2O3, to determine the dissolution mechanism of gold in the calcium silicate-based slags.
Experimental
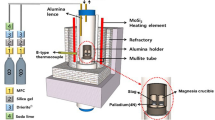
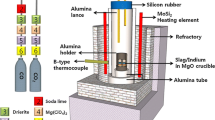
The thermochemical equilibration experiments were carried out using a super-kanthal electric furnace with an MoSi2 heating element. A schematic diagram of the experimental apparatus is shown in Figure 1. The temperature was controlled within ±2 K (±2 °C) using a B-type (Pt-30 pct Rh / Pt-6 pct Rh) thermocouple and a proportional integral differential controller. Pure gold (99.99 pct purity) was used, and slag samples were prepared by mixing reagent-grade SiO2, Al2O3, MgO, and CaO calcined from CaCO3 at 1273 K (1000 °C) for 10 hours.
Gold (0.38 g) and slag (3 g) were loaded into a fused magnesia (99.9 pct purity) crucible (OD; 18 mm, ID; 14 mm, HT; 50 mm) placed in a porous alumina holder (OD; 50 mm, ID; 45 mm, HT; 70 mm). The oxygen partial pressure of the system was controlled by the following reaction:[12]
The gas flow rate was controlled by a mass flow controller. As the (CO2/CO) ratio varied from 0.66 to 0.12, the oxygen partial pressure changed from \( p_{{{\text{O}}_{2} }} \) = 3.2 × 10−10 to \( p_{{{\text{O}}_{2} }} \) = 1.0 × 10−8 atm at 1773 K (1500 °C). Impurities in the CO2 and CO gasses were removed by passing the gases through Drierite (W.A. Hammond Drierite Co. Ltd., Xenis, OH), silica gel, and soda lime.
After 12-hour equilibration, which was determined from Han and Park [12 hours for indium-slag equilibration at 1573 K (1300 °C)][13] and Swinbourne et al. [8 hours for gold-slag equilibration at 1573 K (1300 °C)] results,[8] the samples were quickly extracted from the furnace and quenched by plunging the crucible into brine. The slag and gold samples were carefully separated from the crucible. The interface between the gold and slag was clearly defined and allowed a clean separation. The slag was ground to a fine powder for chemical analysis. The gold content in the slag was analyzed by inductively coupled plasma-optical emission spectroscopy (OPTIMA 8300; PerkinElmer, Waltham, MA), and the equilibrium composition of the slag was analyzed with an X-ray fluorescence spectroscopy (S4 Explorer; Bruker AXS Inc., Madison, WI). The results of all experiments are listed in Table I.
Results and Discussion
Influence of Oxygen Partial Pressure on Gold Solubility in the CaO-SiO2-Al2O3-MgOsat Slag
Gold dissolution reaction into the slag can be described by the following general equation:
The equilibrium constant for Eq. [3] is expressed by Eq. [4]:
where a i , f i , and \( p_{{{\text{O}}_{2} }} \) are the activity and activity coefficient of component i, and the oxygen partial pressure, respectively. Because pure gold was used in the experiments, the activity of gold is unity. Therefore, the above Eq. [4] can be expressed as follows:
From Eq. [5], the solubility of gold is expected to have a linear relationship with the oxygen partial pressure in logarithmic form (slope = m) at a fixed temperature and slag composition. The solubility of gold in the 23CaO-38SiO2-13Al2O3-MgOsat (mass pct) system at 1773 K (1500 °C) is plotted against the oxygen partial pressure in logarithmic form in Figure 2. Gold solubility, log (mass pct Au), linearly increases with increasing oxygen partial pressure, \( \log p_{{{\text{O}}_{2} }} \), with a slope of 0.20 (±0.02).
Gold has two known oxidation states, Au+ and Au3+. If gold oxide is Au2O, viz. gold is stable as Au+, the stoichiometric coefficient m will be 0.25. Otherwise, if Au2O3 is the case, viz. as Au3+, m will be 0.75. From the measured results in the present study (Figure 2), the theoretical slope of 0.25 is more reasonable than 0.75, indicating that the ‘O2(g)’ term should have the stoichiometric coefficient ‘m = 1/4.’ It is significant that the oxygen potential dependence of gold solubility in the CaO-SiO2-Al2O3-MgOsat slag is very similar to that in the lead oxide slag at 1373 K (1100 °C), which was investigated over a more extensive \( p_{{{\text{O}}_{2} }} \) range, and supports the view that gold dissolves as the Au+ oxidation state.[8]
The measured results in the present slag system at 1773 K (1500 °C) were extrapolated to 1373 K (1100 °C), at which temperature the system is assumed to be supercooled, based on the temperature dependence of gold solubility, which will be discussed in detail later in Section III-B. In brief, from the linear relationship between gold solubility (log (pct Au)) and inverse temperature (1/T), viz. van’t Hoff relationship, the gold solubility of the slag at 1373 K (1100 °C) was obtained under the condition of \( p_{{{\text{O}}_{2} }} = 10^{ - 8} \;{\text{atm}} \). Assuming that temperature dependence of gold solubility is not significantly affected by the oxygen partial pressure under the present experimental conditions, i.e., \( p_{{{\text{O}}_{2} }} = 10^{ - 10} \;{\text{atm}} \) to \( p_{{{\text{O}}_{2} }} = 10^{ - 8} \;{\text{atm}} \), the gold solubility measured at 1773 K (1500 °C) could be extrapolated to 1373 K (1100 °C).
It is very interesting in Figure 2 that the extension of gold solubility in the MgO-saturated calcium aluminosilicate melts at moderately reducing atmosphere, i.e., \( p_{{{\text{O}}_{2} }} \) = 10−10 to 10−8 atm, is surprisingly consistent with the solubility in the PbO slag under oxidizing atmospheres, i.e., \( p_{{{\text{O}}_{2} }} \) = 10−3 to 0.2 atm at 1373 K (1100 °C).[8] This means that the experimental results in the current study and Swinbourne et al.’s results are in good agreement and that the influence of oxygen partial pressure on the dissolution mechanism of gold in the CaO-SiO2-Al2O3-MgOsat slag and PbO slag is identical through a very wide range of oxygen potentials, i.e., from \( p_{{{\text{O}}_{2} }} \) = 10−10 to 0.2 atm, irrespective of the slag system.
Temperature Dependence of Gold Solubility in the CaO-SiO2-Al2O3-MgOsat Slag
The temperature dependence of gold solubility in the 24CaO-40SiO2-14Al2O3-MgOsat (mass pct) slag system is shown in Figure 3. The gold solubility, log (mass pct Au), decreases linearly with increasing temperature. The enthalpy change for the dissolution of gold (ΔH °r,Au ) in the present slag system was calculated from the slope of the line based on the van’t Hoff equation, Eq. [6]:[14]
The enthalpy change calculated from Figure 3 and Eq. [6] was about −80 kJ/mol, indicating that the reaction of gold dissolution into the slag is exothermic. As the stoichiometric coefficient m was defined as 0.25 in the previous section, gold oxide must be Au2O regardless of the basicity dependence of gold solubility, viz. the coefficient ‘n.’ The oxidation and dissolution reactions of gold can therefore be written as follows:[15–18]
The heat of formation of Au2O, \( \Delta H_{{{\text{f,Au}}_{2} {\text{O}}}}^{^\circ }, \) was calculated by Shi et al.[15] using the ab initio density functional theory, and the value was 0.228 eV per stoichiometric unit, which can be converted to 22 kJ/mol. Thus, they concluded that the formation of metastable Au2O was endothermic, which is very different from that of Au2O3 (\( \Delta H_{{{\text{f,Au}}_{2} {\text{O}}_{3} }}^{^\circ } = - 50\;\;{\text{kJ/mol}} \)).[15] Although the value of the heat of melting, \( \Delta H_{{{\text{m,Au}}_{2} {\text{O}}}}^{^\circ }, \) is also unknown, it should be a positive value, i.e., endothermic. Since the crystal structure of Au2O is the same as Cu2O (cuprite),[15] the enthalpy change during melting of Au2O is assumed to be similar to that of Cu2O, i.e., 57 kJ/mol.[17,18] Even though this assumption should be further updated, the value of \( \Delta H_{{{\text{d,Au}}_{2} {\text{O}}}}^{^\circ } \) was estimated to be approximately −134 kJ/mol. A similar analysis has been carried out for the oxidation and dissolution of silver into the slags.[18]
The temperature dependence of silver solubility in borate melts is also compared in Figure 3. The enthalpy change for dissolution of silver (ΔH r,Ag°) in the 30CaO-70B2O3 and 40BaO-60B2O3 (mass pct) slags was measured by Park and Min,[18] and the values were −67 and −56 kJ/mol, respectively. Comparing the dissolution enthalpy of Ag2O into the borates and that of Au2O into the aluminosilicates, viz. \( \Delta H_{{{\text{d,Ag}}_{2} {\text{O}}}}^{^\circ } ( = - 71\sim - 59\;{\text{kJ/mol}}) \) and \( \Delta H_{{{\text{d,Au}}_{2} {\text{O}}}}^{^\circ } ( = - 134\;{\text{kJ/mol}}) \),[18] the dissolution of Au2O is significantly more exothermic than that of Ag2O. This is mainly due to the fact that the oxidation of gold is an endothermic (i.e., \( \Delta H_{{{\text{f,Au}}_{2} {\text{O}}}}^{^\circ } = 22\;{\text{kJ/mol}} \)), whereas the oxidation of silver is exothermic (\( \Delta H_{{{\text{f,Ag}}_{2} {\text{O}}}}^{^\circ } = - 31\;\;{\text{kJ/mol}} \)).[12] Not only from the ab initio calculations of Shi et al.[15] but also from the experimental findings in the present study, the large difference between gold and silver dissolution behavior into the slags possibly originated from the metastable characteristics of Au2O.
Effect of Slag Composition on Gold Solubility in the CaO-SiO2-Al2O3-MgOsat Slag System
In Figure 4, the solubility of gold in the CaO-SiO2-Al2O3-MgOsat slag system is plotted against the content (mass pct) of CaO at 1773 K (1500 °C). The solubility of gold in the present slag system increases with increasing content of CaO, and it markedly increases at CaO contents greater than about 30 mass pct. This means that gold oxide (Au2O) qualitatively behaves as an acidic component in the MgO-saturated calcium aluminosilicate melts. The quantitative analysis for the influence of basicity on the dissolution behavior of gold can be given using the following equation, which is modified from Eq. [5]:
Because the basicity of slag, i.e., the activity of free oxygen, cannot be experimentally measured due to thermodynamic constraints, it can be replaced by the activity of CaO, assuming that the CaO activity is proportional to the O2− ion activity at fixed oxygen partial pressure and temperature.[13,18–24] In the current study, the CaO activity in the slag was calculated by FactSageTM6.4 (ESM Software, Hamilton, OH), which is a commercial thermochemical computing program.[25,26] This software has been successfully used for computing the gas–slag–metal multiphase equilibria in ferrous and non-ferrous metallurgical systems.[22–31]
The solubility of gold is plotted against the activity of CaO on logarithmic axes in Figure 5, wherein a good linear relationship between log (pct Au) and log a CaO is found. The slope of the line obtained from a linear regression analysis is 0.48 (r 2 = 0.90), indicating that the stoichiometric coefficient n in Eq. [11] is 1/2 in the CaO-SiO2-Al2O3-MgOsat system. In Figure 5, the experimental results for the gold solubility in the PbO-SiO2 slag measured at 1373 K (1100 °C) under atmospheric condition are shown for the sake of comparison.[8]
In the original work by Swinbourne et al.,[8] the Kapoor–Frohberg model was used to calculate the fraction of oxygen (\( N_{{{\text{O}}^{2 - } }} \)) in the PbO-SiO2 slag system. They obtained a good linear relationship between log (pct Au) and \( \log \;N_{{{\text{O}}^{2 - } }} \) with a slope of 2.0, from which they determined the stoichiometric coefficient n = 2.0. However, if the activity of PbO, which can be obtained using FactSageTM6.4, in the PbO-SiO2 slag were employed as an indirect basicity index assuming that the PbO activity is qualitatively proportional to the O2− activity, the slope of the line obtained from a linear regression analysis is 1.54 (r 2 = 0.88), which is close to 1.5, viz. n = 3/2.
Therefore, based on the above thermodynamic discussion, the dissolution mechanism of gold into the silicate melts can be rewritten as follows according to the activity of basic oxides (BO):
A difference between Eqs. [13] and [14][8] in the relatively basic composition range, i.e., a BO > 0.1, possibly originates from the fact that the activity coefficient of O2− ion is not a constant but a function of slag composition, which cannot be fully considered in the Kapoor–Frohberg model used in previous study.[8] Nevertheless, from the present authors’ findings, it is concluded that the predominant species of gold is strongly dependent on the basicity of slag.
Optical basicity has widely been used to evaluate the influence of slag composition on the physicochemical properties of metallurgical slags since it was originally proposed as a measure of the basicity of glasses by Duffy and Ingram.[32,33] The theoretical optical basicity of the silicate melts (Λmelt) was calculated from Eq. [15] using the theoretical optical basicity of each component listed in Table II.[32–34]
where x i , n i , and Λ i are the mole fraction of oxide, number of oxygen in each oxide, and theoretical optical basicity of pure component i, respectively. As shown in Figure 6, the gold solubility linearly increases with increasing optical basicity of the slags, indicating that the optical basicity represents the basicity index well in the CaO- and PbO-based silicate melts. A discrepancy in the gold solubility between two slag systems originated not only from different temperatures but also from different oxygen partial pressures.
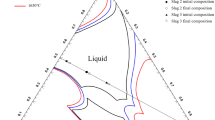
The iso-gold solubility (ppm) contours in the CaO-SiO2-Al2O3-MgOsat slag system at 1773 K (1500 °C) under \( p_{{{\text{O}}_{2} }} \) = 10−8 atm are shown in Figure 7. Considering the similar behavior of CaO and MgO, i.e., BOs, ‘CaO + MgO’ are combined in the phase diagram. The gold solubility in the present slag system increases with increasing ratio of (CaO + MgO) to SiO2, whereas the influence of Al2O3 addition on the solubility of gold is negligible.
Solubility of Gold and Silver in (Alumino-)Silicate and (Alumino-)Borate Melts
In Figure 8, the solubility of gold, log (pct Au), in the CaO-SiO2-Al2O3-MgOsat slag at 1773 K (1500 °C) under \( p_{{{\text{O}}_{2} }} \) = 10−8 atm measured in the present study and in the PbO-SiO2 at 1373 K (1100 °C) under \( p_{{{\text{O}}_{2} }} \) = 0.21 atm measured by Swinbourne et al.[8] is plotted against the mole pct of the BO such as CaO and PbO. As discussed in previous section (Eqs. [12] through [14]), the dissolution of gold into the slags is promoted by increasing the content of BOs, indicating that the less basic slags and fluxes are necessitated to improve the recovery of gold, viz. to minimize gold loss to the slag phase. Moreover, the higher the processing temperature in conjunction with strongly reducing atmosphere, the lower the gold loss expected during pyro-processing of Au-containing e-wastes. However, the viscosity of slag is also an important parameter affecting the recovery of gold. It generally increases with increasing concentration of SiO2 and decreases with increasing temperature. Thus, the optimization of slag composition and processing temperature is required in practice to maximize gold recovery.
It is useful to compare the solubility of gold and silver in the metallurgical slags and fluxes because gold and silver are commonly present in e-wastes. The solubility of silver in the MO-B2O3 (MO = CaO, BaO, and Na2O) binary melts at 1773 K, 1698 K, and 1373 K (1500 °C, 1425 °C, and 1100 °C), respectively, was measured under the condition of strongly reducing atmosphere, \( p_{{{\text{O}}_{2} }} \) = 10−16 atm, by Park and Min.[18] Silver solubility in the Na2O-B2O3-Al2O3 ternary system at temperatures ranging from 1273 K to 1423 K (1000 °C to 1150 °C) under atmospheric condition was measured by Wakasugi et al.[17] The solubility of silver in borate melts exhibits minima at a specific content of BO in each system under a strongly reducing atmosphere, indicating that the dissolution mechanism of silver into the borate melts changed at this critical composition. The more detailed discussion for the dissolution mechanism of silver is available in a previous article.[18]
However, the solubility of silver in the Na2O-B2O3-Al2O3 melts under atmospheric conditions decreases with increasing content of Na2O.[17] Hence, from the viewpoint of silver recovery during pyro-processing of silver-containing e-wastes, a strongly reducing atmosphere is recommended to minimize silver loss to the slag phase. However, the composition of slag should be carefully designed to obtain the highest silver recovery, which corresponds to the lowest solubility.
Comparing the present results for gold solubility and the previous results for silver solubility, the future work for the solubility of silver in the calcium aluminosilicate slag, which can be easily reused from iron and steel meltshops and thus be cost-effective, is needed to propose the best operating conditions for the pyrometallurgical recycling processes of e-wastes containing precious elements such as gold and silver.
Conclusions
Gold solubility in the CaO-SiO2-Al2O3-MgOsat slag system was measured at 1773 K (1500 °C) from \( p_{{{\text{O}}_{2} }} \) = 10−10 atm to \( p_{{{\text{O}}_{2} }} \) = 10−8 atm to determine the dissolution mechanism of gold in the CaO-based metallurgical slags. The major findings can be summarized as follows:
-
1.
Gold solubility in the CaO-SiO2-Al2O3-MgOsat slag system at 1773 K (1500 °C) increased with increasing oxygen partial pressure and increasing activity of CaO. From the thermodynamic analysis for the gold solubility in the present slag system as well as in the PbO-SiO2 system, the dissolution mechanism of gold into the (alumino-)silicate melts is proposed as follows according to the activity of BO:
$$ {\text{Au}}(s) + \frac{1}{4}{\text{O}}_{2} (g) + \frac{1}{2}\left( {{\text{O}}^{2 - } } \right) = \left( {{\text{AuO}}^{ - } } \right),\quad \left( {a_{\text{BO}} < 0.1} \right) $$$$ {\text{Au}}(s) + \frac{1}{4}{\text{O}}_{2} (g) + \frac{3}{2}\left( {{\text{O}}^{2 - } } \right) = \left( {{\text{AuO}}_{2}^{3 - } } \right),\quad \left( {a_{\text{BO}} > 0.1} \right). $$ -
2.
The enthalpy change for the dissolution of gold into the CaO-SiO2-Al2O3-MgOsat slag system was measured to be approximately −80 kJ/mol, indicating that the gold dissolution is exothermic. From the experimental results in conjunction with literature data, it is suggested that gold initially oxidized to metastable Au2O, followed by dissolution into the molten slag with dissolution enthalpy of approximately −134 kJ/mol.
-
3.
From the iso-Au solubility contours in the CaO-SiO2-Al2O3-MgOsat slag system, the dominant factor affecting the gold dissolution behavior is the (CaO + MgO)/SiO2 ratio, whereas the influence of Al2O3 is negligible.
-
4.
The less basic slags and the higher processing temperature in conjunction with a strongly reducing atmosphere are recommended to increase gold recovery during pyro-processing of gold-containing e-wastes. Because the viscosity of slag increases with increasing content of SiO2 and decreases with increasing temperature, optimization of the slag composition and processing temperature is required in practice.
References
Gold, Wikipedia, http://en.wikipedia.org/wiki/Gold. Accessed March 2015.
F. Habashi: Encyclopedia of Metalloproteins, 2014, pp. 932–33.
C. Hageluken and C. W. Corti: Gold Bull., 2009, vol. 43, pp. 209–20.
C. Louis, O. Pluchery: Gold Nanoparticles for Physics, Chemistry and Biology, World Scientific, Singapore, 2012, p. 3.
L. Barbieri, R. Giovanardi, I. Lancellotti and M. Michelazzi: Environ. Chem. Lett., 2010, vol. 8, pp. 171–78.
E. Yamasue, R. Minamino, T. Numata, K. Nakajima, S. Murakami, I. Daigo, S. Hashimoto, H. Okumura and K. Ishihara: Mater. Trans., 2009, vol. 50, pp. 1536–40.
F.D. Richardson, and J.C. Billington: Miner. Process. Ext. Metall. (Trans. Inst. Min. Metall. C), 1956, vol. 65, pp. 273–97.
D.R. Swinbourne, S. Yan, and S. Salim: Miner. Process. Ext. Metall. (Trans. Inst. Min. Metall. C), 2005, vol. 114, pp. 23–39.
J. M. Toguri and N. H. Santander: Metall. Trans., 1972, vol. 3, pp. 586–88.
R. Altman and H.H. Kellogg: Miner. Process. Ext. Metall. (Trans. Inst. Min. Metall. C), 1972, vol. 81C, pp. 163–75.
M. Nagamori and P. J. Mackey: Metall. Trans. B, 1978, vol. 9B, pp. 567–79.
E. T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, pp. 1–24.
Y. S. Han and J. H. Park: Metall. Mater. Trans. B, 2015, vol. 46, pp. 235–42.
C. H. P. Lupis: Chemical Thermodynamics of Materials, Prentice Hall, New York, NY, 1993, pp. 114–20.
H. Shi, R. Asahi and C. Stampfl: Phys. Rev. B, 2007, vol. 75, pp. 1–8.
O. Kubaschewski and C. B. Alcock: Metallurgical Thermochemistry, 5th ed., Pergamon Press, Oxford, UK, 1979, pp. 326-34.
T. Wakasugi, A. Hirota, J. Fukunaga and R. Ota: J. Non-Cryst. Solids., 1997, vol. 210, pp. 141–47.
J. H. Park and D. J. Min: Metall. Mater. Trans. B, 1999, vol. 30B, pp. 689–94.
S. H. Lee, S. M. Moon, J. H. Park, and D. J. Min: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 55–59.
J. H. Park and D. J. Min: ISIJ Int., 2004, vol. 44, pp. 223–28.
J. H. Park and D. J. Min: Mater. Trans., 2006, vol. 47, pp. 2038–43.
J. H. Park, G. H. Park and Y. E. Lee: ISIJ Int., 2010, vol. 50, pp. 1078–83.
G. H. Park, Y. B. Kang, and J. H. Park: ISIJ Int., 2011, vol. 51, pp. 1375–82.
J. H. Heo, S. S. Park and J. H. Park: Metall. Mater. Trans. B, 2012, vol. 43B, pp.1098–1105.
FactSageTM6.4: www.factsage.com. Accessed March 2015.
C. W. Bale, E. Bélisle, P. Chartrand, S. A. Decterov, G. Eriksson, K. Hack, I. H. Jung, Y. B. Kang, J. Melancon, A. D. Pelton, C. Robelin, and S. Petersen: CALPHAD, 2009, vol. 33, pp. 295–311.
J. H. Park: Met. Mater. Int., 2013, vol. 19, pp. 577–84.
J. H. Park: Steel Res. Int., 2013, vol. 84, pp. 664–69.
J. H. Park: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 938–47.
J. H. Heo, B. S. Kim and J. H. Park: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 1352–63.
J. S. Park and J. H. Park: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 953–60.
J. A. Duffy and M. D. Ingram: Phys. Chem. Glasses, 1975, vol. 16, pp. 119–23.
J. A. Duffy, M. D. Ingram, and I. D. Sommerville: J. Chem. Soc. Faraday Trans. I, 1978, vol. 74, pp. 1410–19.
N. Iwamoto, Y. Makino, and S. Kasahara: J. Non-Cryst. Solids, 1984, vol. 68, pp. 389–97.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 13, 2015.
Rights and permissions
About this article
Cite this article
Han, Y.S., Swinbourne, D.R. & Park, J.H. Thermodynamics of Gold Dissolution Behavior in CaO-SiO2-Al2O3-MgOsat Slag System. Metall Mater Trans B 46, 2449–2457 (2015). https://doi.org/10.1007/s11663-015-0421-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0421-z