Abstract
Summary
This study aimed to identify risk factors for the collapse of osteoporotic vertebral fractures (OVFs). We analyzed data from conventional radiography and computed tomography in patients with OVFs and found that older age and two radiological measurements were predictive for vertebral collapse. These factors can be useful for clinical practice.
Purpose
To identify risk factors for collapse of osteoporotic vertebral fractures (OVF) on computed tomography (CT) and conventional radiography (CR).
Methods
This is a retrospective case–control study including a series of patients with OVF diagnosed at the emergency department of our institution from January to September 2019. Inclusion criteria were to have standing CR and supine CT within 2 weeks after the diagnosis of OVF and a follow-up CR at 6 months or later. We evaluated different imaging measurements at the initial diagnostic examinations, including vertebral height loss, local kyphosis, vertebral density, and fracture type according to the grading systems of Genant, Sugita, Association of Osteosynthesis (AO) Spine, and the German Society for Orthopaedics and Trauma. Vertebral collapse was defined as loss of ≥ 50% of vertebral area or height. Cases and controls were defined as OVFs which collapse and do not collapse, respectively, on follow-up.
Results
Fifty-six patients were included in the study, with a mean age of 72.6 ± 1.2 years, including 48 women. Twenty-five (44.6%) OVFs developed collapse on follow-up. None of the fracture classification systems were found to be predictive of collapse. Multivariate analysis showed that older age, increased density ratio (≥ 2) between the fractured and non-fractured vertebral bodies, and a ≥ 6% difference in posterior vertebral height (PVH) loss between standing CR and supine CT exhibited 88% discriminative power in predicting vertebral collapse.
Conclusions
Age over 72.5 years, a density ratio ≥ 2 between the fractured and non-fractured vertebral bodies, and a difference equal to or higher than 6% in PVH loss between standing CR and supine CT, are risk factors for developing vertebral collapse after OVF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoporotic vertebral fractures (OVFs) are the most frequent osteoporotic fractures [1], and their detection is sufficient to make the diagnosis of osteoporosis even in the absence of bone mineral density studies [2]. Ninety percent of all vertebral fractures are osteoporotic and may occur without any noticeable trauma or following a low-energy injury such as falling to the ground [3]. One of the main current challenges in radiology regarding OVFs is to correctly classify them and to identify the imaging features that imply a risk of collapse or increased local vertebral kyphosis. These complications may adversely affect the clinical and functional recovery of the patient. In fact, loss of sagittal balance due to increased kyphosis seems to be the main cause of persistent pain and gait disturbances [4].
Conventional radiography (CR) is still considered the initial screening examination to detect OVFs [5], although its reported sensitivity in vertebral fracture detection is lower compared to CT or MRI [6,7,8], depending on different factors such as anatomical location, technical parameters, or bone density quality.
Computed tomography (CT) has diagnostic advantages compared with CR, allowing the identification of more subtle signs of vertebral fracture [3] such as discontinuity or buckling of the cortex and impaction of the fractured trabeculae. In addition, several studies have found that Hounsfield unit (HU) values of the vertebral marrow (trabecular bone) correlate with bone mineral density, and that routine CT examination can be used to identify patients with osteoporosis [9, 10]. Normal HU values at the vertebral bone marrow range from 256.7 ± 41.8 (mean ± standard deviation) in the second decade of life to 90.0 ± 25.5 in the ninth decade of life in men, and from 253.5 ± 29.6 to 67.3 ± 41.2 in women, respectively [11]. Lower HU values are suggestive of osteoporosis, which increases the risk of developing more severe OVFs [12].
On magnetic resonance imaging (MRI), several findings have been associated with absence of consolidation and persistence of pain, including the presence of an intravertebral cleft, location at the thoracolumbar transition, or a diffuse area of low T2 signal intensity probably secondary to fibrosis and impaction of trabeculae [13].
Several classification systems have been developed to categorize and quantify the severity of OVFs. However, there is no universal agreement on which of these systems is the most useful for appropriate patient management. For instance, the semiquantitative Genant’s classification is mainly used in epidemiological studies. This classification categorizes vertebral fractures based on the morphology and degree of area and height loss, but it does not provide indications for clinical management [14]. On the other hand, Sugita et al. classified fractures into five morphological types based on radiographic findings, with swelled, bow-shaped, and projecting types being frequently associated with the presence of intravertebral cleft and late collapse, as well as with worse prognosis [15].
Recently, the German Society for Orthopaedics and Trauma proposed a classification system for OVFs that offers a comprehensive score based on the type of fracture and clinical factors to decide between surgical or medical management [16, 17]. This classification scores bone density and progressive fracture sintering, among other factors. Preliminary results suggest that this score is an appropriate tool for the preoperative assessment of OVFs [18].
Different risk factors for the development of collapse in the case of traumatic vertebral fractures have been published in the literature, including burst fractures, location in the thoracolumbar transition (T12-L1), and age over 50 years [19, 20]. Osteoporosis is also a risk factor for vertebral collapse, although most OVFs heal well with conservative treatment. Nevertheless, between 8.9 and 20% of cases may develop painful non-union or pseudoarthrosis, progressive kyphosis, and neurological damage [21,22,23,24,25]. Vertebral pseudoarthrosis usually presents with intravertebral cysts or clefts and injury of the posterior vertebral wall [23, 25].
The aim of this study was to identify risk factors related to the development of vertebral collapse of OVFs based on radiological findings in CR and CT.
Materials and methods
Study design and participants
This study was approved by our Institutional Review Board (code TFG-FX-2019). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [26, 27] were followed when designing and reporting this study. We designed a retrospective single-center case–control study including a series of patients with acute OVF of the thoracolumbar spine diagnosed by imaging studies performed at the emergency department in our institution from January to September 2019. The following inclusion criteria were established:
-
1.
Patients diagnosed with acute OVF by both standing CR and supine CT in our emergency department.
-
2.
Patients with imaging follow-up of their fracture at least 6 months after diagnosis by standing CR to confirm or exclude delayed vertebral body collapse.
-
3.
Type A fractures of the AO classification.
-
4.
Management through conservative medical treatment, including the use of drugs or braces.
The exclusion criteria were as follows:
-
1.
Fracture with vertebral collapse (more than 50% of vertebral area or height loss) at initial diagnosis.
-
2.
Lack of follow-up CR at least 6 months after initial diagnosis.
-
3.
More than one acute OVF.
-
4.
Patients who underwent surgery or vertebral augmentation within 6 months of the fracture.
-
5.
Patients with poor quality of images, such as rotated, non-parallel radiographs.
Cases and controls were defined as OVFs which collapse and do not collapse, respectively, on follow-up X-rays. Eighty-four patients with OVF managed conservatively and with proper imaging quality were initially included. Of them, 28 were excluded (11 due to vertebral collapse at the time of initial diagnosis, 6 due to the presence of more than one acute vertebral fracture, and 11 due to lack of follow-up CR 6 months after the fracture). Therefore, a total of 56 cases were analyzed in the study.
Variables of the study
The dependent variable was vertebral collapse, defined as the presence of > 50% loss of vertebral body area or height at the end of follow-up:
The qualitative independent variables included were sex, vertebral fracture location, cause (spontaneous, exertion, fall), presence of cleft, fracture of lateral walls (absent, unilateral, bilateral), involvement of vertebral endplates (absent, superior, inferior, both), and involvement of anterior and posterior walls (absent, anterior, posterior, both).
We also assessed several qualitative variables related to the type of fracture at the time of initial diagnosis and at the end of follow-up based on the following grading systems:
-
1.Genant’s morphological (wedge, biconcave, crush) and quantitative (grade 0, 1, 2, 3) classifications.
-
2.Classification of the German Society for Orthopaedics and Trauma (OF1, OF2, OF3, OF4, OF5).
-
3.The AO Spine classification (A1 to A4), which was only used at the initial evaluation because it does not allow to quantify fracture progression.
-
4.Sugita’s classification (swelled front, bow-shaped, projecting, concave, dented), which was also used only at the initial assessment because it is not applicable when severe posterior height loss or wall damage develops.
Quantitative variables included patient age, local kyphosis, and percentage loss of vertebral area, anterior, middle, and posterior height in standing CR and supine CT images (Fig. 1). To calculate the percentage loss of vertebral area and height in CR and CT, measurements of the fractured vertebral body were divided by the mean of measurements made at the normal cephalad and caudal vertebrae. To compare the variability between standing measurements on radiography and supine measurements on CT, we calculated the differences in height and area loss as well as in local kyphosis obtained in both imaging techniques.
Measurements on computed tomography (CT) and conventional radiography (CR). A Example of area measurement on CT. B Example of measurement of the anterior, middle, and posterior vertebral height on standing CR. C Example of local kyphosis measurement on CR. D Example of local kyphosis measurement on CT
HU values were measured with oval region of interest (ROI) areas of approximately 1.5–2 cm2 at two different levels of the trabecular bone of the fractured vertebra, the adjacent upper and lower normal vertebrae, and the aortic lumen. The mean value of these measurements was used as the final density value of the fractured vertebra, the normal vertebra, and the aorta (Fig. 2). For measurements on the fractured vertebra, care was taken to avoid cystic cavities or sclerotic fracture impaction lines in the ROI area. The aorta showed an almost constant density in all patients and was chosen as the internal reference standard.
All CT examinations were performed on 16-slice Brightspeed or 64-slice Lightspeed CT scanners. Helical CT images with 0.63–1.25-mm thickness and 0.63–1.25-mm interval reconstructions were obtained. Two radiologists (FRS and AJLRB) with 30 and 5 years of experience independently performed measurements using an on-screen digital pointer with the Carestream Vue Picture Archiving and Communication System. For the sake of consistency, mean values of both measurements were used as final values. Fracture classification was also performed by both radiologists independently. In case of disagreement, the case was revised and discussed until agreement was reached.
Statistical analysis
We performed a descriptive analysis of the frequency of the qualitative categorical variables, as well as a numerical analysis of the continuous variables. The Kolmogorov–Smirnov test was applied to verify the normal distribution of the quantitative variables. In the bivariate analysis, quantitative variables were compared using Student’s t test for independent variables and the chi-square test for qualitative variables. Then, we performed a multivariate analysis based on a binary logistic regression model. Goodness of fit was calculated using the Hosmer–Lemeshow test. Finally, receiver operating characteristic (ROC) curves were used for discriminant analysis.
All data were collected and analyzed using SPSS v.20 software, with a p value of less than 0.05 deemed as statistically as significant.
Results
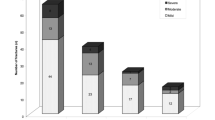
Of the 56 patients included in the study, 8 (14.3%) were men and 48 (85.7%) were women. The mean age was 72.6 ± 1.2 years. The mean follow-up for X-rays was 19 months (SD, 14.1; minimum 6, maximum 59). The fracture was spontaneous or after exertion in 6 cases (10.7%), and after falling to the ground from a standing position or lower in 50 cases (89.3%). Figure 3 shows the number of fractures identified and the vertebral endplates and walls affected. Both endplates were fractured in six cases (10.7%), and none of the endplates was involved in only one case (1.8%). Both anterior and posterior walls were fractured in 21 cases (37.5%), and none of the walls were involved in 4 cases (7.1%). Lateral wall fracture was absent in 12 cases (21.4%). At the time of initial diagnosis, 25 fractures (44.6%) showed an intravertebral cleft.
Twenty-five OVFs (44.6%) developed vertebral collapse on follow-up. Vertebral collapse was associated with fracture of the inferior (three of three cases, 100%; p = 0.040) and both endplates (five of six cases cases, 83.3%; p = 0.044).
Tables 1 and 2 show the quantitative and qualitative variables at the time of initial diagnosis of the patients, respectively.
Numerical variables associated with vertebral collapse were older age, initial percentage of posterior vertebral height (PVH), area loss on standing CR, low density of the non-fractured vertebral body, height density of the fractured body, and the ratio between the density of the fractured body and the aorta. The density ratio of the fractured versus non-fractured vertebral body and the difference between the percentage of vertebral area and PVH loss on CR versus CT were also significantly associated with vertebral collapse.
No fracture classification was predictive of vertebral collapse.
The results of logistic regression are shown in Table 3. The model showed that age, fractured/non-fractured body density ratio, and the difference in the percentage of PVH loss between CR and CT were positively correlated with the development of vertebral collapse (Fig. 4). This model correctly classified 89.8% of the cases and predicted collapse with a sensitivity of 79% and a specificity of 81% (Fig. 5).
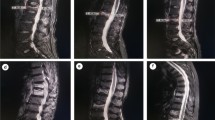
Two cases of vertebral collapse. Case 1: fracture of the L1 vertebral body (arrow) in a 74-year-old patient who fell from a standing position. A Standing radiography showed 16% posterior vertebra height (PVH) loss compared to CT (B). The CT density ratio between the fractured vertebral body versus the non-fractured vertebra was 2.2. C Follow-up radiography revealed collapse of the vertebral body. Case 2: fracture of the T12 vertebral body (arrow) in a 73-year-old patient. D Standing radiography showed 6% PVH loss compared to CT (E). The CT density ratio between the fractured vertebral body versus the non-fractured vertebra was 3.2. F Follow-up radiography revealed collapse of the vertebral body
The discriminative power to correctly classify the collapse of OVFs was 72.5% for age, 75.5% for the fractured/non-fractured body density ratio, and 67.8% for the difference of percentage loss of PVH on CR versus CT (Table 4). For predicting vertebral collapse, a cutoff value of 72.5 years showed a sensitivity of 72% and specificity of 58.1%, a ratio > 2 for the fractured/non-fractured body density ratio showed a sensitivity of 40% and specificity of 90.3%, and a cutoff value of 6% for the difference in the percentage of PVH loss between CR and CT showed a sensitivity of 40% and specificity of 87.1%.
Discussion
Vertebral fractures are the most common type of osteoporotic fractures. Inappropriate or insufficient treatment of OVFs may lead to progressive collapse of the vertebral body with increased kyphotic deformity, which has been associated with impaired quality of life and even higher mortality rates due to cardiopulmonary complications [28, 29]. Previous randomized trials have shown that vertebral augmentation techniques prevent further vertebral height loss over time compared to conservative management [30, 31]. Therefore, determining predictive factors for vertebral collapse may help select appropriate candidates for early interventional or surgical management.
Several classification systems can be used to guide the management of OVFs. The AO Spine classification system was developed to evaluate traumatic fractures and considers osteoporosis as a modifier that may preclude surgery due to poor bone quality [22,23,24,25]. Therefore, specific classifications for OVFs may be considered more appropriate in this setting if they can provide useful guidance to choose the correct treatment, avoiding the progressive sintering of the vertebral body and clinically meaningful kyphotic deformity. Genant’s classification was devised for epidemiological purposes, with no prognostic implications [14]. Sugita’s morphological classification (mainly the projecting, swelling, and bow-shaped fracture types) was reported to have predictive value for vertebral collapse [15]. However, this has not been supported in posterior works [13, 26, 27], including ours. Although a recent study reported that the classification proposed by the German Society for Orthopaedics and Trauma is useful for selecting patients for kyphoplasty [18], there is no current evidence demonstrating its predictive value for vertebral collapse, and this has not been supported in our present study either.
According to the AO Spine classification system, five out six A4 fractures (fracture of posterior wall and both endplates) collapsed in our series, and therefore would have required interventional treatment (percutaneous or open) shortly after diagnosis instead of conservative management. Nevertheless, the rate of collapse in A3 fractures (fracture of the posterior wall with only one fractured endplate) was found to be only slightly higher than in A1 fractures (42.1% versus 35.5%, respectively). This emphasizes the difficulty of choosing an appropriate and standardized treatment in osteoporotic burst fractures (i.e., A3 and A4). In a previous survey, 96.2% of A3 factures were operated in Germany compared to 41.2% in the Netherlands [28, 29]. Another study reported that 50% of A3/A4 fractures were operated due to progressive kyphosis and persistent pain [32].
Therefore, factors other than the type of fracture need to be considered to standardize the most appropriate treatment. Fracture of the inferior endplate was found in our work to be associated with vertebral collapse in the bivariate analysis, with high specificity. Nevertheless, it is an infrequent finding in traumatic (1.5%) and osteoporotic fractures (9.8–17.4%) [33]. In our series, it was present only in 9 out 56 cases (16.1%), precluding its inclusion in the multivariate analysis.
Imaging plays an important role in defining factors predictive of vertebral non-union and collapse. Most of these factors have been studied in MRI and include mid-portion-type fracture [26, 27], middle-column injury, and confined high-intensity or diffuse low-intensity areas in the fractured vertebra on T2-weighted images [13, 34]. However, as our work demonstrates, information predictive of vertebral collapse can also be obtained from measurements on standing CR and supine CT. Differences in measurements between both techniques may be explained by the fact that vertebral height and area tend to recover from compression in supine position, especially in the most unstable vertebral fractures. These differences, mainly in PVH, showed predictive value for vertebral collapse in our study.
Previous studies have suggested that pseudoarthrosis, manifested as the presence of intravertebral clefts or cysts, are risk factors to consider as they indicate lack of consolidation with probable instability [35, 36]. Dynamic instability and consequent hypermobility at the fracture site can lead to collapse and retropulsion of the bone fragments into the spinal canal [37, 38]. This factor was not statistically significant in our study despite the higher percentage of collapse in fractures with intravertebral cleft or cyst (53.8% versus 33.3%). Other authors have noted that this instability may be demonstrated by determining the differences of local kyphosis in standing and supine spine images [39, 40]. In our work, the difference in the percentage of PVH loss between standing CR and supine CT reached statistical significance and should therefore be considered in the decision-making process. This is in agreement with a previous work reporting that involvement of the middle column in vertebral fractures is associated with development of vertebral collapse [41]. In that study, vertebral collapse occurred in 31.2% of the fractures, which is lower than in our series. This discrepancy might be because the majority of our cases involved osteoporotic fractures resulting from low-energy trauma.
We observed in the bivariate analysis an association between the low density of the non-fractured vertebra with development of vertebral collapse in the fractured vertebra. Previous studies have reported low vertebral body density values in patients with OVFs [12, 42] and a negative correlation between HU values and osteopenia/osteoporosis [43], in agreement with our findings. This supports the idea that lower vertebral density may be a risk factor of collapse when a fracture occurs. However, the novelty of our work lies in demonstrating that an increased ratio density of the fractured vertebral body versus the non-fractured osteoporotic vertebral body is a risk factor for vertebral collapse, with a ratio of 2 showing 90.3 specificity. We hypothesize that this is the homologous finding on CT of the low signal intensity of fractured vertebra on MRI described in previous works as a predictive sign of vertebral collapse [13, 34], resulting from impaction of the trabeculae and sclerosis at the fractured body in comparison with the low density of the non-fractured vertebral bodies.
Finally, patient age also needs to be considered in the decision-making process. The relationship between age with osteoporosis and low bone density on CT has been clearly established in the literature [43, 44]. In our work, age over 72.5 years showed the highest discriminative power value for predicting vertebral collapse. Therefore, older patients with other factors predictive of vertebral collapse on CT and CR should be selected for early non-conservative treatment.
The main limitations of our study are its retrospective, single-center nature, and the limited sample size, which calls for further studies to verify our findings. In addition, the fact that the data from our study were selected from symptomatic patients attended in the emergency department, mostly after a fall from standing height or less, precludes the generalizability of our results to other scenarios. This includes patients without prior trauma and those with incidental radiographic findings, where the rate of symptomatic cases ranges between 14 and 30% [45,46,47].
Conclusion
Our work strongly suggests that there is an increased risk of vertebral collapse in fractures with differences of more than 6% in PVH loss between standing CR and supine CT, a CT density ratio between the fractured and non-fractured vertebral body > 2, and age over 72.5 years. Accordingly, non-conservative management (i.e., surgery or percutaneous augmentation) of OVFs must be considered when these findings are present.
Data Availability
All data used in this work are available upon reasonable request to the corresponding author.
References
Diacinti D, Guglielmi G (2019) How to define an osteoporotic vertebral fracture? Quant Imaging Med Surg 9(9):1485–1494. https://doi.org/10.21037/qims.2019.09.10
LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES (2022) The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 33(10):2049–2102. https://doi.org/10.1007/s00198-021-05900-y
Wáng YXJ, Santiago FR, Deng M, Nogueira-Barbosa MH (2017) Identifying osteoporotic vertebral endplate and cortex fractures. Quant Imaging Med Surg 7:555–591. https://doi.org/10.21037/QIMS.2017.10.05
Le Huec JC, Thompson W, Mohsinaly Y et al (2019) Sagittal balance of the spine. Eur Spine J 28:1889–1905. https://doi.org/10.1007/S00586-019-06083-1
Oei L, Rivadeneira F, Ly F et al (2013) Review of radiological scoring methods of osteoporotic vertebral fractures for clinical and research settings. Eur Radiol 23:476–486. https://doi.org/10.1007/s00330-012-2622-z
Karul M, Bannas P, Schoennagel BP, Hoffmann A, Wedegaertner U, Adam G, Yamamura J (2013) Fractures of the thoracic spine in patients with minor trauma: comparison of diagnostic accuracy and dose of biplane radiography and MDCT. Eur J Radiol 82(8):1273–1277. https://doi.org/10.1016/j.ejrad.2013.01.016
Jung CW, Lee J, Ham DW, Kang H, Chang DG, Kim YB, Ahn YJ, Shim JH, Song KS (2022) Forward bending in supine test: diagnostic accuracy for acute vertebral fragility fracture. Healthcare (Basel) 10(7):1215. https://doi.org/10.3390/healthcare10071215
Lentle B, Trollip J, Lian K (2016) The radiology of osteoporotic vertebral fractures redux. J Clin Densitom 19:40–47. https://doi.org/10.1016/J.JOCD.2015.08.009
Pickhardt PJ, Lee LJ, Muñoz Del Rio A et al (2011) Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res 26:2194–2203. https://doi.org/10.1002/JBMR.428
Pickhardt PJ, Pooler BD, Lauder T et al (2013) Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 158:588–595. https://doi.org/10.7326/0003-4819-158-8-201304160-00003
Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG (2011) Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am 93(11):1057–1063. https://doi.org/10.2106/JBJS.J.00160
Zou D, Ye K, Tian Y et al (2020) Characteristics of vertebral CT Hounsfield units in elderly patients with acute vertebral fragility fractures. Eur Spine J 29:1092–1097. https://doi.org/10.1007/S00586-020-06363-1
Tsujio T, Nakamura H, Terai H et al (2011) Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: a prospective multicenter study. Spine (Phila Pa 1976) 36:1229–1235. https://doi.org/10.1097/BRS.0B013E3181F29E8D
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/JBMR.5650080915
Sugita M, Watanabe N, Mikami Y et al (2005) Classification of vertebral compression fractures in the osteoporotic spine. J Spinal Disord Tech 18:376–381. https://doi.org/10.1097/01.BSD.0000168716.23440.61
Schnake KJ, Blattert TR, Hahn P et al (2018) Classification of osteoporotic thoracolumbar spine fractures: recommendations of the Spine Section of the German Society for Orthopaedics and Trauma (DGOU). Glob spine J 8:46S-49S. https://doi.org/10.1177/2192568217717972
Blattert TR, Schnake KJ, Gonschorek O et al (2018) Nonsurgical and surgical management of osteoporotic vertebral body fractures: recommendations of the Spine Section of the German Society for Orthopaedics and Trauma (DGOU). Glob Spine J 8:50S-55S. https://doi.org/10.1177/2192568217745823
Palmowski Y, Balmer S, Hu Z et al (2022) Relationship between the OF classification and radiological outcome of osteoporotic vertebral fractures after kyphoplasty. Glob Spine J 12:646–653. https://doi.org/10.1177/2192568220964051
Yaman O, Zileli M, Şentürk S et al (2021) Kyphosis after thoracolumbar spine fractures: WFNS Spine Committee Recommendations. Neurospine 18:681–692. https://doi.org/10.14245/NS.2142340.170
Curfs I, Grimm B, van der Linde M et al (2016) Radiological prediction of posttraumatic kyphosis after thoracolumbar fracture. Open Orthop J 10:135–142. https://doi.org/10.2174/1874325001610010135
Viswanathan VK, Shetty AP, Sindhiya N et al (2022) Prospective study to identify the clinical and radiologic factors predictive of pseudarthrosis development in patients with osteoporotic vertebral fractures. World Neurosurg 167:e350–e359. https://doi.org/10.1016/J.WNEU.2022.08.011
Inose H, Kato T, Ichimura S, Nakamura H, Hoshino M, Togawa D, Hirano T, Tokuhashi Y, Ohba T, Haro H, Tsuji T, Sato K, Sasao Y, Takahata M, Otani K, Momoshima S, Yuasa M, Hirai T, Yoshii T, Okawa A (2020) Risk factors of nonunion after acute osteoporotic vertebral fractures: a prospective multicenter cohort study. Spine (Phila Pa 1976) 45(13):895–902. https://doi.org/10.1097/BRS.0000000000003413
Wakao N, Sakai Y, Watanabe T, Osada N, Sugiura T, Iida H, Ozawa Y, Murotani K (2023) Spinal pseudoarthrosis following osteoporotic vertebral fracture: prevalence, risk factors, and influence on patients’ activities of daily living 1 year after injury. Arch Osteoporos 18(1):45. https://doi.org/10.1007/s11657-023-01236-8
Vaccaro AR, Oner C, Kepler CK et al (2013) AOSpine thoracolumbar spine injury classification system: fracture description, neurological status, and key modifiers. Spine (Phila Pa 1976) 38:2028–2037. https://doi.org/10.1097/BRS.0B013E3182A8A381
Santiago FR, Muñoz PT, Sánchez EM et al (2016) Classifying thoracolumbar fractures: role of quantitative imaging. Quant Imaging Med Surg 6:772–784. https://doi.org/10.21037/QIMS.2016.12.04
von Elm E, Altman DG, Egger M et al (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349. https://doi.org/10.1016/J.JCLINEPI.2007.11.008
Ha KY, Kim YH (2013) Risk factors affecting progressive collapse of acute osteoporotic spinal fractures. Osteoporos Int 24:1207–1213. https://doi.org/10.1007/S00198-012-2065-Z
Hinde K, Maingard J, Hirsch JA et al (2020) Mortality outcomes of vertebral augmentation (vertebroplasty and/or balloon kyphoplasty) for osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Radiology 295:96–103. https://doi.org/10.1148/RADIOL.2020191294
Pishnamaz M, Curfs I, Balosu S et al (2015) Two-nation comparison of classification and treatment of thoracolumbar fractures: an Internet-based multicenter study among spine surgeons. Spine (Phila Pa 1976) 40:1749–1756. https://doi.org/10.1097/BRS.0000000000001143
Firanescu CE, de Vries J, Lodder P et al (2019) Percutaneous Vertebroplasty is no Risk Factor for New Vertebral Fractures and Protects Against Further Height Loss (VERTOS IV). Cardiovasc Intervent Radiol 42:991–1000. https://doi.org/10.1007/s00270-019-02205-w
Farrokhi MR, Alibai E, Maghami Z (2011) Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine 14:561–569. https://doi.org/10.3171/2010.12.SPINE10286
Joaquim AF, Daubs MD, Lawrence BD et al (2013) Retrospective evaluation of the validity of the Thoracolumbar Injury Classification System in 458 consecutively treated patients. Spine J 13:1760–1765. https://doi.org/10.1016/J.SPINEE.2013.03.014
Wáng YXJ (2022) An update of our understanding of radiographic diagnostics for prevalent osteoporotic vertebral fracture in elderly women. Quant Imaging Med Surg 12(7):3495–3514. https://doi.org/10.21037/qims-22-360
Kanchiku T, Imajo Y, Suzuki H, et al (2014) Usefulness of an early MRI-based classification system for predicting vertebral collapse and pseudoarthrosis after osteoporotic vertebral fractures. J Spinal Disord Tech 27 https://doi.org/10.1097/BSD.0B013E318292B509
Kim DY, Lee SH, Jang JS et al (2004) Intravertebral vacuum phenomenon in osteoporotic compression fracture: report of 67 cases with quantitative evaluation of intravertebral instability. J Neurosurg 100:24–31. https://doi.org/10.3171/SPI.2004.100.1.0024
Peh WCG, Gelbart MS, Gilula LA, Peck DD (2003) Percutaneous vertebroplasty: treatment of painful vertebral compression fractures with intraosseous vacuum phenomena. AJR Am J Roentgenol 180:1411–1417. https://doi.org/10.2214/AJR.180.5.1801411
Kaneda K, Asano S, Hashimoto T et al (1992) The treatment of osteoporotic-posttraumatic vertebral collapse using the Kaneda device and a bioactive ceramic vertebral prosthesis. Spine (Phila Pa 1976) 17:295–303. https://doi.org/10.1097/00007632-199208001-00015
Baba H, Maezawa Y, Kamitani K et al (1995) Osteoporotic vertebral collapse with late neurological complications. Paraplegia 33:281–289. https://doi.org/10.1038/SC.1995.64
Cho JH, Shin SI, Lee JH et al (2013) Usefulness of prone cross-table lateral radiographs in vertebral compression fractures. Clin Orthop Surg 5:195–201. https://doi.org/10.4055/CIOS.2013.5.3.195
McKiernan F, Jensen R, Faciszewski T (2003) The dynamic mobility of vertebral compression fractures. J Bone Miner Res 18:24–29. https://doi.org/10.1359/JBMR.2003.18.1.24
Hoshino M, Tsujio T, Terai H, Namikawa T, Kato M, Matsumura A, Suzuki A, Takayama K, Takaoka K, Nakamura H (2013) Impact of initial conservative treatment interventions on the outcomes of patients with osteoporotic vertebral fractures. Spine (Phila Pa 1976) 38(11):E641-8. https://doi.org/10.1097/BRS.0b013e31828ced9d
Lee SJ, Binkley N, Lubner MG et al (2016) Opportunistic screening for osteoporosis using the sagittal reconstruction from routine abdominal CT for combined assessment of vertebral fractures and density. Osteoporos Int 27:1131–1136. https://doi.org/10.1007/S00198-015-3318-4
Lee H, Park S, Kwack KS et al (2023) CT and MR for bone mineral density and trabecular bone score assessment in osteoporosis evaluation. Sci Rep 13(1):16574. https://doi.org/10.1038/s41598-023-43850-z
Emohare O, Cagan A, Morgan R et al (2014) The use of computed tomography attenuation to evaluate osteoporosis following acute fractures of the thoracic and lumbar vertebra. Geriatr Orthop Surg Rehabil 5:50–55. https://doi.org/10.1177/2151458514525042
Fink HA, Litwack-Harrison S, Ensrud KE, Shen J, Schousboe JT, Cawthon PM, Cauley JA, Lane NE, Taylor BC, Barrett-Connor E, Kado DM, Cummings SR, Marshall LM, Osteoporotic Fractures in Men (MrOS) Study Group (2017) Association of incident, clinically undiagnosed radiographic vertebral fractures with follow-up back pain symptoms in older men: the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res 32(11):2263–2268. https://doi.org/10.1002/jbmr.3215
Sawicki P, Tałałaj M, Życińska K, Zgliczyński WS, Bogołowska-Stieblich A, Krakowiak J, Wierzba W (2021) Characteristics of osteoporotic vertebral fractures in association with symptomatic status in postmenopausal women - a retrospective study of a single centre in Poland. Ann Agric Environ Med 28(4):654–658. https://doi.org/10.26444/aaem/133230
de Klerk G, Hegeman JH, Bronkhorst P, van der Palen J, van der Velde D, Duis HJ (2012) The (a)-symptomatic vertebral fracture: a frequently discovered entity with clinical relevance in fracture patients screened on osteoporosis. Geriatr Orthop Surg Rehabil 3(2):74–78. https://doi.org/10.1177/2151458512449833
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruiz Santiago, F., Láinez Ramos-Bossini, A.J. & Moraleda-Cabrera, B. Factors influencing vertebral collapse in osteoporotic vertebral fractures: a case–control study of symptomatic patients attended in the emergency department. Arch Osteoporos 19, 6 (2024). https://doi.org/10.1007/s11657-023-01365-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-023-01365-0