Abstract
Centrifugal force was used to produce open-cell Zn-22Al-2Cu alloy foams by the replication method. Three different sizes (0.50, 0.69, and 0.95 mm) of NaCl spherical particles were used as space holders. A relatively low infiltration pressure was required to infiltrate completely the liquid metal into the three pore sizes, and it was determined based on the centrifugation system parameters. The infiltration pressure required was decreased when the diameter of the particle was increased. The porosity of the foam was increased from 58 to 63 pct, when the pore size was increased from 0.50 to 0.95 mm, while the relative density was decreased from 0.42 to 0.36. The NaCl preform was preheated to avoid the freezing and to keep the rheological properties of the melt. The centrifugal-replication method is a suitable technique for the fabrication of open-cell Zn-Al-Cu alloy foams with small pore size. The compressive mechanical properties of the open-cell Zn-22Al-2Cu foams increased when the pore size decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metal foams have emerged as an important field of research due to their special structure for impact energy absorbing and low weight, particularly aluminum alloy foams. Metal foam application generally depends on its macroscopic structure (pore size, pore morphology, overall porosity, and distribution) as well as the alloy’s inherent properties (yield strength, Young’s modulus, and hardness).[1] The interest in the practical applications of foamed metals drives the development of several methods to manufacture metallic foams from aluminum alloys. Among them, a simple and versatile method is the replication process, where the liquid metal is infiltrated through the pre-existing network of a leachable preform. One of the attractive features of the replication process is the high degree of control in the size and shape of the pore.[2] The preform is usually comprised of NaCl particles, which result in the formation of a porous structure once the metal solidifies and after leaching of the preform. NaCl particles are widely used to produce open-cell foams because of their high degree of control in the size and shape of the pore, high melting point, and nonchemical reaction with the alloy.
The driving of the liquid metal into the spaces left by the preform requests the application of pressure on the metal, since the metal does not infiltrate spontaneously because liquid metals usually do not wet the interstitial spaces of the preform.[3] This application of pressure or driving force for the infiltration of liquid metal into a porous material can be generated several ways, such as by vacuum-assisted pressure, gas pressure, and extrusion pressure.[4,5,6] Nowadays, most researchers have focused their work on foaming aluminum alloys[6,7]; the basic principles of foaming technology have been transferred to other low-melting metals, especially in Zn-Al alloys since they exhibit good capacity to absorb energy and mechanical damping. Zinc and its alloys have a lower melting point than aluminum alloys, and they have compatibility with steel regarding corrosion resistance, which could be suitable for filling hollow steel sections to improve their stiffness.[8] Kitazono and Takigushi[9] pointed out that in the case of metallic materials, an increase in strain rate sensitivity (m) is desirable for energy absorbing materials. They reported that the m value of nanocrystalline Zn-22Al alloys is as large as 0.2 at room temperature, while aluminum alloys have a small m value. The compressive properties of the Zn-22Al superplastic alloy foam were evaluated at high temperature by Sekido and Kitazono.[10] They found that the compressive flow stress increased with increasing the strain rate. The strain rate sensitivity exponent was determined as 0.55, which is identical to dense Zn-22Al alloy. Muñoz et al.[11] determined that the addition of copper to the Zn-Al alloy enhances its corrosion resistance under the service conditions of stress and temperature and increases the strength and creep resistance without seriously affecting the superplasticity property of the alloy. Negrete and Torres[12] pointed out that the Zn-22Al-2Cu alloy named zinalco shows a unique combination of properties, such as high strength, good machinability, and toughness, that make it suitable to produce metallic foams. The production of new types of Zn-Al alloys, combined with foaming metallic technology, has led to a new class of materials with an optimal combination of mechanical properties and functionality at minimum weight. Casolco et al.[13] manufacture Zn-Al-Cu porous alloys by the replication method using NaCl crystals as space holders. Foams were produced with porosities in the range of 52 to 64 pct and final pore size between 2 and 7 mm. The mechanical properties of the evaluated foams showed a high dependence on the macroscopic pore size and porosity. Siron et al.[14] manufacture open-cell Zn-22Al foams by the replication process using NaCl preform. The foams produced have the equivalent cell size to salt particles in the range from 840 to 3900 μm. Despite the fact that the generation of pressure by centrifugal force has proven the feasibility of manufacturing metal-matrix composites, this method has not yet been used to produce metallic foams or is scarcely documented. Therefore, in this work, the manufacturing of open-cell Zn-22Al-2Cu alloy foams by a centrifugal-replication process was investigated. NaCl particles were used to prepare the preform, and the pressure on the molten metal was generated by the application of a centrifugal force field. The effect of three different spherical salt sizes on the percentage of porosity relative to the pore size in the foams produced was evaluated. Moreover, the effect of the centrifugal pressure on the complete infiltration, final microstructure, and mechanical properties was determined as a function of the pore size of the open-cell Zn-22Al-2Cu foams.
2 Methodology
2.1 Base Alloy
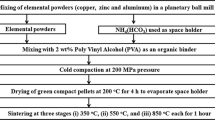
A master Zn-22Al-2Cu alloy was manufactured by conventional melting in an electric furnace at 813.15 K (540 °C) from pure metals. The following chemical composition was obtained in mass pct (76.27Zn, 21.92Al, and 1.81Cu). Two kilograms of alloy were melted in a SiC crucible and poured into an iron mold to obtain ingots of approximately 180 g each. The microstructure examination of Zn-22Al-2Cu alloy and a qualitative chemical analysis of the phases formed were determined with the JEOLFootnote 1 6300 scanning electron microscope (SEM) and with the energy dispersive spectra (EDS) analysis. Images were obtained to different magnifications with backscattering electrons of 15 kV and 10 A. The Zn-22Al-2Cu alloy was analyzed in an X-ray Bruker D8 Focus with monochromatic Cu K α radiation working in a θ/2θ configuration. Data were collected in an angular range from 30 to 90 deg with a step size of 0.02 deg and a counting time of 2 deg min−1.
2.2 Spherical NaCl Particles
In order to manufacture spherical particles, the as-received NaCl particles were melted using a propane gas torch; the liquid was dispersed in drops by an airstream, and the drops were solidified in flight as they fell. The salt particles produced retain a spherical shape due to the surface tension of the liquid after solidification. Then the spherical NaCl particles were sieved to obtain the size fractions of 0.39 to 0.63 mm, 0.56 to 0.89 mm, and 0.49 to 1.27 mm with the average particles sizes of 0.50, 0.69, and 0.95 mm, respectively. The salt circularity was determined by optical microscopy and the analyzer Image J 4.1 along with Eq. [1]:
where A is the area and P is the perimeter of the particle. A circularity (θ) = 1 is for a circle.
2.3 Infiltration Casting
A centrifugal-replication process was used for the manufacture of open-cell foams from Zn-22Al-2Cu alloy. Figure 1 shows a scheme of the experimental arrangement used to produce the composites and further the open-cell foams after leaching the preform. This arrangement is constituted by two electrical resistance furnaces (Figure 1(a)) and a centrifugation system (Figure 1(b)). One of the furnaces contains a SiC crucible loaded with the alloy, and the other one contains a stainless steel cylinder loaded with salt previously produced. Both furnaces are equipped with a control temperature unit. Figure 2 shows the apparatus used for the centrifugal casting. The system is constituted by the centrifugation chamber, a variable frequency drive, and a motor.
The NaCl particles were placed in a stainless steel cylinder (0.06 m in diameter and 0.15 m in length) and vibrated to ensure efficient packing. The stainless steel cylinder is a bipartite dispositive with a hanging system that allows removal and its easy installation in the centrifugation chamber, as can be seen in Figure 3. It is worth noting that the centrifugation chamber contains two additional stainless steel cylinders that act as counterweights. Besides, the cylinder has a small orifice located alongside the wall cylinder, which allows removal of the air contained in the preform as the liquid metal front advances.
During the trials, the inner walls of the cylinder were previously coated with a graphite film to facilitate the removal of the solidified composite from the mold. The system (salt cylinder) was heated at 923.15 K ± 283.15 K (650 °C ± 10 °C) for about 3 hours in an electric furnace; in this stage, a sintered preform of 0.07 m in height and 0.06 m in diameter was obtained. Simultaneously, in the second electric furnace, an amount of 650 g of Zn-22Al-2Cu alloy was melted at 455 °C, and then the temperature was raised to the pouring temperature of 873.15 K ± 283.15 K (600 °C ± 10 °C). The stainless steel cylinder holding the salt preform and the crucible with the molten alloy were both extracted from their respective furnaces once the work temperature was reached (283.15 K (600 °C) for the preform and 283.15 K (600 °C) for the liquid alloy). Afterward, the molten alloy was poured into the stainless steel cylinder, which was transferred immediately into the centrifugation system. The samples were infiltrated using a rotational speed in the range of 100 to 150 rpm determined by the NaCl particle size during 5 minutes. Once the stainless steel cylinder was cooled at room temperature, a Zn-22Al-2Cu/NaCl composite was extracted from the cylinder. The salt was removed by introducing the composite in hot water using ultrasonic equipment to leave an open-cell foam.
Salt and liquid metal interfaces are usually low-wetting systems where the infiltration is a process not spontaneous; therefore, it requires an external force to drive the metal into the interstitial spaces of the preform.[3] In this work, the driving force to carry out the alloy infiltration was the pressure generated by a centrifugal force. Nishida et al.[15] introduced a relationship between the revolution number and the fluid pressure, which acts on the preform surface (Eq. [2]), considering that ω = 2πN:
where ω is the angular velocity, N is revolutions per second, ρ m is the density of the molten metal (ρ m = 5400 kg m−3), and r 1 is the distance from the center of rotation to the inner surface of the preform (r 1 = 0.275 m). The cylinder was placed at 0.215 m from the center of rotation, and r 0 is the thickness of the molten alloy at the beginning of the operation (r 0 = 0.055 m). A scheme with dimensions of the system under centrifugation is shown in Figure 4.
The rotational speeds (N) used to achieve complete infiltration were 100, 120, and 150 rpm, which are equivalent to 1.7, 2.0, and 2.6 rev s−1 for particles of 0.50, 0.69, and 0.95 mm, respectively.
Inserting the previous data in Eq. [2], the experimental fluid pressure, which acts on the preform top surface (the surface in contact with the molten metal column at the beginning of the operation), was obtained for each particle size and is listed in Table I. In order to perform a constant infiltration pressure experiment, the angular velocity was raised as fast as possible up to a given value and kept constant.
2.4 Foam Characterization
The Zn-22Al-2Cu alloy foams were cut on the cross section in order to obtain cylindrical samples to evaluate their density and cell structure. The Zn-22Al-2Cu foam density (ρ*) was determined by a volumetric method considering the weight and the geometry of the cylindrical samples. The relative density (ρ rel) was obtained from the ratio between the Zn-22Al-2Cu foam density and the metal matrix density (ρ Zn-22Al-2Cu = 5400 kg m−3). The porosity (Pr pct) of the Zn-22Al-2Cu foams was calculated according to Eq. [3]:
The cell structure and microstructure were observed using the JEOL 6300 SEM along with EDS analysis.
Cylindrical compression samples were obtained from metallic foams of 20 mm in diameter and 16.6 mm in height, according to the ASTM E-09 Standard. Uniaxial compression tests were performed using a universal testing machine (Shimadzu 100 kN/10 ft capacity). All tests were applied under displacement control with a constant crosshead speed of 0.5 mm min−1 (with strain rate of 3 × 10−3 s−1). Stress strain was deduced from the recorded load-displacement data, which were recorded using a data acquisition unit and a computer. The stress-strain data are reported in terms of engineering stress and strain.
3 Results and Discussion
3.1 Base Alloy
Figure 5 shows SEM micrographs of the Zn-22Al-2Cu alloy as cast. The Zn-22Al-2Cu alloy consists of a eutectic microstructure, where dark and bright areas correspond to aluminum-rich (α) phase and zinc-rich (η) phase, respectively. The microstructure shows a fine inter lamellar structure characterized by regions of α and η phases. Also, the presence of small copper amounts was observed by SEM microanalysis results and confirmed by the XRD pattern of the Zn-22Al-2Cu alloy, as observed in Figure 6. In addition, an aluminum-rich (α) phase, a zinc-rich (η) phase, and the intermetallic (ε) phase corresponding to CuZn4 are observed in Figure 6. Torres-Villaseñor et al.[16] reported that copper addition to Zn-Al alloy originates new intermetallic phases (t’ and ε), which undergo time-dependent transformations into equilibrium phases, and it increases the strength and creep resistance in the material without seriously compromising the superplasticity.
3.2 NaCl Morphology and Particle Size Distribution
Figure 7 shows the morphology and particle size distribution of NaCl particles used as space holders for the preparation of the preforms. The circularity of NaCl particles of 0.940, 0.946, and 0.933 corresponding to an average size of 0.50, 0.69, and 0.95 mm, respectively, was determined. It can be concluded that the methodology used for the manufacture of spherical particles of NaCl allows satisfactorily obtaining round particles.
3.3 Foams Produced
Figure 8 shows open-cell Zn-22Al-2Cu alloy foams manufactured by the centrifugal casting process using a NaCl preform of the three spherical NaCl particle sizes evaluated. Images of the Zn-22Al-2Cu/NaCl composite and Zn-22Al-2Cu foams produced by the centrifugal-replication method, after they were cut and machined for the three different spherical NaCl sizes, are shown in Figure 9. It can be observed from Figures 8 and 9 that all samples had a homogeneous infiltration of the Zn-22Al-2Cu alloy into the space holder for the three different NaCl sizes. SEM images of foams produced using the 0.50-, 0.69-, and 0.95-mm spherical NaCl particles are shown in Figure 10.
For all samples, the structure is typical of open-cell metallic foams with interconnected pores. For the three NaCl particle sizes, the pores of the foams have a near-spherical shape with equivalent size and circularity to the NaCl particle size distribution shown in Figure 10.
The experimental pressures determined based on Eq. [2] are shown in Table I. The optimal temperature of the preform and the alloy during the casting are also summarized.
During the infiltration of a liquid in a porous medium, infiltration pressure can also be considered as a measure of the resistance of the porous medium to the infiltration of the liquid. From Table I, it can be seen that the experimental pressure is decreased when the diameter of the particle is increased. The behavior of this system is explained according to Darcy’s Law (Δp = −µv/K), where the pressure needed to infiltrate a liquid in a porous medium is inversely proportional to the permeability, and the permeability (K) of the preform is proportional to the square of the mean particle diameter (\( K \propto d_{f}^{2} \)).[17] The results obtained showed that the permeability of the preform is very sensitive to the particle size, especially for the particle size of 0.95 mm, where only 7.429 kPa was necessary to achieve complete infiltration. On the other hand, it is expected that the pressure at the infiltration front will be increased from the beginning to the end of infiltration due to the centrifugal pressure being proportional to the distance from the center of rotation, and the additional response of the air compression must also be considered. It can be concluded that the pressure needed to manufacture Zn-22Al-2Cu/NaCl composites under the conditions studied in this work is relatively low, obtaining a complete infiltration of the molten alloy into the interstitial spaces of the preform.
3.4 Foam Characterization
The as-cast Zn-22Al-2Cu alloy foam was cut along the longitudinal direction to observe the microstructure at different zones according to the metal front advancement, as can be observed in Figure 11. It was observed that the microstructure in different regions through the infiltration front is composed of dendrites as well as fine lamellar eutectic structures. SEM micrographs of the open-cell Zn-22Al-2Cu alloy foams were captured from the different regions presented in Figure 12.
The air-cooled foam shows the same microstructure identified in the as-cast alloy condition for the different regions evaluated. These are bright regions that correspond to the zinc-rich phase (η), and the darker gray regions relate to the (α) solid solution richer in Al; the addition of copper formed a copper-rich (ε) phase in the interdendritic regions. Despite the fact that different centrifugal force fields were needed in each case according to the permeability of the bed, it was observed that the rate of cooling imposed by the angular velocity of the cylinder and its surroundings did not show any appreciable effect on the final microstructure of the Zn-22Al-2Cu foam. In our case, the same temperature between the preform and the molten alloy [873.15 K (600 °C)] did not form a thermal gradient; therefore, a slow solidification process was carried out, allowing similar microstructures of the whole casting ingot to be obtained.
Table II summarizes the experimental results of the foams obtained with different pore sizes. It can be observed that the density (ρ*) and the relative density (ρ rel) of the open-cell Zn-22Al-2Cu foams decrease when the pore size is increased from 0.50 to 0.95 mm. Furthermore, the porosity increased with increasing pore size, reaching a value up to 63 pct for the NaCl size of 0.95 mm. The size and shape of NaCl particles have a considerable effect on large scale structural features, such as cell-strut thickness, pore size overall porosity fractions, and mechanical properties of the open-cell foams.
The relationship between the pore size and porosity are observed in Table II, where the values obtained in this work are compared with others previously reported.[13,14] In this work, the porosity was increased from 58 to 63 pct, when the pore size was increased from 0.50 to 0.95 mm. Siron et al.[14] manufactured Zn-22Al foams by the replication method and NaCl as a preform. They obtained a pore size in the range from 0.84 to 3.9 mm with porosities in the range from 64 to 74 pct, respectively, under an infiltration pressure of 6 MPa. Casolco et al.[13] manufactured Zn-Al-Cu foams by the replication method and obtained pore sizes in the range from 2.3 to 3.9 mm without any external force. This helps infiltration because the resistance of the porous medium to the infiltration of the liquid is low based on the particle size used to pack the preform; on the contrary, no use of external force requires a column of metal relatively high, considering that the pressure exerted in the preform surface is proportional to the height of the fluid column (ρgh). They reported that porosity was increased with the smallest grain size, contrary to the results obtained in this work. It is commonly accepted that the porosity is increased when the pore size is increased.[17] It is clear that in order to decrease the pore size by the replication method, an external force is required to achieve an adequate infiltration. The porosity results obtained in this work are in good agreement with the results reported by Siron et al.[14]; however, in this work, the pressure required to achieve completely was significantly lower. It has been reported[18] that high infiltration pressures may lead to the presence of faults as small intrusions into the center of the cell foams. In this work, the SEM images of Figure 10 did not show any evidence of cracking or chipping of the particles or faults due to the low infiltration pressure applied (Figure 13).
The influence of the pore size on the mechanical properties of the open-cell Zn-22Al-2Cu foams was also analyzed in the present work. The compressive stress-strain curves of open-cell Zn-22Al-2Cu foams with different relative densities and pore size are shown in Figure 14. In all cases, the curves display (I) an initial linear elastic region, where stress increased almost linearly at very low stress (smaller than 0.05); followed by (II), a plastic plateau stage, where the stress increased slowly as the strain proceeded, due to the plastic deformation of the cell struts; then a densification stage registered at 0.5 to 0.7 pct strain (III), where stress raised strongly with a slight increase of the strain, due to the densification of the collapsed cells. The foam compression curves obtained are smooth, without the presence of oscillations or serrations in the plateau regions that are commonly observed in open-cell Zn-Al foams manufactured by powder metallurgy[9] or by melt route adding a blowing agent.[19,20]
The mechanical properties of the Zn-22Al-2Cu foams obtained by compression test are listed in Table III. The results show that all mechanical parameters, yield stress σ *y , plateau stress σ pl, and Young’s modulus E, increase when the relative density increases and the pore size decreases. The reduction of the pore size increased the relative density and decreased the cell strut thickness of the open-cell Zn-22Al-2Cu foam; consequently, the mechanical properties were also increased. For instance, the highest mechanical properties, yield strength σ *y (8.6 MPa), Young’s module E (0.559 GPa), and average Plateau stress σ pl (19.3 MPa), were obtained for the open-cell Zn-22Al-2Cu foam with the lowest pore size of 0.50 mm.
A similar behavior was reported in a previous study for the open-cell magnesium foams[21] and open-cell steel foams.[22] These researchers discovered that the mechanical properties were increased when the porosity and pore size were decreased. This behavior was attributed to the hardening capacity of the cell struts of the foams; i.e., as the pore size decreases, the porosity decreases and the cell strut of the open-cell foams becomes thinner, obtaining a refined microstructure resulting in a higher hardening capacity. Therefore, an effective hardening of the cell-strut metal of the foams is produced causing higher stress in the plastic region.[23]
Another important parameter influencing mechanical properties is the morphology of the pores. According to Goodall et al.,[18] open-cell aluminum foams with spherical pores exhibit higher mechanical properties compared with open-cell aluminum foams with angular pores due to stiffness of the angular pores. According to the results of the open-cell Zn-22Al-2Cu foams of this work, the mechanical property results were consistent with those reported for the open-cell Zn-22Al alloy foams with irregular pore morphology.[24]
A centrifugal infiltration process was used in this work for the manufacturing of open-cell Zn-22Al alloy foams. This technique requires simple and economical casting equipment that allows the infiltration of low melting point alloys into a salt preform by applying low infiltration pressure. Some limitations of the present technique are that the preform needs to be at high temperature to improve wetting, since the column of metal is small, and the pressure achieved with such fluid column is relatively lower than necessary to improve wetting. Moreover, when permeability of the preform is low because the NaCl particle used is smaller, the angular velocity needs to be increased, and it is believed that this could cause agglomeration of the intermetallic phases and cracking of the preform.
4 Conclusions
The centrifugal-replication method is a suitable technique for the fabrication of open-cell Zn-22Al-2Cu foams. The salt particles melted and solidified in air allow production of a wide range of spherical particle sizes. Low infiltration pressures were required to obtain fully dense composites Zn-22Al-2Cu/NaCl. It was possible to obtain open-cell Zn-22Al-2Cu foams having nearly spherical cells with 0.50-, 0.69-, and 0.95-mm diameter. The infiltration pressure required was decreased as the diameter of the particle was increased. The porosity of the open-cell Zn-22Al-2Cu foams were increased from 58 to 63 pct, when the pore sizes were increased from 0.50 to 0.95 mm. In order to achieve an adequate melted infiltration in the NaCl preform, it was necessary to preheat the NaCl preform. The rate of cooling imposed by the angular velocity of the cylinder and its surroundings did not show any appreciable effect on the final microstructure of the open-cell Zn-22Al-2Cu foam. The highest mechanical properties, yield strength σ *y (8.6 MPa), Young’s modulus E (0.559 GPa), and plateau stress σ pl (19.3 MPa), were obtained for open-cell Zn-22Al-2Cu foams with the smallest pore size of 0.50 mm and the highest relative density, ρ rel = 0.423.
Notes
JEOL is a trademark of Japan Electron Optics Ltd., Tokyo.
References
P. Degisher and B. Kriszt: Handbook of Cellular Metals, Production, Processing, Applications, 1st ed., Wiley-VCH, Weinheim, 2002, p. 2.
C. Gaillard, J.-F. Despois, and A. Mortensen: Mater. Sci. Eng. A, 2004, vol. 374, pp. 250–62.
N. Dukhan: Metal Foams, Fundamentals and Applications, DEStech Publications Inc., 2013.
M. Sánchez, J. Rams, and A. Ureña: Compos. Part A, 2010, vol. 41, pp. 1605–11.
R. Jamshidi and G. Roudini: Mater. Lett., 2012, vol. 76, pp. 233–36.
Z. Ma, F. Han, J. Wei, and J. Gao: Metall. Mater. Trans. A, 2001, vol. 32A, pp. 2657–61.
J. Zhou, P. Shrotriya, and W. Soboyejo: Mech. Mater., 2004, vol. 36, pp. 781–97.
J. Kovacik and F. Simancik: Kovove Mater., 2004, vol. 42, pp. 79–90.
K. Kitazono and Y. Takigushi: Scripta Mater., 2006, vol. 55, pp. 501–04.
K. Sekido and K. Kitazono: Mater. Sci. Forum, 2013, vol. 753, pp. 73–78.
J.D. Muñoz, A. Mendoza, E. Cabrera, G. Torres, and J.A. Montemayor: Mater. Sci., 2007, vol. 45, pp. 7617–20.
J. Negrete and G. Torres: Mater. Manuf. Processes, 1995, vol. 10, pp. 785–93.
S.R. Casolco, G. Dominguez, D. Sandoval, and J.E. Garay: Mater. Sci. Eng., 2007, vol. 471, pp. 28–33.
Y. Siron, J. Liu, M. Wei, X. Zhu, and Y. Liu: Mater. Des., 2009, vol. 30, pp. 87–90.
Y. Nishida, I. Shirayanagi, and Y. Sakai: Metall. Mater. Trans. A, 1996, vol. 27A, pp. 4136–39.
G. Torres-Villaseñor, Y. Hua, and C. Piña-Barba: Proc. 3rd Int. Conf. on Zn-Al Alloys, Instituto de Investigaciones en Materiales, UNAM, Mexico City, 1994, pp. 89–94.
J. Banhart: Progr. Mater. Sci., 2001, vol. 46, pp. 559–632.
R. Goodall, A. Marmottant, L. Salvo, and A. Mortensen: Mater. Sci. Eng. A, 2007, vol. 456, pp. 124–35.
J. Liu, S. Yu, X. Zhu, M. Wei, S. Li, Y. Luo, and Y. Liu: Mater. Lett., 2008, vol. 62, pp. 683–85.
A. Heydari, H. Shahverdi, and S. Elahi: Trans. Nonferrous Met. Soc. China, 2014, vol. 24, pp. 162–69.
C. Wen, Y. Yamada, K. Shimojima, Y. Chino, H. Hosokawa, and M. Mabuchi: Mater. Lett., 2004, vol. 58, pp. 350–60.
N. Bekoz and E. Oktay: Mater. Sci. Eng. A, 2013, vol. 576, pp. 82–90.
K.R. Mangipudi, S.W. Van Buuren, and P.R. Onck: Int. J. Solids Struct., 2010, vol. 47, pp. 2081–96.
S. Yu, J. Liu, M. Wei, Y. Luo, X. Zhu, and Y. Liu: Mater. Des., 2009, vol. 30, pp. 87–90.
Acknowledgments
The authors thank the Institutions Consejo Nacional de Ciencia y Tecnología (CONACyT), Sistema Nacional de Investigadores (SNI), Comisión de Operación y Fomento de Actividades Académicas (COFAA), and Secretaria de Investigación y Posgrado (SIP)-Instituto Politécnico Nacional (IPN) for their permanent assistance to the Process Metallurgy Group at Escuela Superior de Ingeniería Química e Industrias Extractivas (ESIQIE)–Metallurgy and Materials Department.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 6, 2016.
Rights and permissions
About this article
Cite this article
Sánchez, A., Cruz, A., Rivera, J.E. et al. Manufacturing of Open-Cell Zn-22Al-2Cu Alloy Foams by a Centrifugal-Replication Process. Metall Mater Trans A 49, 272–281 (2018). https://doi.org/10.1007/s11661-017-4390-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-017-4390-5