Abstract
In the current study, the effect of an organic additive tetra propyl ammonium bromide (TPAB) on the structural, morphological characteristics of the cobalt metal produced from aqueous sulfate solutions has been investigated. The concentration of TPAB was varied over a range of 1 to 50 mg/L to evaluate its effect on current efficiency, energy consumption, and quality of electrodeposited cobalt. Smooth and bright electrodeposits of cobalt were obtained at low concentration of TPAB (10 mg/L) maintaining a current efficiency of 99.4 pct, with a low energy consumption of 2.42 kWh/kg. X-ray diffraction studies revealed that (100) plane is the most preferred plane of crystal growth during cobalt electrodeposition. However, at higher concentrations, the (101) plane became the most preferred one. Scanning electron micrographs indicated that smooth and uniform deposit of cobalt was obtained at 10 mg/L beyond which the deposit quality deteriorates. The presence of TPAB in the electrolytic bath polarizes the cathode and decreases the cathodic current considerably. AAS results indicated that the cobalt deposits were of high purity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cobalt is considered as a strategic and critical metal due to its numerous industrial applications. It finds use in rechargeable batteries and in the manufacture of corrosion and wear resistant alloys. Other major uses of the metal include the manufacture of catalysts for petroleum and chemical industries, as a drying agent for paint varnishes, ground coats for porcelain enamels, magnetic recording media and steel-belted radial tires.[1] It comprises about 0.02 pct of the earth crust. Owing to its scarce reserve and high demand, considerable research is being carried out for finding out a highly efficient process for commercial production of the metal.[2] Over the last three decades, 70 pct of the World’s cobalt production is met through aqueous processing of the cobalt containing resources where the final step is electrodeposition, mostly from sulfate medium.[3–6] Industrially, pure cobalt metal can be produced either by precipitating with hydrogen or by electrolytic method.[7] However, electrodeposition method is preferred due to its lower capital and operating cost, higher metal recoveries and less stringent operation, and maintenance costs.[8] The various forms of cobalt metal produced by electrodeposition mostly include metallic powder and cathode sheets. Production of cobalt metal as sheets is preferred over powder as the later suffers from numerous problems associated with handling and processing.[9] Cathodic current efficiency of 80 pct has been reported when electrodeposition of cobalt was carried out using undivided cells.[8] Electrodeposition of cobalt from such cells is associated with serious problem of short-circuiting between the anode and the cathode, consequently reducing the current efficiency.[10] This happens as cobalt metal is inherently stressed and prone to peeling from the cathode. Use of divided cells can resolve this problem there by enhancing the cathodic current efficiency and hence the yield. Use of anode bags during cobalt electrodeposition from divided cells has also been reported; however, an adherent deposit is essential to avoid damage to the bags by the peeling of the metal from the cathode surface.[11] In this context, use of additives in the electrolytic bath becomes crucial. The use of additives during the electrodeposition is usually important owing to their potential benefits which include brightening of the deposit, grain size reduction, reducing the tendency of tree formation, increasing the current density, promoting leveling, and reducing stress and pitting.[12,13]
The presence of some common impurities or additives in the electrolytic bath and their effect on cobalt electrodeposition have been reported by many researchers.[14–18] Das et al.[15] have examined the role of boric acid and sodium fluoride as additives in the electrodeposition of cobalt from cobalt sulfate solutions. It was observed that additives, individually or in combination, yielded metal deposits with better surface morphology and higher current efficiencies (CE). Tripathy et al.[16] have examined the role of boric acid and manganese (Mn) in cobalt electrowinning. They observed that the presence of Mn(II) or boric acid alone increased the current efficiency by 2 to 3 pct and also brought a decrease in energy consumption (EC). The effect of the organic additives on surface roughness was studied for the Co-thiourea and Co-saccharin system.[17] Tripathy et al.[18] have investigated the effect of thiourea and saccharin on the electrodeposition of cobalt. The saccharin and thiourea act as smoothing agents and produce a compact, smooth, and high-quality deposit. However, systematic studies based on the influence of organic additives on deposit morphology are few in case of cobalt electrodeposition. Franklin et al.[19] have demonstrated the effectiveness of quaternary ammonium salts as additives in the electrodeposition of metals like copper and tin. The use of quaternary ammonium bromide as an additive in zinc electrodeposition has been reported.[20] Ciszewski et al.[21] have investigated the effect of quaternary ammonium chlorides on the electrodeposition of nickel. Tetra alkyl ammonium salts have also been used in the electrodeposition of MnO2.[22] TPAB has also been used as an additive in the electrodeposition of cadmium.[23] The current study thus investigates the role of the additive tetra propyl ammonium bromide (TPAB) on the current efficiency, EC, and deposit morphology of cobalt.
Experimental
Experimental Setup
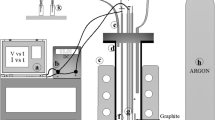
Cobalt electrodeposition was carried out in a rectangular cell made from Perspex, consisting of independent cathodic and anodic chambers separated by a polypropylene diaphragm. Stainless steel (316 grades) and Pb-Sb (1 pct) sheet were used as cathode and anode, respectively. Equal volumes of electrolyte (200 mL) were used in both the cathodic and anodic compartments. The cell was placed in a thermostatic water bath [Julabo, Germany] to maintain the temperature of the electrolytic cell. All the electrodeposition experiments were carried out under galvanostatic conditions. A regulated power supply system [0 to 32 V, 10 A, DC, Aplab Ltd., India] was used for providing constant current. The schematic diagram of the experimental setup is shown in Figure 1.
Reagents
The electrolytic solutions were prepared using doubly distilled water. The cobalt electrolyte was prepared from analytical grade cobalt sulfate (CoSO4·7H2O), sodium sulfate (Na2SO4), and boric acid (H3BO3). The pH of the electrolyte was adjusted to 4 using dilute sodium hydroxide and sulphuric acid. Solutions referred to as “blank” contained 60 g/L cobalt, 15 g/L Na2SO4, and 9 g/L H3BO3. Calculated amounts of TPAB were added to the electrolytic bath from the freshly prepared stock solution of 10 g/L. All chemicals were from Merck Chem. Ltd., India.
Electrode Preparation
The cathode was first polished by 320, then by 600 grade silicon carbide papers to have a mirror-like finished surface. The cathode was then rinsed with 1 M HCl followed by its washing with distilled water. It was then allowed to air dry. The dried cathodes were then weighed prior to electrodeposition.
Electrolysis
The entire sets of electrodeposition experiments were conducted at a temperature of 333 K (60 °C) and at a current density of 200 A/m2 for 3 hours. The distance between the electrodes was kept at about 2.5 cm. Cell voltage was recorded using a multimeter connected across the anode and cathode at various time intervals and finally the average cell voltage was reported. After electrolysis, the cathode was removed from the cell and washed thoroughly with water and acetone and finally dried in an oven at 383 K (110 °C). CE were calculated from the weight gained by the cathode during electrolysis.
Polarization Studies
The polarization behavior of the cathode in the presence and absence of any additive during the electro reduction of cobalt was carried out by cyclic voltammetric technique using a three electrode glass cell. A stainless steel electrode and platinum wire were used as the working and counter electrode, respectively. Standard silver-silver chloride (Ag/AgCl) was used as the reference electrode. The polarization measurements were carried out using Metrohm potentiostat/galvanostat model 128A and the potentials were reported as such. Voltammetric scans were performed in the potential range of −0.6 to −1.0 V vs Ag/AgCl at a scan rate of 10 mV/s. High-purity nitrogen was used to sparge out the dissolved oxygen so as to make the system free from aerial or any dissolved oxygen which may affect the redox reactions.
Physico-chemical Characterization
Sections of cobalt electrodeposits were analyzed by a X-ray diffractometer (PAN ANALYTICAL PW 1830; Philips, Japan) with Cu K α radiation, λ = 1.5404 Å to determine the preferred crystal orientations. The surface morphologies of the deposits were examined by scanning electron microscope, (JEOL JSM 6510, Japan) to investigate the structural and morphological changes in the electrodeposited metal under various deposition conditions. The purity of the cobalt electrodeposits was determined by digesting small portions of the cobalt deposits with nitric acid and then analyzing the solutions with Perkin Elmer A Analyst 200 Atomic Absorption Spectrometer (AAS).
Results and Discussion
Cathodic Current Efficiency
The consequential effects of the presence of the additive TPAB in the electrolytic bath on CE and EC during cobalt electrodeposition are shown in Figure 2. In the absence of TPAB, electrodeposition of the cobalt from the blank solution resulted in a CE of 90.29 pct and EC of 3.03 kWh/kg. Introduction of 1 mg/L of TPAB in the electrolytic bath increased the CE by ~2 to 3 pct. Further increase in the concentration of TPAB in the electrolytic bath increased the CE and at 10 mg/L highest CE of 99.4 pct and lowest EC of 2.42 kWh/kg were observed. Increase in CE during electrolysis is always associated with a decrease in EC which is also evident here (Table I). It should also be noted that at this condition very smooth and bright cobalt deposit was obtained indicating it as the optimum condition for electrodeposition of cobalt pertaining to this system. The increase in CE can be attributed to inhibition of H2 evolution by the additive. Such instances regarding the suppression of hydrogen evolution have already been reported in case of zinc electrodeposition where glue was used as an additive.[24] As usual decrease in CE was observed with increase in the concentration of TPAB in the electrolytic bath. Thus increasing the concentration of TPAB beyond 10 mg/L resulted in a decrease in CE and hence an increase in EC during electrodeposition (Table I). This decrease in the CE at higher additive concentrations can be ascribed to the blocking of the active nucleation sites on the cathode surface by adsorption of the additive and thus inhibiting the electro reduction of cobalt. It was found that all the cobalt electrodeposits were of high purity (Table II).
Polarization Studies
Cyclic voltammetric studies are essential to investigate the behavior of the cathode toward cobalt electrodeposition from cobalt sulfate solutions in the presence and absence of TPAB. The polarization behavior of the cathode during electroreduction of cobalt from sulfate solutions is shown in Figure 3. The cycle started at −0.6 V vs AgCl and the scan swept in the cathodic direction. The nucleation potential (E n) and the crossover potentials (E co) were then determined using the procedures reported earlier.[25] The nucleation overpotential (NOP) which is equal to the difference between E n and E co was calculated and recorded in Table I. The NOP value in the absence of TPAB was −157 mV. The effect of TPAB of varying concentrations can be seen by comparing the shift of NOP (∆NOP) from the baseline value of −157 mV (Table I). Increase in the additive concentration resulted in shifting of the NOP to more negative values indicating polarization of the cathode with simultaneous decrease in cathodic current densities. It was found that ∆NOP was more with more the concentration of TPAB. With TPAB concentration of 10 mg/L the ∆NOP was −14 mV indicating polarization of the cathode. In the current study, highest polarization was observed with 50 mg/L of TPAB where the ∆NOP was highest (−44 mV). Higher the ∆NOP value means lower the cathodic current density. This decrease in current density with increase in additive concentration was due to the increased additive adsorption on the cathode. Similar observations have been reported by Tripathy et al.[20] This behavior was also seen here and was also reflected in the decrease in CE with increased additive concentrations in the solution (Table I).
Crystal Orientations
Figure 4 shows the X-ray diffraction pattern of the cathodic cobalt deposits obtained at various concentrations of TPAB. The crystal growth in the absence of the additive follows the order of preferred orientation (100) (101) (110). There was practically no change on the preferred orientations at lower concentrations of the additive ≤10 mg/L. However, at higher concentrations of the additive the order changed to (101) (100) (110). It was also observed that at higher concentrations of the additive, growth of an extra plane (111) appeared leading to a change in the preferred order of orientation to (100) (101) (110) (111). It is also important to note that the presence of TPAB in the electrolytic bath affects the order of preferred orientation as well as the degree of crystalinity. It was observed that at higher concentrations of the additive (≥20 mg/L) the intensity of the XRD peaks decreased and broadened resulting in non-uniform and poorly crystalline growth (Figures 4(c) and (d)) of the crystallites. This was also supported by the observations made in the surface morphologies of the cobalt deposits as discussed later.
Digital Images
The benefits of quaternary amine TPAB as additive on the surface texture during cobalt electrodeposition can be seen from the digital images of the cobalt electrodeposits (Figure 5). The electrodeposited cobalt obtained in the absence of any additive as seen to the naked eye was dull, full of whitish spots indicating possible hydrogen evolution centers (Figure 5(a)). With the addition of TPAB in the electrolytic bath, we obtain bright smooth and compact deposits (Figures 5(b) and (c)). Further increase in the concentration of the additive to 50 mg/L resulted in a fractured deposit and reappearance of whitish spots (Figure 5(d)), which is associated with lower CE.
Surface Morphology
Effects of TPAB on the CE and EC during cobalt electrodeposition were also reflected on its surface morphologies. Figure 6 shows the SE micrographs of the cobalt metal deposited from acidic sulfate solutions. Figure 6(a) shows that in the absence of any additive dull bright and smooth deposit with randomly oriented crystallites of cobalt were obtained. Introduction of TPAB at 10 mg/L in the electrolytic bath produced uniform, bright, and smooth deposits with increased grain size (Figure 6(b)). It was observed that at higher concentrations of the additive (20 and 50 mg/L) the quality of the deposits deteriorated, resulting in poorly crystalline and non-uniform deposits with fibrous growth on the cobalt deposits (Figures 6(c) and (d)).
Conclusions
The additive tetra propyl ammonium bromide when added in the electrolytic bath at a concentration of 10 mg/L resulted in high CE of 99.4 pct during the electrodeposition of cobalt. The incorporation of additive in small amounts enhanced the CE, produced smooth and compact deposits, and also brought a reduction in EC. The preferred plane of orientation during crystal growth shifted from (100) to (101) at higher concentrations of the additive. Higher concentrations of additive not only decreased the degree of crystallinity but also resulted in deterioration in the deposit quality of the cobalt metal produced from sulfate baths. Cyclic voltammetric studies indicated that the additive TPAB polarizes the cathode. The high purity of the cobalt metal was confirmed by AAS.
References
Minerals Yearbook: Cobalt Statistics and Information, USGS, 2013 http://minerals.usgs.gov/minerals/pubs/commodity/cobalt/.
M. J. Hawkins: Journal of metals, 1998, vol. 50, pp 44-45.
R.R. Moskalyk and A. M Alfantazi: Minerals and Metallurgical Processing, 2000, vol. 17, pp. 205-16.
S.C. Das and T. Subbaiah: Hydrometallurgy, 1984, vol. 12, pp. 317-33.
K.C. Lenthall, and A. W. Bryson: Electrowinning cobalt from sulphate solutions, aqueous electro technologies: Progress in theory and practice, The Minerals Metals and Materials Society, Warrendale, PA, 1997, pp. 305–10.
I.G. Sharma, P. Alex, A.C. Bidaye, and A.K. Suri: Hydrometallurgy, 2005, vol. 80, pp.132-38.
K.G. Fisher: The Southern African Institute of Mining and Metallurgy 6th Southern African Base Metals Conference, p. 237–57, 2011.
B. Swartz, S. Donegan, and S. Amos: Hydrometallurgy Conference, 2009, pp. 385–400.
P.C. Angelo and R. Subramanian: Powder Metallurgy: Science, Technology and Applications, PHI publishers, India, 2008.
N. Mulaudzi and M. Kotze: The Southern African Institute of Mining and Metallurgy Base Metals Conference, 2013, pp. 1–14.
K.G. Fisher and L.G. Treadgold: Alta Nickel-Cobalt Conference, ALTA metallurgical services, 2009.
T. C. Franklin: Surface and coatings technology, 1987, vol. 30, pp.415-28.
L. Oniciu and L. Muresan: Journal of applied electrochemistry, 1991, vol. 21, pp. 565-74.
M.I. Jeffery, W.L. Choo, and P.L. Breuer: Mineral engineering, 2000, vol. 12, pp. 1231-47.
S. C. Das and T. Subbaiah: Journal of applied electrochemistry, 1987, vol. 17, pp. 675-83.
B.C. Tripathy, P. Singh, and D.M. Muir: Metallurgical and material transactions B, 2001, vol. 32B, pp. 395-99.
R.F. Renner and K. C. Liddell: Journal of applied electrochemistry, 2002, vol. 32, pp. 621-27.
B.C. Tripathy and M. Kotze: Australia Patent, 2012, AU 2012101517 A4.
T.C. Franklin, V. Totten, and A. Akten: J. Electrochem. Soc., 1988, vol. 135, pp. 1638-40.
B.C. Tripathy, S.C. Das, P. Singh, and G. T. Hefter: Journal of applied electrochemistry, 1999, vol. 29, pp. 1229-35.
A. Ciszewski, S. Posluszny, G. Milczarek, and M. Baraniak: Surface and coatings technology, 2004, vol. 183, pp. 127-33.
A. Biswal, B.C. Tripathy, T. Subbaiah, D. Meyrick, and M. Minakshi: Journal of solid state electrochem, 2013, vol. 17, pp.1349-56.
T.S.N. Sankara Narayanan: Metal Finishing, 1999, vol. 97, pp.94-99.
D.J. Robinson and T.J. Okeefe: Journal of applied electrochemistry, 1976, vol. 6, pp. 1-7.
B.C. Tripathy, S.C. Das, G.T. Hefter, and P. Singh: Journal of applied electrochemistry, 1997, vol.27, pp. 673-78.
Acknowledgments
The authors are thankful to MOES, New Delhi for partial financial support. The authors would also like to thank Dr. Y.S. Chaudhary and K.K. Nanda for providing help in carrying out polarization studies. Thanks are also due to Jogeswar for XRD and Debadatta for SEM analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 10, 2014.
Rights and permissions
About this article
Cite this article
Patnaik, P., Tripathy, B.C., Bhattacharya, I.N. et al. Effect of Tetra Propyl Ammonium Bromide During Cobalt Electrodeposition from Acidic Sulfate Solutions. Metall Mater Trans B 46, 1252–1256 (2015). https://doi.org/10.1007/s11663-015-0301-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0301-6