Abstract
Thermal stabilization of nanograined metallic microstructures (or nanostructures) can be difficult due to the large driving force for growth that arises from the inherently significant boundary area. Kinetic approaches for stabilization of the nanostructure effective at low homologous temperatures often fail at higher homologous temperatures. Alternatively, thermodynamic approaches for thermal stabilization may offer higher temperature stability. In this research, modest alloying of aluminum with solute (1 pct by mole Sc, Yb, or Sr) was examined as a means to thermodynamically stabilize a bulk nanostructure at elevated temperatures. Following 1-hour annealing treatments at 673 K (400 °C) (0.72 Tm), 773 K (500 °C) (0.83 Tm), and 873 K (600 °C) (0.94 Tm), the alloys remain nanocrystalline (<100 nm) as measured by Warren–Averbach Fourier analysis of X-ray diffraction peaks and direct observation of TEM dark-field micrographs, with the efficacy of stabilization: Sr ≈ Yb > Sc. The disappearance of intermetallic phases in the Sr- and Yb-containing alloys in the X-ray diffraction spectra is observed to occur coincident with the stabilization after annealing, suggesting that precipitates dissolve and the boundaries are enriched with solute.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metals with grain sizes in the nano-scale are of interest for many unique or increased properties they can impart, such as higher strength, that are distinct from the same material with coarser grain sizes.[1] While some processing methodologies such as high-pressure torsion are able to create nanostructured materials in bulk form,[2] many techniques produce material, such as powder or ribbon, that require subsequent consolidation. Consolidation methods and/or post-processing of nanostructured bulk ingot often involve the application of elevated temperatures, and this can provide the kinetic means for grain growth as a result of the strong driving force to reduce the overall free energy of the system. Traditional methods of preventing grain growth have usually employed kinetic barriers by introducing pinning mechanisms to impede the movement of grain boundaries. These methods can include solute drag, in which the grain boundaries drag segregated solute and an associated strain field, and the formation of secondary phases, which increase the energy cost of forming a new boundary as grain boundaries come into contact and interact with these phases.[1,3–6] More recently, thermodynamic-based strategies have been proposed to inhibit grain growth in conjunction with the array of kinetic approaches.[7–10]
One particular thermodynamic approach involves introducing a solute species that will partially saturate the grain boundary surface area. By introducing a solute into the metal that has both a driving force to segregate to the grain boundaries and the appropriate thermodynamic parameters to effectively lower the grain boundary surface energy, it may be possible to mitigate the high free energy of the system attributable to the large grain boundary surface area.[7–10] Predictive models and proposed mechanisms in this area are numerous, with most drawing upon the Gibbs interface equation.[8–18] Works reported by Darling et al.[8,9] have created hierarchal models that aid in structuring practical experiments by identifying key parameters to assist in selecting solute with the potential to thermally stabilize grain size. These parameters include elastic misfit strain energy (as estimated by Friedel’s approximation[19]), equimolar enthalpy of mixing in the liquid state, and free surface energy.
Having an adequate amount of solute for segregation to the grain boundary for stabilization can require metastable concentrations in the matrix. In bulk processes, achieving extended solubility typically involves either mechanical alloying or rapid solidification. Melt-spinning, a rapid solidification process, allows incorporation of these metastable solute concentrations through solute entrapment since it can achieve cooling rates of 105 to 107 K/s.[20] However, despite the rapid cooling rate, the solidification may not impart a uniform nanostructure. Brief mechanical attrition can be used to homogenize the grain size of the melt-spun ribbon in the nanoregime.
Annealing of high-energy ball-milled pure Al samples has indicated some thermal stabilization at lower homologous temperatures as a result of kinetic pinning mechanisms from small oxide and carbide impurities formed during milling.[21,22] However, rapid increases in grain size are then observed at higher homologous temperatures. The research reported in this paper seeks to draw on the current work in thermodynamic stabilization of grain size in metals and apply them to dilute Al alloys by selecting solute additions that should provide varying degrees of thermal stabilization based on the previously described critical solute parameters. The high-energy ball milling experiments with pure Al from the literature will serve as a baseline for comparison.[21,22]
2 Experimental
Selection of stabilizing solute relied on the key input parameters for the models from Darling et al.[8,9] The values of the key parameters for the subsequently selected Sc, Yb, and Sr solutes are provided in Table I. According to the model, Sr and Yb should be the strongest thermodynamic stabilizers with low free surface energies and large elastic misfit strain energies. Sc should provide a modest stabilization, but should be much weaker when compared to Sr and Yb (Figure 1).
Predicted grain boundary surface energy of an Al matrix as a function of solute concentration at the grain boundary for solutes of Sr, Yb, and Sc using the Darling–Koch model: \(\gamma = \gamma_0 + \frac{2x^{*}_{A}} {\sigma}\left[\sigma\frac{\gamma^{S}_{A} - \gamma^{S}_{B}}{6} - \frac{\Delta{H}_{\text{mix}}}{3} \left(17x^*_A + \frac {1} {2}\right) - \Delta {E}_{\text{el}}\right] \) where γ is the surface energy of grain boundary, γ0 is the initial grain boundary energy, \( x^{*}_{A} \) is the equilibrium interfacial solute atomic fraction, σ is the molar area of the monolayer, \( \gamma^{S}_{A} \) is the surface energy of the solute, \( \gamma^{S}_{B} \) is the surface energy of the solvent metal, \( \Delta{H}_{\text{mix}} \) is the equimolar enthalpy of mixing between the solute and solvent, and \( \Delta {E}_{\text{el}} \) is the elastic strain energy, which is represented by Friedel’s approximation
The approach for creating the thermally stabilized nanostructure in this study is relatively novel since some of the solute is incorporated in metastable concentrations before the majority of the initial nanostructure is created. The starting material is first melt-spun to extend the solid solubility of the solute in the Al matrix. The ribbon product is subsequently subjected to high-energy ball milling to mechanically reduce the grain size to the nanoregime. The detailed preparation of the materials for these experiments is outlined below.
Al-1Sc (by moleFootnote 1) samples were sectioned from the as-received ingot (AMG Alloys—Wayne, PA). The Al-1Sr and Al-1Yb charges were prepared from elemental 99.9999 pct (by mass) Al shot (ProChem Inc.—Rockford, IL), with either 99pct (by mass) Yb buttons (Michigan Chem. Corp. —St. Louis, MI) or Al-10Sr (by mass) buttons (AMG Aluminum —Wayne, PA). The 20-30 g charges were arc-melted in a Thermal Analysis Inc. arc-melter on a water-cooled copper hearth. The entire button was used as the charge for melt-spinning.
The charges were loaded into 25-mm-diameter SiO2 quartz tubes with a tip orifice diameter of 0.5 mm. The vacuum chamber of the melt-spinner (Marko Materials, Model 2M) was evacuated to a pressure of 1.33 × 10−3 Pa and subsequently backfilled with 99.9999 pct (by mass) Ar to 2.7 kPa. The wheel was set to ~3100 rpm (50 m/s linear wheel velocity) and an induction unit supplied power at a frequency of 5-6 kHz to induce melting of the charge in the crucible. The melts were held briefly at 1173 K (900 °C), as measured by a Micro-Epsilon CTRM-1SF40-C3 High Performance Glass Fiber Ratio pyrometer. At this point, the induction heating was suspended and 99.9999 pct (by mass) Ar was introduced into the top of the quartz tube to push the molten metal through the orifice and onto the wheel. Ar flow was slightly adjusted during the pouring to ensure a steady jet of metal onto the wheel.
To create the nanostructure, approximately 3 g of ribbon was placed in a 5-cm-diameter steel SPEX jar with 1.19-cm-diameter balls (10:1 ball-to-charge ratio). Liquid N2 was poured into the jar and allowed to evaporate multiple times to chill the jar and the contents within. Stearic acid (5 pct by mass milling agent to charge) was then added to the jar before sealing it. The jar was then placed in a SPEX 8000 high-energy vibratory mill and run for 1200-second intervals up to 2 hours of total milling time, recooling the vial in liquid N2 and flipping it after each interval. While longer milling times could be utilized to further reduce starting grain size, the exponential part of the grinding curve established by Fecht[23] and verified by internal calibration using Al-1Sc ribbon is used to be efficient as possible from a processing perspective.
The resulting powder was emptied into a funnel lined with Whatman #4 filter paper. 2-propanol, a solvent in which stearic acid has a solubility of 0.318 M,[24] was poured onto the samples and stirred multiple times in order to remove residual stearic acid and air dried.
Samples to be subjected to annealing treatments, usually about 1 g of powder, were encapsulated in a SiO2 quartz tube under vacuum. Also included in the tube was Al2O3 refractory cloth to keep the powder in place and TiO2 sponge to absorb excess oxygen and outgassing products. The as-milled samples were annealed for 1 hr at 673 K, 773 K, and 873 K (400 °C, 500 °C, and 600 °C) in a Thermodyne box furnace. After 1 hour, the tubes containing the samples were removed from the furnace and allowed to return to room temperature before being broken open.
The samples were placed in a Scintag 2000 XDS diffractometer equipped with a graphite monochromator and with CuKα radiation. The X-ray tube of the diffractometer was operated with a current of 35.0 A and a potential of −45.0 V for all scans, with a divergence slit width of 1 mm and a scatter slit width of 2 mm. The detector widths were 0.5 mm for the scattering slit and 0.3 mm for the receiving slit. Scan parameters were selected based on the solute being studied, the scan time available, and the expected full width at half maximum (FWHM). However, all scans had scan parameter minimums of >10 steps above FWHM in order to adequately model the top of the peak, ±3 FWHM range in order to adequately model the tails of the peak, and sufficient counts per second in order to have >1000 raw counts for the top of the peak in order to ensure good statistics.
Fitting and deconvolution of the Al and associated precipitate peaks was carried out using the Scintag DMNSTTM software. The background was first subtracted by selecting points along the background signal areas unobstructed by peak contributions and fitting a line under the peaks, followed by peak modeling using a Pearson VII function with a built-in Rachinger correction for contributions from the Kα 2 peaks. The Pearson VII peak parameters were then entered into modeling software that converted the function into X–Y data points of 2θ and intensity. Peak asymmetry was lost with this fit, but is ignored since the primary importance of these asymmetries is for stacking fault and twinning corrections that should be negligible for the materials of interest. For the Warren–Averbach Fourier analysis, data were collected for the (111) and (222) peaks of Al. The reference material used for the Stokes Correction and as the “pure Al” baseline for lattice parameter calculations was a 325-mesh 99.99 pct pure Al powder (AEE Metals) that was annealed for 3 hours at 923 K (650 °C) encapsulated in a SiO2 tube under Ar.
The Warren–Averbach analysis for grain size determination was performed using software and algorithms based on Halder and Wagner[25,26] and Warren and Averbach[27] with the base program being received from C.N.J Wagner in 1988 and since has been modified by staff members in the Materials Science & Engineering Department at Michigan Tech. Before separation of the peak profile into its components, corrections for Lorentz polarization and atomic scattering factor (Hartree–Fock) were applied to correct for their contributions to intensity. The program utilizes a fast Fourier transform to represent the unit cells as equal lengths in reciprocal space.
The area-averaged column length of unit cells, \( \langle L\rangle_{\text{area}} \), and volume-averaged column length of unit cells, \( \langle L\rangle_{\text{vol}} \), determined from the above procedure are “effective” measures of the column lengths. Stacking faults and twins can interrupt the unit cell column and cause a distortion in the column length. The methods for these corrections in translation of the \( \langle L\rangle_{\text{area}} \)and \( \langle L\rangle_{\text{vol}} \) from effective to real column lengths can be found in Warren.[28] These contributions are ignored in this research as aluminum’s high stacking fault energy precludes significant contribution from stacking faults and twins and are seldom observed in coarse-grained materials. While deformation twinning in nanocrystalline aluminum has been predicted and experimentally reported in the literature, it is generally limited to the smallest of grain sizes in the nanoregime (10-25 nm).[29] These grain sizes are below the average grain sizes in this study and therefore any contributions from deformation twinning are ignored for practicality.
Transformation of the measured column lengths (L) to grain sizes (D) require assumptions that the grains have a particular shape and all the grains share the same shape. The simplest method is to model the grains as spheres. In doing so, the difference between the lognormal distribution curves of column lengths and spheres must be bridged. With the grains as spheres, the \( \langle L\rangle_{\text{area}} \)and \( \langle L\rangle_{\text{vol}} \) average column lengths can be transformed into the\( \langle D\rangle_{\text{area}} \) and \( \langle D\rangle_{\text{vol}}, \) respectively, as shown by Krill and Birringer[30] and are converted using the equations:
Once the grains are defined as spherical, \( \langle D\rangle_{\text{area}} \) and \( \langle D\rangle_{\text{vol}} \) can be related based on the distribution of grains. Typical distributions of nanocrystalline materials are lognormal in character, and thus for a lognormal distribution of spheres \( \langle D\rangle_{\text{area}} \)and \( \langle D\rangle_{\text{vol}} \) can be related by the equations:
A distribution (as measured by standard deviation σ) of grains with a higher number of larger grains will lead to larger differences between the number, area, and volume averages since the larger grains will contribute more to the latter averages. A tighter distribution of grains will lead to more similar values for the number, area, and volume averages.
Lattice parameter calculations were performed utilizing the DMNST lattice parameter refinement program using the (111), (311), (222), (331), and (420) peaks. The (311) was not used for the 873 K (600 °C) annealed samples of Sc and Sr due to scan time constraints.
Powder samples for transmission electron microscopy (TEM) were prepared by mixing the powder into epoxy from a Spurr epoxy kit and using a Leica UCT Ultracut ultramicrotome for sectioning. The blade settings were set to a 50-nm cut at 4 mm/s. The sections were placed on a copper grid for TEM analysis. The samples were examined in a JEOL 3010 analytical transmission electron microscope at 300 KeV at the National Institute of Standards and Technology (NIST) in Gaithersburg, MD. Grain size analysis from the TEM micrographs was carried out using ImageJTM software[31] to heighten the contrast between grains and background. Since the grains were non-spherical in shape, the area of the grains was traced and equivalent area circles were created, which were subsequently converted to equivalent spheres. Grains were selected from three separate 25 kx micrographs of each alloy system after the 873 K (600 °C) annealing treatment based on which grains’ edges could be reasonably resolved.
3 Results
3.1 Grain Size and Microstrain
Verification of the grain size reduction via mechanical milling was established by analyzing Al-1Sc ribbon by X-ray diffraction at 900-second milling intervals up to 2 hours (Figure 2) and is in good agreement with previous work performed by Fecht.[23] The first few increments of milling times did not yield an output of coherent data, likely due to very large grains remaining and a very large grain size distribution at the start of milling. Grain size drops below 50 nm after about 2 hour of milling time.
Initial grain sizes from Warren–Averbach analysis of the 2-hour milled ribbons were on the order of 30-45 nm with RMS strains of 0.21-0.29 pct as shown in Figures 3 and 4(a) and (b). An initial drop of RMS strain is observed for all alloys after annealing, with only slight oscillations in RMS strains as annealing temperature increased.
(a) Average grain size for Al-1X (X=Sr, Yb, Sc) samples as a function of annealing temperature (1-h anneals) estimated from Warren–Averbach area-averaged approximated column length of crystallites. (b) Average grain size for Al-1X (X=Sr, Yb, Sc) samples as a function of annealing temperature (1-h anneals) estimated from Warren–Averbach volume-averaged approximated column length of crystallites. Error bars indicate a 95 pct confidence interval
Average grain sizes are approximated using both the area-averaged diffracted column length of the crystallites and the volume-averaged column length of the crystallites using Warren–Averbach analysis and are shown in Figures 4(a) and (b). Error bars represent the 95 pct confidence interval from the error propagation combined with the error from peak fitting. The standard error agrees well with previous Warren–Averbach analysis of nanocrystalline metals.[30,32] Both the Al-1Yb and Al-1Sr alloys undergo little grain growth at 673 K (400 °C) as measured by both methods, with the Al-1Sc alloy showing some growth in the volume-averaged method. At 773 K (500 °C), the Al-1Sc alloy exhibits significant grain growth as compared to the Al-1Yb and Al-1Sr alloys for the area-averaged method, but only slightly more growth using the volume-averaging method. At 873 K (600 °C), all the alloys undergo significant growth relative to their starting size, but still remain nanocrystalline. Using the area-averaging method, the Al-1Sr alloy exhibits the lowest final grain size, \( \langle D\rangle_{\text{area}} \), of 72 nm, followed by Al-Yb at 83 nm and Al-1Sc at 89 nm. Using the volume-averaging method, the Al-1Yb alloy exhibits the lowest final grain size, 〈D〉vol, of 64 nm, followed by Al-1Sr at 74 nm and Al-Sc at 90 nm.
TEM micrographs (Figures 5(a) through (c)) reveal grain size following a lognormal distribution for all three alloys after the 873 K (600 °C) annealing treatment, with possible bimodal character developing in the Al-1Sc sample (Figures 6(a) through (c)). Comparisons with the Warren–Averbach \( \langle D\rangle_{\text{area}} \) and \( \langle D\rangle_{\text{vol}} \)using the determined distribution from TEM are shown in Figures 7 and 8. The number-averaged grain size from equivalent sphere diameters, D0, were 46, 49, and 70 nm for the Al-1Sr, Al-1Yb, and Al-1Sc alloys, respectively, and are shown in Figure 9. It should be noted that the error bars for the TEM analysis represent the 95 pct confidence interval as determined by a 1-sample sign test.[33] All the measured and determined values from the X-ray and TEM data are presented in Tables II, III, IV, and V.
Comparison of average grain size for Al-1at. pct X (X=Sr, Yb, Sc) for the 1-h 873 K annealing treatment between Warren–Averbach \( \langle D\rangle_{\text{area}} \) (area-averaged column length of crystallites) and TEM \( \langle D\rangle_{\text{area}} \) (determined from D 0 using the grain distribution). Error bars indicate a 95 pct confidence interval
Comparison of average grain size for Al-1at. pct X (X=Sr, Yb, Sc) for the 1-h 873 K annealing treatment between Warren–Averbach integral breadth \( \langle D\rangle_{\text{vol}} \) (volume-averaged column length of crystallites) and TEM \( \langle D\rangle_{\text{vol}} \) (determined from D 0 using the grain distribution). Error bars indicate a 95 pct confidence interval
3.2 Lattice Parameter
Changes in lattice parameter through the processing and annealing steps are displayed in Figure 10. The Al-1Yb and Al-1Sr alloys begin with lattice parameters below that of the pure Al standard, followed by increases in lattice parameter after milling. Subsequent increases in the lattice parameters to above that of the pure Al standard are observed after annealing. For the Al-1Sc alloy, a lattice parameter larger than that of the pure Al standard is observed followed by an increase after ball milling. The lattice parameter decreases toward that of the pure Al standard after the first annealing treatment, with only small variations after subsequent treatments.
Lattice parameter for Al-1at.pct X (X=Sr, Yb, Sc) samples as a function of annealing temperature (1-h anneals) using X-ray diffraction peak positions. The as-spun ribbon samples are denoted by arrows. The remaining data points are measurements of the ball-milled ribbon. Error bars represent standard error of the least-squares fit of the refinement slope. *Pure Al refers to the reference sample described in the experimental section. Error bars indicate a 95 pct confidence interval
3.3 Phase Identification from X-ray Diffraction
The diffraction patterns of the Al-1Sr, Al-1Yb, and Al-1Sc in Figures 11(a) through (c) of the as-milled ribbons (RT) show the characteristic peaks of the (200) Al4Sr and (111) of the Al3Yb and Al3Sc precipitates along with the characteristic peaks of Al. After the annealing treatments, the precipitate peaks are significantly reduced or no longer observed for Al4Sr and Al3Yb. Inspection of extended 2θ ranges of the diffraction patterns at the 773 K (500 °C) anneals does not reveal the formation of any new phases as no additional significant peaks in Figures 12(a) and (b) are above background. In contrast, the (111) Al3Sc precipitate peak shows increasing intensity with annealing (Figure 11(c)).
X-ray diffraction spectra for the annealing treatments at room temperature (RT), 673, 773, and 873 K of the samples: (a) Al-1at.pct Sr, (b) Al-1at.pct Yb, and (c) Al-1at.pct Sc. Precipitates for Al-Sr and Al-Yb are observed to disappear after annealing, while Sc appears to precipitate out of the Al matrix into Al3Sc after annealing
4 Discussion
4.1 Grain Growth Analysis from X-ray Diffraction
The grain growth behavior in this study fits well with the observations of previous work on ball-milled pure nanocrystalline Al. At low homologous temperatures (<0.78 Tm), only very minor grain growth is observed in both the pure Al from[21] and the alloyed Al samples in this study. However, at higher homologous temperature, the alloyed samples exhibit less growth than the pure Al (as compared with[21,22]), and are therefore likely not the sole product of kinetic stabilization as a result of pinning by impurity particles. Grain growth behavior has been reported to change to a more rapid rate at about 0.78 Tm,[21] indicating that the previously effective kinetic pinning mechanisms of the impurities were no longer sufficient to reduce the driving force for grain growth. The solute additions predicted to provide a strong thermodynamic stabilization (Yb and Sr) show only minimal growth at 773 K (500 °C) (~0.83 Tm), while the solute addition predicted to provide modest thermodynamic stabilization (Sc) leads to much larger grain growth at 773 K (500 °C) according to the measured \( \langle D\rangle_{\text{area}} \). It is possible that the segregation of the Yb and Sr solutes is taking place and providing stabilization to the grain structure. Based on the disappearance of the intermetallic phases in the Al-1Sr and Al-1Yb alloys, and the emergence of the intermetallic phase in the Al-1Sc alloy, it stands to reason that while all three solute additions have been effective at keeping the material nanocrystalline, the Sr and Yb additions likely do so through a thermodynamic mechanism, and the Sc does so through a kinetic pinning mechanism related to its intermetallic precipitates.
The differences between the average grain size measurements of \( \langle D\rangle_{\text{area}} \) and \( \langle D\rangle_{\text{vol}} \) can usually be used to determine the grain size distribution. However, given that the \( \langle D\rangle_{\text{vol}} \) value is likely less accurate for the materials in this study due to the difficulty in deconvolving the broadening contribution from grains approaching and exceeding 100 nm, this calculation of this distribution has not been performed. The average grain sizes begin to approach 100 nm and therefore will be difficult to deconvolute their contribution to broadening from the strain and instrumental contributions. As the strain contributions are somewhat moderate, this is a non-trivial deconvolution. While evidence of abnormal grain growth is not seen from the TEM micrographs here, it is possible that the presence of a higher number of larger grains was not accounted for due to the small sample size.
The case of Al-Sc alloy is more uncertain. In the case of the large difference between the values of average grain size for the Al-Sc alloy at 773 K (500 °C), abnormal grain growth may be occurring, in which the larger grains are growing with minimal change in overall number and size of the smaller grains. The TEM results may give credence to this as a bimodal hump appears to be forming, at 873 K (600 °C), but the number of grains collected is not high enough to make any definitive conclusions. Another challenge with the Al-Sc alloy is the tight overlap of the Al3Sc superlattice precipitate peaks with the Al peaks. Deconvolution could be causing fitting issues for the Pearson VII function and lead to a decrease in the accuracy of these results as well.
4.2 Grain Growth Analysis from Transmission Electron Microscopy
TEM measurements of grain size of the alloys after the 873 K (600 °C) annealing treatments are in fairly good agreement with the results from X-ray diffraction, both in the absolute values of the means and the relative difference between the mean grain size of each of the alloys. Both the area-averaged and the volume-averaged values determined from the number average using the appropriate distribution equation calculation fall close to the values from Warren–Averbach analysis. It is possible that a bi-modal distribution is beginning to develop in the Al-1Sc system; however, the sample size is currently too small to draw any definitive conclusions. The grain size measurements from the TEM serve as a verification of the general size of the grain from X-ray diffraction. Limited sample size of resolvable grains and the difficulty of resolving defined edges of diffracting grains in the dark field introduce some uncertainty in both the accuracy and precision of these measurements. Since not all of the grains diffract perfectly for resolving the edges, some grains must be left out of the analysis for lack of resolution, while others may be underestimated since the definition of the edge may decay into unresolvable space. Furthermore, many of the grains are superimposed on top of one another, which may either inflate the size of the grain measurement if considered one grain, or decrease the size of the grain measurement if inadvertently considered separate grains. However, these uncertainties should still be rather small and consistent across each alloy.
4.3 Phase Analysis from X-ray Diffraction
The reduction and disappearance of the intermetallic phases after annealing of the Al-1Sr and Al-1Yb alloys was an unexpected result. Possible explanations for the disappearance of the Al3Yb and Al4Sr precipitate peaks would be dissolution of the precipitates into their elemental components or formation of other compounds such as oxides or carbides. However, characteristic peaks of oxides or carbides are not observed. Since relative grain size stabilization is observed at the annealing temperatures as compared to that of nanostructured pure Al, solute segregation to the grain boundary is likely taking place. However, alternative explanations cannot be completely eliminated at this stage of the research. It should be noted that the reduction of other oxides by the particularly stable Sr-, Yb-, and Sc-based oxides could provide an alternative explanation for the disappearance of the intermetallics that may not be easily observed in an X-ray diffraction pattern at small volume fractions. However, where this the case, it is curious that the Sr and Yb intermetallics would be reduced, while the Sc intermetallic is not given the similarity of the reducing potential of the oxides of all three solutes. This difference could possibly be attributed to kinetics rather than thermodynamic potential and ultimately requires further study.
In the 873 K (600 °C) annealing treatment of the Al-1Sc alloy, it appears that there may be a separation of the (111) Al3Sc precipitate peak and the (111) Al peak. These may be due to the shift of the Al peak as more Sc comes out of solution and the reduction of broadening of the Al3Sc precipitate peak as the precipitates coarsen. Further experimental work would be needed to completely resolve the uncertainty of the peak profiles at this annealing temperature.
4.4 Thermodynamic and Kinetic Feasibility of Disassociation of Precipitates
As previously indicated, the observations from X-ray diffraction spectra concerning the dissociation of the Al4Sr and Al3Yb intermetallics are contrary to the expected precipitation of these phases. The possible alternatives of oxidation of these phases have been noted and discussed in the previous section. There are some similar reports in the literature, such as Smith et al.[34] in the area of nanostructured dual-phase bronze alloys where the apparent equilibrated structure contained a heterogeneous microstructure of alpha and beta rather than an expected single homogenous phase. While kinetic limiting factors could not be completely ruled out, the authors indicate that the calculated diffusion distances should have been sufficient to allow sufficient mass transport for equilibration. In the present work, if the intermetallic phases have disassociated and broke local equilibrium to satisfy a global equilibrium which includes the surface energy contributions of the nanostructured grain boundaries, the experimental grain sizes determined should support the calculation of a necessary surface energy of the grain boundary that is consistent (reasonable values) with the Darling model’s prediction of the added solute’s ability to lower the grain boundary energy.
To establish a basic thermodynamic feasibility of dissolution of the precipitates, a simple calculation can be done using the experimentally determined grain sizes to determine if the calculated surface energy values \( \gamma_{\text{grain}}^{ *} \) are at least in the correct range to support the evidence of the dissolution of the normally stable precipitates. If the starting and final states of grain sizes and precipitates are taken into account, the maximum grain boundary energy required to produce the final microstructure should be able to be established, albeit approximate as some assumptions must be made in order to both simplify the calculation and account for the lack of literature values for input parameters. The starting and finishing states are defined in terms of Gibbs free energy by G 1 and G 2:
where n is the number of moles at equilibrium, \( G_{\text{precip}}^{f} \) is the Gibbs free energy of formation of the precipitates, γprecip|Al is the interfacial energy between the precipitate and the matrix, γAl|GB is the grain boundary energy of Al, N is the number of atoms in each formula unit of intermetallic, Ω is the molar volume, r is the radius of either the precipitate or the grain (r 1 and r 2 indicating before and after annealing, respectively), and \( G_{{\alpha - {\text{Al}} - 1 {\text{sol}}}}^{M} \) is the Gibbs free energy of bulk mixing between the solute and solvent.
For Al4Sr, \( G_{\text{precip}}^{f} \) is calculated from Eq. [7]. For Al3Yb, the enthalpy of formation (\( H_{\text{precip}}^{f} \)) is used as an estimation of \( G_{precip}^{f} \).[35]
\( G_{{\alpha - {\text{Al}} - 1 {\text{sol}}}}^{M} \) is calculated from Eq. [9]:
where \( H_{{L - {\text{Al}},{\text{sol}}}}^{M} \) is the equimolar enthalpy of mixing in the liquid state, X Al and Xsol are the mol. fractions of Al and solute (X Al + X sol = 1), respectively, R is the universal gas constant, and T is the temperature (773 K (500 °C) for these calculations).
To determine the grain boundary energy required in order to dissipate the precipitates, the Gibbs free energy is set G 2 = G 1 so that \( \gamma_{\text{grain}}^{ *} \) can be solved for. \( \gamma_{\text{grain}}^{ *} \)can be solved for a range of solute segregation as \( G_{{\alpha - {\text{Al}} - 1 {\text{sol}}}}^{M} \) will vary based on the X sol’s value. X sol is varied in these calculations from 0.0004 (equilibrium bulk solubility, all excess at grain boundary) to 0.01 (entire solute concentration in the bulk).
The previous equations take into account the assumptions for calculation. First, grain size and precipitate size are assumed to be uniform and spherical for the calculation, which allows the radius of curvature to be approximated as 2/r for both the precipitates and the grain. Although not experimentally determined, precipitate radii are assumed to be 5 nm (10 nm diameter) since they must be embedded in the grains themselves, but still provide measurable scattering from X-ray diffraction. Enthalpy of formation is used as an approximation of the Gibbs free energy of formation for Al3Yb due the lack of literature values for the Gibbs free energy. Table VI displays the input parameters and Table VII the \( \gamma_{\text{grain}}^{ *} \) result. Al4Sr is of the D13 structure and is not coherent with the α-Al matrix; however, no literature values could be found for its interfacial energy with Al. The interfacial energy between Al4Sr and the Al matrix is arbitrarily selected as 0.5 J/m2, at the rough junction for semi-coherent/incoherent precipitates. This is a relatively safe estimate as this parameter does not greatly affect the result (about 20 pct difference between the values of 0.1 and 0.9 J/m2, therefore ±10 pct from 0.5 J/m2). Equilibrium solubility for Sr in Al uses the same value as Yb since good literature values could not be found (given the magnitude size order, this value is essentially trivial). For calculation, the moles of atoms are set to be n Al + n precip = 1. Atomic volume (Ω) must undergo a division operator, N (4 and 5 for Al3Yb and Al4Sr, respectively), to normalize to the appropriate atomic basis. Final grain size is also larger than the initial grain size as there is some grain growth.
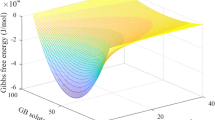
For comparison with the determined required values of \( \gamma_{\text{grain}}^{ *} \), the predicted values of \( \gamma_{\text{grain}}^{ *} \)from use of the Darling–Koch model are presented in Table VII as well. To arrive at the model-predicted values, Eqs. [10] through [12] are used for complete segregation. Eq. [11] can be ignored for concentration values less than complete segregation as the volume associated with the particular atomic concentration can be input directly into Eq. [12]. From the curve generated from this equation (Figure 13), the solute concentration at the grain boundary can be determined for a grain size of 48 nm. The concentration derived from the curve can be used as \( x_{A}^{ *} \) in Eq. [13] to determine the predicted \( \gamma_{\text{grain}}^{ *}. \)
Figure 13 shows the solute concentration at the grain boundary as a function of grain size for 1 at. pct solute. The available space for solute is approximated using geometric descriptions and the relative volume fractions of the crystalline region and grain boundaries as described by Boylan et al.[3,36] The curves are calculated from the following equations:
where v GB is the molar volume of the grain boundaries, v Al and v A are the molar volumes of aluminum and the solute, respectively, \( v_{\text{GB}}^{\text{frac}} \) is the volume fraction of the grain boundaries as determined using the equations from Boylan et. al.,[3] \( v_{A}^{ *} \) is the molar volume fraction of solute atoms at the grain boundary, \( x_{A}^{\text{eq}} \) is the bulk equilibrium concentration of solute, and \( x_{A}^{ *} \) is the fraction of solute at the grain boundaries.
This figure also assumes that non-binary impurity atoms do not take up space at the grain boundary and that grain size is uniform. From these assumptions, it will follow that smaller grain sizes can accommodate more solute than larger grain sizes since the volume of grain boundary area is larger, and that solutes with larger atomic volumes will take up more space, and therefore saturate the grain boundary at smaller concentrations. Deviations from this model in practice may include distributions of grain sizes, other impurity atoms taking up space at the grain boundary, and possible solute clustering. Distribution of grain sizes may give rise to some grains being large enough to start rejecting solute. This may lead to abnormal grain growth if enough solute is not present to stabilize the grain boundary. The formation of precipitates from rejection of solute by these larger grains may or may not occur if enough smaller grains lower than the critical grain size threshold exist in order to accommodate these rejected solute atoms.
For complete segregation of the solute to the grain boundary, the predicted values of \( \gamma_{\text{grain}}^{ *} \) are far below what is predicted to be capable of the solutes by the model. Furthermore, the result of a negative surface energy would imply that the grains would have a propensity to shrink, which is not the case from these results. For complete solubility of the solute in the matrix, the \( \gamma_{\text{grain}}^{ *} \) values that result are above those of the grain boundary energy for aluminum. This result would imply an acceleration of growth of the grains, while the experimental evidence suggests a retardation of growth. However, there is a range of segregated solute concentration that results in \( \gamma_{\text{grain}}^{ *} \) values that are below that of the grain boundary energy of aluminum and are reasonably predicted by the model. These values are close to zero and are indicated in Figures 14 and 15. Therefore, it is at least plausible that the precipitates can dissolve, with a reduction in grain boundary energy serving as a possible mechanism for the inhibition of grain growth.
Grain boundary energy as a function of 1 at.pct solute in the bulk solution (balance at the grain boundary) for Al-1Sr. The grain boundary energy determined from the experimentally determined grain sizes and equations derived in the body text of this dissertation is compared with the grain boundary energy predicted by the Darling–Koch model
Eventually, as the average grain size increased, a point may arise where a critical amount of solute is rejected that cannot be accommodated by the grain boundaries and exceeds a thermodynamic threshold from residing in the grain interior and precipitates would reform. A more expansive aging study of these alloys in future experiments is needed to determine the point in which this would occur. Further complicating the point of “re-precipitation” of the intermetallic phase may be the local energy considerations at the grain boundary since the surface energy between two grains can affect the dihedral angle between the grain boundary and the precipitating phase.[37] Even if the initial dissolution of the precipitates were a result of bulk equilibrium and subsequent grain growth caused enough solute to be rejected to favor precipitation, a situation might exist where the surface energy between grains is low enough that it would limit the ability of the grain boundaries to serve as heterogeneous nucleation sites.
Another possible factor contributing to solute segregation could be the role of dislocation density. Residual microstrain remains as determined from Warren–Averbach analysis and therefore provides another possible interface for more preferential accommodation of solute. Integration of the role of the dislocation density in solute segregation is beyond the scope of this current research as the objective of these analyses is to present a feasibility of the dissipation of the precipitates as a function of grain boundary surface area. The contributions and interactions with the dislocation density will require as solute concentration profiles of the interior and at the grain boundary to adequately assimilate into the simplified equations presented earlier. These concentration profiles obtained by atomic probe topography are the next logical step to both confirm solute segregation and determine the extent of solute in the interior of the grain as opposed to that at the grain boundary and are planned as a follow-up study to the research presented in this paper.
4.5 Lattice Parameter Analysis
Examination of the lattice parameter measurements at the annealing temperatures indicates distortions to the Al lattice that result in lattice parameters greater than those of Al. However, the substitution of Sr and Yb in the matrix results in lattice parameters less than those of pure Al. Similar observations of the Al lattice parameter decreasing with the incorporation of Sr into the Al matrix through melt-spinning were made by Zhang et. al.,[38] and were attributed to the strong Sr-Al bond. However, they also observe an expansion of the lattice with increasing levels of Sr in solution, suggesting that the effect of the larger Sr atom as compared to Al may be revealed at certain concentrations. These phenomena may be similar to the findings of Moriarty and Widom,[39] who explain the deviations from the expected trends of atomic volume along the periodic table in transition metal intermetallics through general pseudopotential theory (GPT) by examining the sp-d hybridized bonding between the Al atoms and the other transition metal atoms.
Examination of the lattice parameter measurements at the annealing temperatures indicates distortions to the Al lattice that result in lattice parameters greater than those of pure Al. The “pure Al” lattice parameter indicated in Figure 10 may not serve as a good baseline for the end-point of a fully annealed sample as impurity atoms (other than the intended solute) are likely introduced during the creation of these samples. These impurity atoms would likely cause an increase in the lattice parameter and some variation would likely be present depending on the impurity atom’s affinity for both the grain boundary and the precipitates. Furthermore, other studies have shown that in nanocrystalline materials, the strain fields introduced by solute segregation can cause uniform lattice expansion, causing an increase in the lattice parameter.[36] This result would be consistent with segregation of Sr and Yb atoms to the grain boundary. For the Al-1Sc alloy, it is known that small coherent L12 particles can form, which could also act as a mechanism for an observed increase in lattice parameter. However, because of the multiple confounding factors, it is difficult to draw any definitive conclusions about exact quantitative amounts of solute in solution.
5 Summary/Conclusions
In summary, Al-1X (X=Sr, Yb, Sc) charges were melt-spun into ribbon and ball-milled to the starting grain size range of 30-45 nm. Annealing treatments at 673 K (400 °C), 773 K (500 °C), and 873 K (600 °C) for 1 hour were applied to the powder samples. Relative thermal stability of the grain size (<100 nm) is observed in all samples as approximated by Warren–Averbach analysis of area-averaged column length of crystallites from X-ray diffraction data. Little to no grain growth is observed at 673 K (400 °C) for all samples. Significant growth at 773 K (500 °C) is only observed for the Al-1Sc alloy. At 873 K (600 °C), the final grain sizes for the Al-1X (X=Sr, Yb, Sc) alloys were 72, 83, and 89 nm, respectively, from the WA area-averaging method (\( \langle D\rangle_{\text{area}} \)) and 74, 64, and 90 nm from the Warren–Averbach volume-averaged method (〈D〉vol), respectively. Final grain size after the 873 K (600 °C) anneal from the indirect measurements was confirmed using dark-field micrographs from TEM, which show good agreement with both X-ray-derived values. The grain size of the alloys remained in the nanoregime (<100 nm) for all annealing treatments, with the effectiveness of solute following, Sr≈Yb>Sc, which was consistent with the predictions from the Darling thermodynamic model. X-ray diffraction spectra reveal the disappearance of the precipitate phase peaks of Al3Yb and Al4Sr after annealing of the Al-1Yb and Al-1Sr alloys. The precipitation of the Al3Sc phase after annealing of the Al-1Sc is observed. These phase observations are attributed to Sr and Yb having thermodynamic attributes that lead to greater solute segregation to the grain boundary than Sc. Therefore, the effective stabilization for the Sc is more likely a pinning mechanism due to its precipitates, as opposed to the thermodynamic stabilization of the Sr and Yb alloys.
Notes
Al-1X (X=Sr, Yb, Sc) alloys are described in terms of mole fraction percent throughout the remainder of the text.
References
C. Koch: J. Mater. Sci., 2007, vol. 42, pp. 1403–1414.
P.V. Liddicoat, X.-Z. Liao, Y. Zhao, Y. Zhu, M.Y. Murashkin, E.J. Lavernia, R.Z. Valiev, and S.P. Ringer: Nat. Commun., 2010, vol. 1, p. 63.
K. Boylan, D. Ostrander, U. Erb, G. Palumbo, and K. Aust: Scr. Metal. Mater.,1991, vol. 25, pp. 2711–2716.
R. Averback, H. Höfler, and R. Tao, Mater. Sci. Eng. A, 1993, vol. 166, pp. 169–177.
Z. Gao, and B. Fultz, Nanostruct. Mater., 1994, vol. 4, pp. 939–47.
A. Michels, C. Krill, H. Ehrhardt, R. Birringer, and D. Wu, Acta Mater., 1999, vol. 47, pp. 2143–2152.
P.C. Millett, R.P. Selvam, and A. Saxena, Acta Mater., 2007, vol. 55, pp. 2329–2336.
Darling, K., B. VanLeeuwen, C. Koch and R. Scattergood, Mater. Sci. Eng., 2010, vol. 527, pp. 3572–80.
K. Darling, B. VanLeeuwen, J. Semones, C. Koch, R. Scattergood, L. Kecskes, and S. Mathaudhu, Mater. Sci. Eng. A 2011, vol. 528, pp. 4365–71.
T. Chookajorn, H.A. Murdoch, and C.A. Schuh, Science, 2012, vol. 337, pp. 951–54.
P. Knauth, A. Charai, and P. Gas, Scr. Metall. Mater., 1993, vol. 28, pp. 325–30.
P. Wynblatt, and D. Chatain, Metall and Mat Trans A 2006, vol. 37, pp. 2595-2620.
J. Weissrmiller, J. Mater. Res., 1994, vol. 9.
Kirchheim, R., Acta materialia 2002, vol. 50, pp. 413-419.
Liu, F. and R. Kirchheim, Journal of crystal growth 2004, vol. 264, pp. 385-391.
Liu, F. and R. Kirchheim, Scripta materialia 2004, vol. 51, pp. 521-25.
A. Sutton, and R. Balluffi, Interfaces in Crystalline Solids, Clarendon: Oxford 1995
Saber, M., H. Kotan, C. C. Koch and R. O. Scattergood, Journal of Applied Physics 2013, vol. 113, pp. 063515-063515-10.
Friedel, J., Advances in Physics 1954, vol. 3, pp. 446-507.
Jones, H., Reports on Progress in Physics 1973, vol. 36, p. 1425.
Zhou, F., J. Lee and E. Lavernia, Scripta materialia 2001, vol. 44, pp. 2013-2017.
Abdoli, H., M. Ghanbari and S. Baghshahi, Materials Science and Engineering: A 2011, vol. 528, pp. 6702-6707.
Fecht, H.-J., Nanostructured Materials 1995, vol. 6, pp. 33-42.
Liu, W. J., C. Sun, P. X. Zhao and S. F. Wang, Advanced Materials Research 2012, vol. 550, pp. 71-74.
Halder, N. and C. Wagner, Adv. X-ray Anal 1966, vol. 9, pp. 91-102.
Halder, N. and C. Wagner, Acta Crystallographica 1966, vol. 20, pp. 312-313.
Warren, B. and B. Averbach, Journal of Applied Physics 1952, vol. 23, pp. 497-497.
B. Warren, and X.-R. Diffraction, New York, 1990, p. 251.
Chen, M., E. Ma, K. J. Hemker, H. Sheng, Y. Wang and X. Cheng, Science 2003, vol. 300, pp. 1275-1277.
Kril, C. and R. Birringer, Philosophical Magazine A 1998, vol. 77, pp. 621-640.
Abràmoff, M. D., P. J. Magalhães and S. J. Ram, Biophotonics international 2004, vol. 11, pp. 36-43.
Wagner, C. N., E. Yang and M. S. Boldrick, Journal of non-crystalline solids 1995, vol. 192, pp. 574-577.
Gibbons, J. D., Gibbons nonparametric methods for quantitative analysis, Holt, Rinehart and Winston: New York, 1976.
Smith, N. A., N. Sekido, J. H. Perepezko, A. B. Ellis and W. C. Crone, Scripta materialia 2004, vol. 51, pp. 423-426.
Zhong, Y., C. Wolverton, Y. Austin Chang and Z.-K. Liu, Acta materialia 2004, vol. 52, pp. 2739-2754.
Weissmüller, J. and C. Lemier, Physical review letters 1999, vol. 82, p. 213.
Balluffi, R. W., S. Allen and W. C. Carter: Kinetics of Materials. John Wiley & Sons, Hoboken, 2005.
Zhang, Z., X. Bian, Y. Wang and X. Liu, J Mater Sci 2002, vol. 37, pp. 4473-4480.
Moriarty, J. A. and M. Widom, Physical Review B 1997, vol. 56, p. 7905.
F. De Boer, R. Boom, W. Mattens, A. Miedema, and A. Niessen: Cohesion in Metals: Transition Metal Alloys: Cohesion and Structure. (North-Holland, Amsterdam, 1989).
Inoue, A., T. Zhang, K. Kita and T. Masumoto, JIM Mater. Trans. 1989, vol. 30, pp. 870-877.
Gale, W. F. and T. C. Totemeier: Smithells metals reference book. Butterworth-Heinemann, Oxford, 2003.
Lide, D. R.: CRC handbook of chemistry and physics. CRC press, Boca Raton, 2004.
Shubin, A.B. and Yu Shunyaev, K, Russ. Metall., 2011, vol. 2011, pp. 109-13. DOI:10.1134/S003602951102011X
Chang, Y., Gao, K., Wen, S., Huang, H., Wang, W., Zhu, Z., Nie, Z., and Zhou, D, J. Alloy Compd., 2014, vol. 590, pp. 26-534.
D. Porter and K. Easterling: Phase Transformations in Metals and Alloys, CRC Press, Boca Raton, 1992.
G. Borzone, A.M. Cardinale, N. Pariodi, and G. Cacciamani, Journal of Alloys and Compounds, 1997, vol. 247, pp. 141-147.
P. Villars and K. Cenzual, Pearson’s crystal data—crystal structure database for inorganic compounds, release 2012/13 Materials Park, Ohio, USA, ASM International.
Acknowledgments
The authors would like to acknowledge Paul Fraley (Michigan Technological University Particulates Processing Laboratory) for his assistance in melt-spinning sample preparation, Ed Laitila (Michigan Technological University Applied Chemical and Morphological Analysis Laboratory) for his assistance in X-ray diffraction analysis, and Owen Mills (Applied Chemical and Morphological Analysis Laboratory) for his assistance in TEM sample preparation.
The following copyright wording should be placed in the block with the author affiliations: E.A. Lass is employed by National Institute of Standards and Technology. U.S. Government work is not protected by U.S. Copyright.
Author information
Authors and Affiliations
Corresponding author
Additional information
E.A. Lass is employed by the National Institute of Standards and Technology. U.S. Government work is not protected by U.S. Copyright.
Manuscript submitted June 30, 2014.
Rights and permissions
About this article
Cite this article
Baker, A.H., Sanders, P.G., Lass, E.A. et al. Solute-Derived Thermal Stabilization of Nano-sized Grains in Melt-Spun Aluminum. Metall Mater Trans A 47, 4287–4300 (2016). https://doi.org/10.1007/s11661-016-3551-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-016-3551-2