Abstract
Diffusion path analysis was applied to the Ni-Si-B system to explain the deviation from classical transient liquid-phase bonding observed when using multicomponent systems containing Boron as a melting point depressant. This was achieved using a combination of differential scanning calorimetry, SEM-EDS microstructural and chemical analysis, and Thermo-Calc modeling software of Ni/Ni-Si-B couples. Compositional analysis identified differing distributions of B and Si across the braze joint which were mapped onto isothermal sections of Thermo-calc-generated phase diagrams. Below 1093 °C, the inevitable formation of diffusionally affected zone (DAZ) and isothermally solidified zone is a direct consequence of the need for phase equilibria in the Ni-Si-B ternary system and is predicted by diffusion path analysis. Above 1100 °C, diffusion path analysis also correctly predicts a persistent liquid phase observed to occur through the melting of the DAZ (i.e., a γ-Ni(Si)-Ni3B eutectic reaction).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The strict performance requirements of aerospace applications typically warrant the use of advanced joining techniques such as vacuum diffusion brazing, as it offers several advantages over other brazing and welding methods. Joining is accomplished through the melting of a low-melting point filler metal (FM) alloy and subsequent diffusionally induced solidification; hence the name Transient Liquid-Phase Bonding (TLPB).[1,2] Most often TLPB of superalloys is achieved using nickel-based brazing alloys containing either Boron, Phosphorous, and/or Silicon in addition to other alloying additions. These alloys generally exhibit an excellent combination of wetting, spreading, low fusion temperatures, and relatively low joining times, similar to the prohibitively expensive Ni-Au (or Pd) alloys.[3] Despite their extensive service, considerable uncertainty remains with regards to the exact mechanism by which TLPB and isothermal solidification occurs in boron-containing systems, since they deviate from classic TLPB theory.[4,5,6,7] In classic TLPB as described by Eager, Tuah-Poku et al.,[1,2,8,9,10,11] the filler metal is an ideal eutectic with melting point depressant (MPD) elements displaying appreciable solid solubility in the base metal (Figure 1(a)). During brazing the MPD solute atoms diffuse from the liquid to the solid solution (SS) matrix and subsequently redistribute and homogenize in the base metal (BM). The high soluble MPD allows for isothermal solidification and maintains a single-phase microstructure in the BM. The solute composition is assumed constant in the liquid phase, but has a decreasing concentration with increasing distance from the solid/liquid interface within the BM.
Conversely, the immiscibility of B in Ni (0.015 wt pct max.) and its rapid interstitial diffusion in Ni[12,13,14] results in the heterogeneous distribution of embrittling borides in the vicinity of the joint (Figure 1(b)). The region of boride precipitates in the BM is known as the diffusionally affected zone (DAZ) and has been widely reported for Ni-base superalloys, in which segregation and progressive coarsening of metal borides adjacent to the original solid/liquid interface occurs—the exact type and composition governed by the materials involved.[15,16,17,18,19] A (γ-Ni) solid solution single-phase layer forms on the opposite side of the original solid/liquid interface and is known as the isothermally solidified zone (ISZ). During brazing, the DAZ and ISZ layers grow in thickness while the liquid layer deceases and eventually removed through diffusional solidification. The compositional profile of elements in the braze that are soluble in the (γ-Ni) have a similar distribution as for the classic case of Figure 1(a). However, the B profile is markedly different, going to near zero in the ISZ layer, and discretely increasing to its concentration in the filler metal liquid. In the DAZ, the boron is contained within the boride compounds (e.g., Ni3B). The volume fraction of boride within the two-phase DAZ layer decreases further into the BM. Therefore, the bulk composition of B is decreasing with distance away from the DAZ/ISZ layer, eventually reducing to zero further into the BM (bottom schematic of Figure 1(b)).
Considerable efforts have been made to adapt conventional TLPB theory and models to Ni-based superalloys with Boron-based filler metals (FM)s.[4,5,6,7,20,21,22,23] In most instances, however, the adoption of Boron FMs results in either the in-situ formation of intermetallics precipitates or a significant reduction in the rate of isothermal solidification.[4,5,6,7] The former believed to be an artifact of the rapid diffusivity of B in Ni as compared to other solute elements; the latter related to the melting of intermetallic precipitates thereby increasing the quantity of liquid. Nonetheless, the typical approach is to assume that the interface structure and compositional profile can be approximated by the arrangement depicted in Figure 1(a), and considering B as the diffusing species that controls IS. Little effort has been spent to understand the more complex interface microstructure of Figure 1(b) that actually occurs in the joints. In this study, it is proposed that this interface microstructure can be analyzed and understood on the basis of diffusion path theory as it applies to multicomponent systems.[24,25,26] The diffusion path approach requires a comparison between the experimentally determine interface structure and composition profile with the relevant multicomponent phase diagram. To facilitate this comparison, a ternary alloy system containing Ni was considered. The Ni-Si-B ternary composition was chosen since Si and B are the most common MPD additions in superalloy filler materials. The experimental TLPB behavior of a pure Ni BM/Ni-Si-B filler braze couple was determined using differential scanning calorimetry (DSC), metallography and Scanning Electron Microscopy and Energy Dispersive Spectrometry (SEM-EDS). The results were compared to various ternary phase diagram predictions developed using Thermo-Calc Software.
2 Experimental Procedure
All Ni/Ni-Si-B couples used in the current study were created using Ni200 (99.8 pct Ni) 161.588-mm-thick plate (McMaster Carr) electrical discharge milled to 4.763 mm diameter pucks, and the BNi-3 (Ni-4.5Si-3.2B, wt pct) amorphous brazing foil (approx. 48 μm thick) procured from Vitta Corporation (AMS/AWS 4778/BNi-3) the compositions of which are provided in Table I.
All solidification/melting tests implemented a Netzsch Pegasus 404 differential scanning calorimeter (DSC) under continuous flowing high-purity (99.9999 pct) argon gas at a rate of 20 cc/min, following a dual evacuation/pressurization procedure (P = 10−5 mbar). The DSC itself was calibrated beforehand using the onset and enthalpy values obtained from the melting of a series of high-purity reference metals. Alumina crucibles (and lids) were used in all instances as it prevented wetting and chemical interaction with the Ni-based brazing alloy. Assembling a brazing couple involved placing a 4.763 mm diameter punched disk of the ≈ 48 µm thick BNi-3 foil within the crucible followed with a Ni puck. This arrangement, shown schematically in Figure 2, was designed to improve the DSC signal as described by Turriff et al. and facilitate incorporation of the Ni into the FM during melting.[27] Interrupted tests were performed in the DSC to examine the microstructure at a desired temperature via furnace quenching (< 50 °C/min), otherwise cyclic thermal profiles were implemented at 20 K/min between 870 °C and the brazing temperature (TB), as to ensure complete solidification (solidus of BNi-3 is 993 °C). The purpose of this cyclic method was to allow repeated enthalpy measurements during solidification (ΔHS), as discussed in greater detail by other authors.[27,28,29,30,31] This technique enables one to examine the rate of isothermal solidification with time (above solidus) using a single specimen, thus minimizing experimental variation while greatly reducing experimental preparation. The influence of cycling vs a constant hold has been repeatedly confirmed to be negligible for the current system. Microstructural characterization involved grinding/planning, followed by sequential polishing; 9, 3 μm, and finishing with colloidal silica suspension. Etching was achieved using waterless Kalling’s reagent. All imaging procedures were performed in the unetched condition, using either an optical or scanning electron microscope (Hitachi S4700 CFE). All compositional measurements were acquired via Oxford X-max (80 mm2) energy dispersive spectrophotometer (EDS) during SEM examination using an acceleration voltage and emission current of 5 kV and 20 μA, respectively. the lower voltages were adopted to improve detection of Boron. Also, the main detector was supplemented with a electron backscatter detector (EBSD) to further improve the contrast between the boron-rich phases (borides) All compositional measurements were acquired via energy dispersive spectroscopy (EDS) during SEM examination (Hitachi) using an acceleration voltage and emission current of 5 kV and 20 μA, respectively. Backscatter was adopted to improve the contrast between the various phases as described by Vargas et al.[32] Lastly, Thermo-Calc® thermodynamic modeling (Database: TCNI9/2019.01.25.12.06.45) was adopted to assist in explaining experimental results.
3 Results and Discussions
3.1 Thermal and Microstructural Characterization of BNi-3
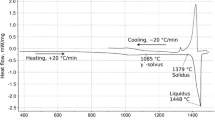
Figure 3 illustrates the representative DSC results obtained after repeated cycling of a BNi-3 foil between 870 °C and 1100 °C, wherein melting/solidification involves three thermal events; a sharp melting event between 994 °C and 1010 °C, a second broader melting (994 °C to 1050 °C) event believed to initiate concomitantly with the first event, and lastly a small melting event between 1050 °C and 1065 °C (liquidus). The shape of the overall melting peak is characteristic of off-eutectic (hypo) melting behavior.[33]
From Figure 3, the cooling trace also indicates three solidification peaks. The high-temperature peaks onset below the liquidus of 1065 °C due to the influence of undercooling. However, these peaks are broad and shallow, much like the 2nd and 3rd melting peaks. A more intense peak with a sharp onset (much like the 1st melting peak), completes solidification. This comparison of the melting and solidification traces indicates that the same events occur during heating and then in reverse sequence during cooling. The individual peaks correspond to the individual melting/solidification events of primary and binary and ternary eutectics events as confirmed by thermodynamic modeling latter.
Figure 4 presents the microstructure of the BNi-3 sample after solidification from 1100 °C. Numerous SEM-EDS spot scans were performed on the various microstructural constituents visible in the solidified BNi-3. It must be acknowledged, however, that owing to Boron’s size and electronic structure, obtaining quantitative results is notoriously difficult. Also, the relatively low accelerating voltages required for quantitative measurements of B (< 5 kV) may compromise the accuracy of other species, e.g., Kα(Ni), Lα(Ni), and Kα(Si) = 7.46, 0.85, and 1.74 eV, respectively. Two distinct phases, γ-Ni(Si) and Ni3B and an additional Si-rich region (TE) likely corresponding to the last of the liquid phase to solidify are labeled in Figure 4. SEM-EDS analysis performed on the Ni3B, γ-Ni(Si), and TE phases yielded concentrations of 26.9 ± 1.6 at. pct B, 9.5 ± 1.6 at. pct Si, and 15.8 at. pct Si, respectively. The sub-stoichiometric Si content measured for what was believed to be the β phase (β-Ni3Si) may be an artifact of the operating conditions i.e., the inability to measure low conc. of boron of Ni-Si-B phase. However, in the context of the current study it is likely attributed to limitations of EDS to measure the very fine β-Ni3Si precipitates alone, thereby yielding results intermediate between that of β-Ni3Si (25 at. pct) and γ-Ni(Si) (8 at. pct Si max.).[34,35,36] As expected, the composition of the Ni3B borides are nearly stoichiometric (25 at. pct B) and represent the only Boron-bearing phases, while the saturated γ-Ni(Si) is approx. that of the solubility limit at the given temperature.[12,13,14,37,38]
To assist in understanding the sequence of solidification events observed on the DSC cooling trace and the resultant solidified structure of BNi-3, a Ni-rich isopleth with a constant Si:B ratio of 1:1.4 (wt pct) was generated using Thermo-Calc software (Figure 5). The composition and solidification sequence of BNi-3 is traced as a vertical line on the figure. The equilibrium sequence starts with solidification of; (1) primary γ-Ni(Si), (2) Ni3B + γ-Ni(Si) binary eutectic (BE), and (3) Ni3B + γ-Ni(Si) + β-Ni3Si ternary eutectic (TE). Nonequilibrium freezing would produce the same sequence but modifying the relative quantities of phase(s). It should also be noted that the wide spread precipitation of the γ′-Ni3Si; the semi-coherent intermediate of βx-Ni3Si infers a significant degree of supersaturation of Ni with Si, preserved by the rapid solidification rate, as clearly evident in Figure 4.
3.2 Influence of Temperature on the TLPB Behavior of Ni/Ni-Si-B Couples
Following the thorough characterization of the BNi-3 FM, focus shifted towards assessing the TLPB response of Ni/Ni-Si-B (Ni/BNi-3) couples as a function of peak brazing temperature. Recommended brazing temperatures for BNi-3 range from 1010 °C to 1175 °C;[39] thus, peak cyclic temperatures of 1020 °C, 1050 °C, 1075 °C and 1100 °C were examined. Figure 6 illustrates the DSC heating (A) and cooling traces (B) associated with the first two heating/cooling cycles as well as that obtained after the final 19th cycle. It should be noted that the “hook” like shape of the DSC curve immediately upon cooling, is an experimental artifact caused by the transient nature of the cooling rate immediately upon cooling. Once the cooling rate becomes constant at the prescribed rate of 20 k/min, the baseline becomes stable. This hook persists throughout the additional cycling further confirming that it is a condition of the transient cooling rate and not an actual solidification event.
The 1020 °C cyclic trace indicates that only the ternary eutectic portion of the BNi-3 foil melts and solidifies. This melting/solidification event persists throughout most of the cycles, but the peak decreases in magnitude, indicating a reduced amount of liquid and an isothermal solidification (IS) process. Despite only partial melting of the BNi-3 foil, wetting, bonding and joint formation with the Ni200 BM occurs at 1020 °C (see Figure 7). Even with the short brazing time (Figure 7(a)), some diffusion of B into the Ni200 BM has occurred at 1020 °C as evidenced by the Ni3B precipitation in the solid BM forming the diffusionally affected zone (DAZ). The joint region of Figure 7(a) (which represents a brazing time of less than 3 minutes) exhibits a matrix of resolidified TE with dispersed, coarse Ni3B particles throughout. Since complete melting of the BE
(which involves Ni3B) requires a temperature of approx. 1045 °C (Figure 3), the coarse residual Nickel Borides remain as a solid fraction at 1020 °C. It should be noted that the microstructure of the filler metal in Figure 7(a) is similar to that observed in the foil only, when furnace quenched from 1020 °C.
Despite only the partial melting of the filler at 1020 °C, the last heating/cooling cycle (cycle 19 in Figure 6) exhibits no melting or solidification event, indicating complete isothermal solidification of the TE liquid phase. Figure 7(b) confirms this observation microstructurally, where the filler region consists of a γ-Ni(Si) ISZ region with residual, unmelted Ni3B dispersoids. The DAZ layer has grown in thickness through continued B diffusion and precipitation into the pure Ni BM.
For the sample brazed at 1050 °C, the DSC trace also exhibits repeatable melting/solidification peaks in the vicinity of the TE with the addition of a broad BE melting peak up to 1050 °C (Figure 6). The magnitude of the peaks decreased with brazing time, also indicating a reduced liquid fractions and IS process. The microstructure of Figure 8(a) indicates that near-complete melting of the BNi-3 occurred, when compared to the morphology observed in Figure 4 (i.e., well developed BE and TE structures are evident). Otherwise, the 1050 °C sample exhibited similarities with the 1020 °C sample in that complete IS occurred within 19 cycles, a DAZ was formed with the BM and some unmelted dispersed Ni3B was present (Figure 8(b)).
The sample cycled at 1075 °C (slightly above the liquidus of BNi-3) exhibited a distinctly different DSC trace following the first melting event (see Figure 6). The melting and solidification behavior shift to a higher temperature range and there is no longer a distinct thermal event near the temperature associated with the TE. This is more evident when plotting all full melting/solidification cycles of this sample shown in Figure 9 wherein the peak associated with TE melting/solidification disappears after 2 cycles. This demonstrates that the high-temperature melting/solidification event persists but diminishes in magnitude until complete IS occurs by the 19th cycle. With complete melting of the BNi-3 foil at 1075 °C, the complete IS joint was free of residual Ni3B dispersoids as indicated in Figure 10(b).
The 1100 °C sample also exhibited different behavior than the other samples. The DSC traces of Figure 6 indicate the development of a strong, melting/solidification event in the temperature range of 1090 °C to 1100 °C. This event persists in a similar magnitude even after 19 cycles. The microstructure of this sample near the faying surface after 19 cycles is shown in Figure 11. The layered DAZ/ISZ structure present in the other samples is completely disrupted and absent in the 1100 °C cycle. As will be discussed in more detail below, the DAZ layer of the joints at 1020 °C, 1050 °C and 1075 °C have a composition and structure close to that associated with the Ni-Ni3B binary eutectic which has a melting point of 1092 °C.[12,13,14,37,38] Therefore, it is likely that heating to 1100 °C melted the DAZ layer as observed by Ruiz-Vargas et al.[35]
3.3 DSC Examination of Base Metal Dissolution Phenomena
The DSC trace for the sample brazed at 1075 °C exhibited a distinct shift in its melting behavior. It is assumed that this is a result of BM dissolution which enriches the Ni content of the liquid phase. To confirm this assumption, pure Ni powder was added to the BNi-3 foil in increasing amounts. These mixtures were then heated to 1350 °C to completely melt and homogenize the mixtures. Samples were cycled up to 1350 °C a total of three times to ensure homogenization and to determine the influence of Ni content on the melting behavior. The melting traces for the last heating cycle for each mixture are illustrated in Figure 12. The results show the changing melting characteristics due to increasing Ni additions (i.e., simulating BM dissolution). Increasing the Ni wt pct reduces the magnitude of the invariant TE melting peak, which onsets at 994 °C and continuously shifts the 2nd broader BE melting event to higher temperatures. An even broader peak extends to higher temperatures as the Ni content increases, reaching a temperature near 1300 °C when the Ni content reaches 96.3 pct The TE melting event disappears from the DSC trace at and above a Ni content of 94.7 wt pct.
The microstructures corresponding to the 93.2, 93.8, 94.7 wt pct Ni samples from Figure 12 are displayed in Figure 13. Consistent with the DSC results, the 93.2 and 93.8 pct Ni samples exhibit regions of TE and BE microstructure. Conversely the 94.7 pct Ni sample indicated only the BE structure and a pronounces primary γ-Ni phase. Ni compositions of 96.3 and 99.8 pct Ni had microstructures similar to the 94.7 pct sample accept the primary γ-Ni phase increased in volume fraction. EDS analysis of the gamma-Ni grains yielded consistent Si concentrations of less than < 0.5 at. pct both in primary γ-Ni and eutectic γ-Ni phases. Comparison of the melting curve of the 1075 °C braze sample (Top plot of Figure 9) indicates that the absence of the TE and onset temperature of the BE melting event is very close to the a liquid phase diluted to 94.7 wt pct Ni
Figure 14 reintroduces the Ni-Si-B isopleth prediction from Thermocalc for the case of a fixed Si:B ratio of 4.5:3.2 = 1.4, shown previously, however, also including the experimental compositions and corresponding peak temperatures acquired from the DSC dilution studies. The solidus temperatures for the TE and BE events were determined from the temperature at which the endothermic peaks on the DSC traces of Figure 12 first deviated from the baseline. The BE and Primary liquidus temperatures were measured from the point of the maximum endothermic energy of the appropriate peaks (i.e., the “peak” temperature) to avoid an overestimation of the values that could be caused by thermal lag. The experimental values appeared to agree very well with the calculated predictions considering that the heating and cooling rate within the DSC was 20 K/min (i.e., nonequilibrium heating and cooling). More importantly, the comparison reinforces the melting/solidification sequence as a function of Ni composition observed and validates the Thermo-calc predictions
3.4 Solidification/Diffusion Path Studies in the Ni/Ni-Si-B System
The isopleth analysis in the previous section is most applicable to alloys with a homogeneous composition or to help interpret the solidification behavior of any remaining liquid in the braze joint upon cooling. Interpretation of diffusional or isothermal solidification of the braze joints, where the composition varies across the diffusion couple and phase equilibria at interfaces is imposed, requires isothermal sections of the ternary Ni-Si-B phase diagram along with diffusion path theory. Figure 15 presents a 1075 °C isothermal section of the Ni-Si-B ternary phase diagram using the Thermo-Calc software. The isopleth used previously corresponds to a straight line intersecting the Ni apex and the nominal composition of the BNi3 foil (dashed line 1 to 4 in Figure 14) and is very useful for monitoring the phenomena of BM dissolution. However, the heterogenous microstructure i.e., BM → DAZ → ISZ observed can be best understood using diffusion path theory. The microstructure of an additional sample cycled at 1075 °C for only 6 cycles is shown in Figure 16 with the corresponding solute distribution as determined through EDS to assist in the analysis. The concentration of B and Si in the liquid are assumed to be nominal composition of the BNi-3 FM, while the boron concentration within the DAZ is predicted based on the bulk B content as inferred by the volume fraction of Ni3B with position. The microstructure of the joint consisting of, (i) a solute-free BM, γ(Si = 0) transitioning to (ii) a region of isolated Ni3B borides near the original faying surface γ-Ni with increasing Si concentrations (iii) a boron free region of γ-Ni with an ever increasing Si composition and (iv) terminating at a continuous interface of athermally solidified BE eutectic (γ-Ni(Si)/Ni3B) corresponding to remaining liquid phase. A discrete change in the B and Si composition occurs which would be expected for the solid/liquid interface. The measured B and Si compositions from Figure 16 are mapped onto the isotherm of Figure 15 as a diffusion path, the solid line 1—1a—2—3—4.
The main rules restricting the number of possible diffusion paths relates to; the morphology of the interfaces (columnar, lamellar, continuous) and the requirement that mass conservation be maintained as described in the conventions established by Clark.[26] Two of the most likely diffusion paths are the dashed line (1 to 4) and solid line (1—1a—2—3—4) of Figure 15 given the microstructure and solute distribution displayed in Figure 16. The resultant microstructure for these two paths are represented schematically in Figure 17 center and right.
The simplest path would involve a diffusion couple between the Ni apex and the nominal composition of the BNi3 (diffusion path 1 to 4) thereby complying with mass conservation. However, this configuration poorly represents that observed experimentally in Figure 16, as it would result in the structure shown in Figure 17 (right). In this case, the simultaneous, interpenetrating growth of γ-Ni and Ni3B during isothermal solidification and considerably greater concentrations of Si within the DAZ would occur. These two phases would extend to the solid/liquid interface such that a three-phase equilibrium between of γ-Ni, Ni3B and liquid could be established. No ISZ layer is predicted with this diffusion path.
The microstructure of the 1075 °C joints clearly shows that the growing ISZ layer is the same phase as the pure Ni base metal (see the bottom images of Figures 7, 8 and 10). Furthermore, the interface with the liquid is that of this same single phase. Therefore, the Ni3B within the DAZ represent isolated precipitates within the single-phase γ-Ni(Si) solid solution. This DAZ structure is predicted by the diffusion path measured and traced in Figure 15i.e., 1 → 1a → 2 since it corresponds to diffusion path convention No. 7 described by Clark.[26] Namely a diffusion path ‘traversing a two-phase field in a curved path and returning to the same single-phase field (e.g., line d to f) represent isolated precipitates in a single-phase layer’. The growing ISZ layer is represented by the diffusion path 2 → 3 which is a compositional variation within a single-phase region. Diffusion path 3 → 4 with a dashed line crossing a two-phase region parallel to the tie lines, follow convention No. 4 described by Clark,[26] with a corresponding interface separating the two phases, in the current case, phase γ-Ni(Si) and convention. Consequently, the application of the Ni-Si-B diffusion path analysis fully explains the experimental diffusion couple and TLPB braze microstructure.
The measured Si levels repeatedly show to be below those predicted by the calculated equilibrium data (approximately 11 at. pct at point 3 in Figure 15), this is believed to be a consequence of inaccuracies measuring Si using EDS and possibly the reduced solubility of Si at room temperature (the condition measured in the SEM).
The 1100 °C Ni-Si-B isotherm shown Figure 18 was also created to assist in explaining the persistent liquid phase observed at this brazing temperature. EDS line scans gave a maximum Si concentration of 2 at. pct Si within the γ-Ni(Si) of the athermally solidified BE displayed in Figure 11. In most instances, Si concentrations were below 1 at. pct, inferring a significant degree of base metal dissolution resulting from liquefaction of the DAZ. Figure 18 reveal that new phase fields develop at 1100 °C adjacent to the Ni-B axis and in the compositional range of 15 to mole pct B, including a single-phase liquid field appearing near 0 pct Si, representing the melting of the binary eutectic Ni-Ni3B. (Tm = 1093 °C). With a small increase in Si content, a three-phase region consisting of L + γ-Ni + Ni3B develops. The triangular area of this field extends down toward the Ni-Si binary axis. Superimposing the 1 → 1a → 2 diffusion path measured for the 1075 °C couples indicates that the region near the DAZ-ISZ transition falls within the three-phase field. Therefore, this region of the diffusion couple would melt at 1100 °C. Furthermore, the two-phase field adjacent to the Ni-B binary axis, which was γ-Ni + Ni3B, has now become L + γ-Ni. These changes demonstrate that the DAZ-ISZ layer undergoes bulk melting creating a new liquid phase that is in equilibrium with a γ-Ni(Si) solid solution. The 1100 °C isotherm indicates that the solubility of B in this phase is very low. The main sink of B in the solid phase at 1075 °C and below was the precipitated DAZ layer. With the melting of this layer and particularly Ni3B, the low solubility of B in the remaining solid results in a persistent liquid phase. Such findings are further confirmed by the DSC results.
4 Conclusions
The application of diffusion path analysis in conjunction with SEM-EDS and thermodynamic modeling proved successful in explaining the underlying mechanisms responsible for the deviation from conventional TLPB theory in B-containing diffusion couples. The primary findings are summarized as follows:
-
1.
Below the binary γ-Ni/Ni3B eutectic temperature of 1093 °C, TLPB of Ni/Ni-Si-B couples resulted in a microstructure consisting of a network of Ni3B intermetallics along the faying surface (DAZ) and related opposing growths of single phase (ISZ)
-
2.
Above 1093 °C, melting of the DAZ occurred along with the formation of a persistent liquid phase as predicted by calculated ternary phase diagrams.
-
3.
The deviation from conventional TLPB theory is attributed to the need to establish phase equilibria at the interface of a multicomponent alloy system. In the case of the Ni-Si-B system, this necessitates the formation of B-rich DAZ layer and Si-rich ISZ layer at the former pure Ni/BNi-3 liquid interface. This interface structure is immediately developed as soon as the solidus temperature of the filler metal is exceeded.
-
4.
During Isothermal solidification, the widths of the DAZ and ISZ layers increase, while that of the liquid layer decreases. This results in isothermal solidification.
References
W.D. Macdonald, T.W. Eagar, Annu. Rev. Mater. Sci., (1992) Vol. 22 pp. 23-46.
D.S. Duvall, W.A. Owczarski, D.F. Paulonis, Welding Journal, (1974), Vol. 53-4 pp. 203-214.
P. Roberts: 2nd ed., CRC Press, Boca Raton, 2013, pp. 266–314.
N.P. Wikstrom, O.A. Idowu, O.A. Ojo, M.C. Chaturvedi: in Proc. 3rd Int. Brazing and Soldering Conf. TX, 2006.
C.W. Sinclair, J. Phase Equilibira. (1999), 20(4), pp. 361–69.
C.W. Sinclair, G.R. Purdy, J.E. Morral, Metall. Mater. Trans. A., (2000), Vol. 31A pp. 1187-1192.
X.J. Yuan, M.B. Kim, C.Y. Kang, J. Mater. Sci. Tech., (2011), Vol.27(7), pp. 1191-1197.
W.A. Owczarski, W.H. King, D.S. Duvall: U.S. Patent# 3,530,568, 1970.
I. Tuah-Poku, M. Dollar, T. B. Massalski, Metall. Trans. A., (1988), Vol. 19A, pp. 675-686.
J. T. Niemann, R.A. Garett, Welding Journal, (1974), Vol. 53(4), Part 1., pp. 175-183.
H. Nakagawa, C.H. Lee, T.H. North, Metall. Trans. A, (1991), Vol. 22A, pg. 543-555.
J.D. Schobel, N.H. Stadelmaier, Z. Metallkd., (1965), Vol. 56, pp. 856.
T. Tokunaga, K. Nishio, H. Ohtani, M. Hasebe, Materials Trans., (2003), Vol. 44(9), pp. 1651-54.
B. Jansson, J. Agren, A, Mat. Sci. and Eng., (1984), Vol. 63, pp. 51-60.
M. Pouranvari, A. Ekrami, J. Weld. Res. (2014), vol. 93, pp. 60–68.
M. Pouranvari, A. Ekrami, Canadian Metallurgical Quarterly, (2014), Vol. 53(1), pp. 38-46.
A. Ghasemi, M. Pouranvari, Sci. and Tech. of Welding and Joining, (2018), 23(5), pp. 441-448.
M. Pouranvari, A. Ekrami, A.H. Kokabi, J. Sci. Tech. Weld. Join. (2018), 23(1), pp. 13-18.
A.T. Egbewande, C. Chukwukaeme, O.A. Ojo, J. Materials Characterization, (2008), Vol. 59, pg. 1051-1058.
J.E. Ramirez, S. Liu: Weld. J. Suppl. 1992, pp. 365–76.
Y. Zhou, W.F. Gale, T.H. North, Int. Mat. Rev., Vol. 40(5), (1995), pp. 181-196.
K. Ohsasa, T. Shinmura, T. Narita, J. Phase Equilibria, (1999), 20(3), pp. 199-206.
M. Abdelfatah, O.A. Ojo: in Proc. 3rd Int. Brazing and Soldering Conf., TX, 2006.
R. Metselaar, J. Mater. Ed. (1984), 6, p. 229–260.
R. Metselaar, J. Mater. Ed. (1985), 7(4), p. 653–685.
J. B. Clark, Transactions of the Metallurgical Society of AIME, (1963), Vol. 227, pp. 1250–1251.
D.M. Turriff, S.F. Corbin, M. Kozdras, Acta. Mater., (2010), Vol. 210(8), pp. 1332-1341.
Corbin, P. Lucier, Metall. Mater. Trans. A, (2001), Vol. 32(4), pp. 971-978.
M.L. Kuntz, S.F. Corbin, Y. Zhou, Acta Mater., (2005), Vol. 53(10), pp. 3071-82.
D.C. Murray, S.F. Corbin, J. Mater. Proc. Tech., (2017), Vol. 248, pp. 92-102.
B.A. Lindner, Thermodynamic and Kinetic Simulation of Transient Liquid-Phase Bonding. M.A.Sc. Thesis Dissertation University of Wisconsin Milwaukee, 2015.
J. Ruiz-Vargas, N. Siredey-Schwaller, P. Noyrez, S. Mathieu, P. Bocher, N. Gey, J. Mater. Charac., (2014), Vol. 94, pp. 46-47.
W. J. Boettinger, U.R. Kattner, K.W. Moon, J.H. Perepezko, DTA and Heat-Flux DSC Measurements of Alloy Melting and Freezing: Practice Guide. National Institute of Standards and Technology, Special Publication, 2006, pp. 960–15.
J. Ruiz-Vargas, N. Siredey-Schwaller, P. Bocher, A Hazotte, J. Mater. Proc. Tech., (2013a), Vol. 213, pp. 2074-80.
J. Ruiz-Vargas, N. Siredey-Schwaller, N. Gey, P. Bocher, A Hazotte, J. Mater. Proc. Tech., (2013), Vol. 213(1), pp. 20-29.
S.K. Tung, L.C. Lim, M.O. Lai, Scripta Materialia, (1996), Vol. 34, pp. 763-769.
S. Lebaili, S. Hamar-Thibault, Acta Metall., (1987), Vol. 35(3), pp. 701-710.
S. Omori, Y. Hashimoto, K. Shoji, K. Hidaka, Y. Kohira, Funtai Oyobi Funmatsuyakin, (1972), Vol. 18, pp. 316.
ASM International Handbook Committee, Volume 6—Welding, Brazing, and Soldering. Materials Park, OH 44073-0002, 1993, 6, pp. 924–30.
Acknowledgments
The authors would like to thank the Natural Science and Engineering Research Council of Canada (NSERC) and Pratt and Whitney Canada for their financial support of this research. Also, the authors like to thank Alain Bouthillier, Daniel Turner, and Francesco D’Angelo of Pratt and Whitney Canada for their valuable contributions and guidance with regard to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted April 12, 2019.
Rights and permissions
About this article
Cite this article
Moreau, E.D., Corbin, S.F. Application of Diffusion Path Analysis to Understand the Mechanisms of Transient Liquid-Phase Bonding in the Ni-Si-B System. Metall Mater Trans A 50, 5678–5688 (2019). https://doi.org/10.1007/s11661-019-05459-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-019-05459-8