Abstract
Summary
Identifying secondary causes among osteoporotic patients is crucial. However, there is no simple tool for screening secondary osteoporosis. A predictive model for screening secondary osteoporosis was constructed using simple clinical and biochemical parameters. This predictive model may provide clinicians with guidance to perform further investigations for specific causes of osteoporosis.
Purpose
Establishing whether a fragility fracture is secondary to a specific cause of osteoporosis is crucial for treatment outcomes. Therefore, this study aimed to develop a simple screening tool for secondary osteoporosis in the elderly initially presented with fragility fractures.
Methods
A retrospective cohort study including 456 patients with fragility hip and vertebral fractures that occurred between January 2017 and July 2022 was conducted. Demographic, clinical, biochemical, and final diagnostic data were retrieved. Potential predictors for secondary osteoporosis were determined by multivariable logistic regression analysis, and a predictive model for secondary osteoporosis was subsequently developed using identified potential predictors.
Results
This study included 343 females and 113 males with a mean age of 76.9 ± 11.0 years. One hundred and twenty-one patients (26.5%) were diagnosed with secondary osteoporosis. Vitamin D deficiency (71.9%) was the most common cause of secondary osteoporosis, followed by glucocorticoid-induced osteoporosis (23.9%) and primary hyperparathyroidism (9.9%). The potential predictors for secondary osteoporosis included in the predictive model were age, body mass index (BMI), corrected calcium, phosphate, thyroid stimulating hormone, and a 10-year probability of hip fractures calculated by BMI-based FRAX®. With a cut-off level of 0.22, the proposed predictive model has an AuROC of 0.75 (95% CI 0.69 to 0.81) with a sensitivity of 77%, a specificity of 66%, and an accuracy of 68.9%.
Conclusion
A predictive model for screening secondary osteoporosis was constructed using simple clinical and biochemical parameters. This newly developed predictive model may provide clinicians with guidance to perform further advanced investigations for secondary causes of osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a concerning disease worldwide because of its increasing prevalence along with the trend of an aging society, as well as its impact on the independence of individuals who experience osteoporosis-related fractures. Even though primary osteoporosis is the most common type of osteoporosis, specific contributing factors for osteoporosis were identified in 20–30%, 40–50%, and 50–80% in postmenopausal women, premenopausal women, and men, respectively [1,2,3]. Undiagnosed secondary osteoporosis usually does not respond as well to osteoporotic therapy as primary osteoporosis [4]. As a result, the correct diagnosis of secondary osteoporosis before starting pharmacologic treatment for osteoporosis is critical for the treatment’s success.
Multiple clinical practice guidelines, both international and regional, recommend a thorough clinical evaluation and laboratory panel for all osteoporotic cases to determine secondary causes of osteoporosis [5,6,7,8,9,10]. In addition, the specific contributing factors for osteoporosis should be explored in medication-compliance patients who poorly respond to treatment, even those who previously had a negative screening panel for secondary osteoporosis [11, 12]. Almost all clinical practice guidelines recommend basic laboratory investigations, including complete blood count, blood chemistry, liver function tests, and serum 25-hydroxy vitamin D (25(OH)D) in all cases of osteoporosis. Additional investigations, including thyroid function tests and parathyroid hormone levels, should be evaluated upon specific clues identified by either clinical evaluation or a basic laboratory investigation [5, 6, 8,9,10]. Several studies have shown that different screening panels for secondary osteoporosis are beneficial [13,14,15]. Nevertheless, to date, there is no simple tool for screening secondary osteoporosis.
Fragility fracture is the most devastating skeletal outcome of osteoporosis. Patients with previous fragility fractures are at the highest risk for recurrent fractures. Therefore, it is essential to establish a diagnosis of osteoporosis and specify its type for pursuing a proper treatment algorithm. However, many patients have missed opportunities for appropriate investigation and treatment [16,17,18,19]. Therefore, it would be beneficial in the clinic if there were a simple screening tool for secondary osteoporosis to guide physicians in their decision to further investigate the specific cause of osteoporosis. This study aimed to identify specific contributing factors to osteoporosis in the patients who first presented with fragility fractures as well as to develop a predictive model for secondary osteoporosis.
Methods
Study subjects and design
A retrospective cohort study of patients diagnosed with fragility fracture between January 2017 to July 2022 was conducted. We included patients first documented with fragility fractures, including hip and vertebral fragility fractures, aged more than 18 years based on the relevant International Classification of Diseases 10th revision (ICD-10) code: M4840, M4845-4846, M4849-4850, M4854-4856, M4859, M8000-8029, M8040-8059, S2200, S2210, S3200, S7200, S7220, S7230, S7290, S8280, and S8290 (Online Resource 1). Our implemented inclusion is primarily based on ICD10, which includes hip and vertebral fractures diagnosed without a specific cause, allowing us to encompass a wide range of cases. The exclusion criteria were: (1) fracture associated with the preexisting pathological bone lesion or high energy trauma, which is unrelated to falling from a standing height or less, such as a vehicle accident or falling from a stair [8], (2) patients who were already diagnosed with osteoporosis, (3) patients who had been prescribed anti-resorptive or anabolic agents, and (4) patients who received an incomplete evaluation for the secondary osteoporosis according to current guidelines [6, 8,9,10]. The study was approved by the Institutional Board Review of the Faculty of Medicine Chiang Mai University (MED-2564-08627). The study flow is shown in Fig. 1. The study size was calculated based on a pilot study of 20 patients giving a total number of 455 patients to reach the power of 80%.
Measurement and definition
Demographic characteristics and laboratory tests were collected during the admission. Bone mineral density (BMD) was collected if the patient underwent to perform BMD after the fracture and before anti-resorptive or anabolic agent initiation. BMD was performed with the exclusion of the fracture area and determined based on the lowest value. The BMD T-score and Z-score were measured by dual-energy X-ray absorptiometry (DXA) (DiscoveryTM QDR series, Hologic®, Marlborough, MA, USA). The patient’s fracture risk was calculated using the fracture risk assessment tool (FRAX®) based on body mass index (BMI) according to Thailand’s database [6, 20]. We calculated FRAX® without a previous fracture history and used data from the first day of admission to determine the baseline pre-injury fracture risk. According to previous literature, the 10-year probability of major osteoporotic fractures (FRAX-M) and hip fractures (FRAX-H) calculated by FRAX® in type 2 diabetes (T2DM) patients should be adjusted [21]. As a result, in patients with T2DM, we adjusted the FRAX-M and FRAX-H by adding 10 years of age or substituting rheumatoid arthritis. The higher value was subsequently used for further analysis. For patients with prednisolone use, we adjusted the FRAX-M and FRAX-H based on the current dose of prednisolone [6, 20].
Secondary osteoporosis is defined as low bone mass with microarchitectural alterations in bone leading to fragility fractures in the presence of an underlying disease or medication [4]. Therefore, in this study, we defined secondary osteoporosis as osteoporosis with at least one identified contributing factor for osteoporosis according to the clinical evidence recorded in the electronic medical record after the episode of fragility fracture. Every patient included in this study has neither a previous fracture nor documentation of any contributing factors for osteoporosis prior to the episode of fracture. The specific contributing factors for osteoporosis included the following documented factors as causes of secondary osteoporosis: hypercortisolism, hyperparathyroidism, hypogonadism, hypophosphatemia, vitamin D deficiency, drugs induced osteoporosis, myeloma, and early menopause [22]. Vitamin D deficiency was defined by serum 25(OH)D level of less than 20 ng/mL [23, 24]. The 25(OH)D level was measured by electrochemiluminescence immunoassay (ECLIA) (Elecsys® 2010/1010, Modular E 170, cobas e411, Roche Diagnostics International Ltd, Rotkreuz, Switzerland) with a detectable dose ranging from 3 to 70 ng/mL. The coefficient of variation (CV) of intra- and inter-assay was below 5.4% and 5.7%, respectively. The 25(OH)D level was assessed using standardized laboratory procedures. Hypercortisolism was diagnosed based on increased 24-h urinary free cortisol and non-suppressed serum cortisol level by overnight 1 mg dexamethasone suppression test (DST) or low-dose 2-day dexamethasone test (LDDT) [25]. Hyperparathyroidism included primary, secondary, and tertiary hyperparathyroidism. Classic primary hyperparathyroidism (PHPT) was defined by the elevation of serum calcium and non-suppressed parathyroid hormone level with a cut-off level of 10.5 mg/dL and 15 pg/dL, respectively. Normocalcemic PHPT (NPHPT), a well-recognized variant of PHPT, was defined by normal total serum calcium levels without any known etiologies for a secondary elevation of the PTH [26]. According to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, secondary (SHPT) and tertiary hyperparathyroidism (THPT) was defined as the PTH level above nine times the upper normal limit [27]. Drug-induced osteoporosis was defined as osteoporotic patients who received glucocorticoid, aromatase inhibitors, ovarian suppressing agents, androgen deprivation therapy, thiazolidinediones, psychotropic and anticonvulsant therapy, selective serotonin reuptake inhibitors, anticonvulsants, drugs targeting the immune system, calcineurin inhibitor, and anti-retroviral therapy without others cause of osteoporosis [28]. Additionally, early menopause was defined as women who experienced menopause before the age of 45 [29]. If the final diagnosis were inconclusive, a consensus on the diagnosis was made by two endocrinologists who had independently performed a chart review.
Statistical methods
All statistical analyses were performed using STATA (version 16.0, StataCrop LLC., College Station, TX, USA). Continuous data were presented by the mean and standard deviation (SD) for normally distributed data. For non-normally distributed data, median and interquartile ranges (IQR) were used. Continuous data were analyzed using an independent t-test and the Mann-Whitney U test as appropriate. Categorical data were presented by frequency and percentage, which were compared using Fisher’s exact probability test. A comparison of three or more groups was performed using analysis of variance (ANOVA) with Bonferroni correction. Statistically significant was defined as a p-value less than 0.05.
A diagnostic screening tool was developed according to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statements [30]. Candidate predictors were selected according to clinical relevance and statistical significance via multivariable logistic regression modeling with a stepwise backward elimination approach. In addition, all continuous parameters were included in the model without categorization to avoid unnecessary information loss. We employed the multivariable fractional polynomials (MFP) function to identify each continuous variable Field’s most appropriate functional form [31]. As a result, a non-linear model could be generated to reflect the true association between the dependent and independent variables. The developed model’s performance was evaluated using the area under the receiver operating characteristics (AuROC) curve. The model’s calibration, an agreement between the predicted and observed risk, was presented via calibration plot. Internal validation was performed using the bootstrap resampling method to identify the model’s optimism. Overfitting was tested using calibration slope, calibration-in-the-large (CITL) value, and the shrinkage factor.
Results
Specific contributing factors were identified in one-quarter of apparent healthy elderly presenting with fragility fractures
A total of 456 patients were eligible to enter the study (Fig. 1). Most of the study population was female (75.2%), with a mean age of 76.9 ± 11.0 years. Most of the fragility fractures were hip fractures (77.8%), followed by vertebral fractures (22.2%). In general, our enrolled patients had low BMD in the osteoporotic range and had a high risk of hip fractures as defined by the 10-year probability of hip fracture calculated by FRAX® (FRAX-H) of greater than 3%. One hundred and twenty-one (26.5%) patients were diagnosed with secondary osteoporosis. Patients with secondary osteoporosis were younger (73.2 ± 12.0 years vs. 78.3 ± 10.3 years, p < 0.001) and had a higher BMI (22.2 ± 3.7 kg/m2 vs. 21.3 ± 4.5 kg/m2, p = 0.024). Patients with secondary osteoporosis had a significantly higher prevalence of rheumatoid arthritis and systemic lupus erythematosus, as well as a higher usage rate of prednisolone. Although the prevalence of diabetes mellitus was comparable in both groups, patients with secondary osteoporosis tended to receive pioglitazone and sodium-glucose transporter 2 (SGLT2) inhibitors more frequently than primary osteoporosis. In terms of the basic laboratory profile, only serum phosphate (3.4 ± 1.2 mg/dL vs. 3.2 ± 0.6 mg/dL, p = 0.033) and TSH (7.15 ± 30.4 μIU/mL vs. 2.54 ± 2.2 μIU/mL, p = 0.013) were significantly higher in those with secondary osteoporosis (Table 1). Among those with secondary osteoporosis, there were no clues of a specific disease detected by clinical evaluation and basic laboratory investigation.
Vitamin D deficiency leading contributing factor to fragility fractures in the elderly population
Vitamin D deficiency was the most common cause of secondary osteoporosis (87 patients, 71.9%). Other less common etiologies were glucocorticoid-induced osteoporosis (GIO) (29 patients, 23.9%), followed by PHPT (12 patients, 9.9%), SHPT (5 patients, 4.1%), and multiple myeloma (4 patients, 3.3%) (Online Resource: Table 1). There were 21 patients with more than one diagnosis, including eleven patients with vitamin D deficiency plus GIO, eight with vitamin D deficiency plus PHPT, one with vitamin D deficiency plus SHPT associated with chronic renal failure, and one with vitamin D deficiency plus multiple myeloma. The mean duration of prednisolone usage in GIO was 2.76 ±2.83 years before the fracture, with a mean dosage of 8.53±11.1 mg per day and a mean accumulative dosage of 2.81 ± 2.9 g per year. The patient characteristics of each identified secondary cause are shown in Online Resource: Table 1. Hip fractures were predominant in patients with vitamin D deficiency, PHPT, and SHPT. Low BMD within the osteoporosis range was demonstrated in patients with any identified secondary causes; however, patients with hyperparathyroidism tended to have a lower BMD compared to those with other causes.
Among 329 patients tested for serum 25(OH)D levels, 87 (26.1%) patients were diagnosed with vitamin D deficiency. One hundred and twenty-nine (39.5%) patients were diagnosed with vitamin D insufficiency, as defined by serum 25(OH)D levels ranging from 20 to 29.9 ng/mL. The characteristics of the three groups of patients according to their vitamin D levels were demonstrated in Table 2. The levels of serum 25(OH)D levels were significantly different among the three groups (13.83 ± 3.97 ng/mL vs. 25.53 ± 2.8 ng/mL vs. 38.03 ± 8.4 ng/mL, p < 0.001). Since SHPT is related to vitamin D deficiency, PTH levels tended to be higher in the group with vitamin D deficiency compared to the other two groups. All parameters were comparable among the three groups, except BMI and the FRAX-M. The pairwise comparison with Bonferroni adjustment revealed that BMI significantly differed between groups with vitamin D deficiency and vitamin D adequacy (22.5 ± 3.8 kg/m2 vs. 21.1 ± 3.5 kg/m2, p = 0.019) while the FRAX-M significantly differed in both the vitamin D deficiency group compared to the vitamin insufficiency group (9.2 ± 4.6% vs. 7.6 ± 3.9%, p = 0.016) and the vitamin D deficiency group compared to the vitamin D adequacy group (9.2 ± 4.6% vs. 7.6 ± 3.7%, p = 0.020). Twenty-eight patients in the vitamin D deficiency group had severe vitamin D deficiency (mean 25(OH)D 9.1 ± 1.9 ng/mL), as defined by serum 25(OH)D level of lower than 12 ng/mL [32]. The PTH levels were comparable between patients with severe and non-severe vitamin D deficiency (97.3 ± 157.8 ng/mL vs. 112.7 ± 349.5 ng/mL, p = 1.000). Even though osteomalacia frequently occurs in severe vitamin D deficiency, no bone pain and muscle weakness were reported in those with severe vitamin deficiency, and the levels of serum calcium, serum phosphate, and ALP were normal in all those patients in the study, suggesting a clinical diagnosis of osteoporosis but not osteomalacia.
Predictive model as a diagnostic screening tool for secondary osteoporosis in elderly
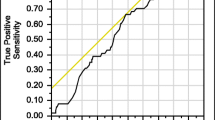
All potential predictors for secondary osteoporosis were included in multivariable logistic regression modeling with the multivariable fractional polynomial (MFP) algorithm to develop a diagnostic screening tool (Online Resource: Table 2). After applying the stepwise backward elimination method, the patient’s age, BMI, corrected calcium level, phosphate level, TSH level, and FRAX-H were shown as predictive factors for secondary osteoporosis. The patient’s age negatively correlated with the risk of developing secondary osteoporosis. In contrast, the BMI, FRAX-H, phosphate level, TSH level, and serum calcium level that was outside the range of 9 to 10 mg/dL positively correlated with the risk of developing secondary osteoporosis. No multicollinearity was identified among predictors. The predicted probability of secondary osteoporosis was calculated according to each included predictor’s covariate transformations and regression coefficient (Table 3). The model’s performance was acceptable, with an AuROC of 0.75 (95% CI 0.69 to 0.81) (Fig. 2). All six factors contributed to the power of the constructed predictive model. The calibration plot illustrated an agreement between observed and predicted probabilities (Online Resource: Fig. 1). In addition, the developed model demonstrated good internal validity with minimal optimism (0.02, 95% CI −0.08 to 0.09) and overfitting (E:O ratio = 1.00, CITL≤0.01, calibration slope = 1.00).
Area under receiver operating characteristic (AuROC) curve of secondary osteoporosis predicted by predictive model (gray line) and 50% chance prediction (diagonal line). Risk categories of secondary osteoporosis diagnosis with sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and their 95% confidence intervals were shown
A risk category was created to provide an appropriate recommendation regarding a patient’s risk (e.g., high and low risk). An optimum probability cut-off level was identified using the Youden index. As a result, a cut-off level of 0.22 demonstrated a maximum sensitivity of 74.4%, a specificity of 65.8%, a positive predictive value (PPV) of 41.6%, and a negative predictive value (NPV) of 88.7% (Fig. 2). The diagnostic indices of other cut-off values were demonstrated in Online Resource: Table 3. For practical use, the developed model was transformed into a web-applicable diagnostic screening tool, which is available at: https://www.calconic.com/calculator-widgets/secondary-osteoporosis-screening-tool/62f7c112d5da86001f8e3a25?layouts=true (Online Resource: Fig. 2).
Discussion
This study reported epidemiological information on secondary osteoporosis in the elderly who presented with fragility fractures. We also identified that the patient’s age, BMI, corrected calcium level, phosphate level, TSH, and 10-year probability of hip fractures calculated by BMI-based FRAX® were contributing factors for secondary osteoporosis. In addition, a diagnostic screening tool derived from the discovered risk factors demonstrated good diagnostic performance for secondary osteoporosis.
This study showed that 26.5% of apparently healthy elderly people who had no clinical clues for secondary osteoporosis but presented with fragility fractures had at least one specific contributing factor for osteoporosis. Since our study involved mainly postmenopausal women (75%), the prevalence of secondary osteoporosis in this study is consistent with the previously reported prevalence of 30% in postmenopausal women [3]. Even though GIO was previously shown as the most common cause of secondary osteoporosis, vitamin D deficiency was the most common etiology related to osteoporosis found in this current study. The prevalence of vitamin D deficiency in our enrolled patients was in the same range as shown in the previous reports done on postmenopausal Thais [33, 34]. Chailurkit and colleagues [33] reported a prevalence of vitamin D deficiency of 31.6% in postmenopausal women (mean age 67.5 ± 6.0 years). Furthermore, their findings demonstrated that osteoporotic patients, defined by the femoral neck BMD, had significantly lower 25(OH)D levels compared to those without osteoporosis.
In our study, one-third of patients diagnosed with vitamin D deficiency had serum 25(OH)D of lower than 12 ng/mL, indicating severe vitamin D deficiency [32]. According to a bone histomorphometric study of iliac crest biopsy, Priemel and colleagues [35] demonstrated mineralization defect in patients with serum 25(OH)D lower than 30 ng/mL but the highest prevalence was illustrated in patients with 25(OH)D lower than 12 ng/mL. However, the mineralization defect was not present in all patients with serum 25(OH)D levels less than 12 ng/mL. SHPT and chronic calcium deficiency would result in hyperphosphaturia and hypophosphatemia, resulting in bone mineralization defects [32]. Therefore, hypocalcemia and hypophosphatemia are indispensable for the occurrence of osteomalacia in cases of vitamin D deficiency. In this study, SPHT secondary was demonstrated in patients with vitamin D deficiency, but the PTH level did not positively correlate with the severity of the vitamin D deficiency. In addition, no patients with severe vitamin D deficiency revealed clinical features and biochemical abnormalities suggesting osteomalacia, including bone pain, muscle weakness, hypocalcemia, hypophosphatemia, and hyperphosphatasia. Accordingly, we are certain that patients with severe vitamin D deficiency had a clinical diagnosis of osteoporosis without osteomalacia in the present study.
Identifying secondary causes among osteoporotic patients is crucial since secondary osteoporosis usually does not respond well to the primary osteoporosis treatment protocol [4]. Secondary osteoporosis could be found in a significant number of patients who had primary osteoporosis by first clinical impression. Therefore, screening for any possible secondary causes should be applied to all patients suspected of osteoporosis. Several studies demonstrated that various evaluations for secondary osteoporosis were beneficial in the diagnostic utility and cost-effectiveness [13,14,15]. For that reason, we developed a diagnostic model for secondary osteoporosis using six simple predictors, including the patient’s age, BMI, FRAX-H calculated by BMI-based FRAX®, corrected calcium level, phosphate level, and TSH level. FRAX-M was not included in the model due to the insufficient data regarding the use of FRAX-M in predicting the risk of osteoporotic fractures in the Thai population [6, 7]. We believe that the developed diagnostic model could provide useful guidance for clinicians to provide further investigations in fragility fracture patients, entailing an appropriate treatment for re-fracture prevention. As we intended to use this predictive model as a screening tool, high sensitivity is crucial for early detection or ruling out a case with secondary osteoporosis. Patients with a risk for secondary osteoporosis of greater than 22% were classified as high-risk, for whom further investigation and specialist consultation were recommended. This cut-off level provides not only high sensitivity but also high NPV. Therefore, if a patient was classified as low risk from our screening tool, the patient is 89% likely to have primary osteoporosis. The developed predictive model is practical since all predictors are simple and available in most scenarios, even in settings where healthcare resources are limited. Patient’s age, BMI, and FRAX-H calculated by BMI-based FRAX® can be collected in routine patient evaluation. At the same time, calcium, phosphate, and TSH levels are simple laboratory tests that can be measured without special patient preparation. Therefore, we can assume that all predictors in the developed prediction model can be easily obtained during hospitalization for fragility fractures.
The older age and corrected calcium level of 9–10 mg/dL were protective factors for secondary osteoporosis. In contrast, the higher BMI, phosphate level, TSH level, and FRAX-H calculated by BMI-based FRAX® were risk factors for secondary osteoporosis. All of them contributed to the power of the predictive model; the power of the model significantly decreased if anyone was deleted. Age and weight are commonly recognized as traditional contributing factors for primary osteoporosis [36, 37]. Accordingly, patients with younger ages or higher BMI demonstrated higher risk and should be suspected of secondary osteoporosis [36]. In terms of FRAX-H, the high probability of hip fracture would derive from older age and a lower BMI [36]. A high probability of fractures in the context of a younger age and a higher BMI, according to our model, would indicate secondary osteoporosis.
Calcium and phosphate levels represent mineral homeostasis, which is tightly controlled by various hormones. Abnormal calcium and phosphate levels indicate abnormal mineral metabolism that usually associates with metabolic bone diseases. For example, hyperphosphatemia is usually demonstrated in chronic kidney disease-related metabolic bone disease (CKD-MBD) [27, 38]. Hypocalcemia usually indicates vitamin D deficiency [32] or SHPT [27, 38], while hypercalcemia is found in PHPT [26]. Multiple studies found high bone turnover and increased fracture risk in cases of hyperthyroidism or subclinical hyperthyroidism, whereas adults with hypothyroidism theoretically had a low bone turnover and a prolonged bone remodeling cycle [39, 40]. However, the adverse skeletal consequences of hypothyroidism in adults have never been established [39, 41]. TSH levels have been previously shown to be useful and cost-effective as part of secondary osteoporosis screening panels in determining overt and subclinical thyroid disease [14, 15]. Although the TSH level itself did not show a statistically significant correlation to secondary osteoporosis in this study, we decided to include this parameter due to its effects on bone turnover and its impact on the power of our constructed predictive model. In our study, 51 (13.9%) patients had elevated TSH with normal free thyroxine (free T4) and free iodothyronine (free T3). In addition, most of them had mild subclinical hypothyroidism (TSH elevation of <10 mIU/L) and were not indicated for thyroid hormone supplementation. No patients had been previously treated with thionamides or radioiodine therapy. This mild subclinical hypothyroidism could be from aging, since an increased prevalence of elevated TSH up to 15% in the population at the age of 70 years or older [42].
There are several strengths in this study. Our web-based diagnostic screening tool is simple and easy to use. With only six simple parameters, our tool allows clinicians to evaluate fragility fracture patients’ risk of secondary osteoporosis. However, there are some limitations to be addressed. First, our prediction tool was developed using a relatively small number of patients, which included only a minimal number for model optimism. Second, our tool was based on a retrospective study, which is subjected to several biases. Excluding patients who received an incomplete evaluation for secondary osteoporosis has the potential for selection bias and could affect the incidence and probability of cases. Third, the method for evaluation for 25(OH)D levels was electrochemiluminescence immunoassay (ECLIA), not the liquid chromatography-tandem mass spectrometry (LC-MS) which is the gold-standard test for 25(OH)D levels. However, the Roche Elecsys® Vitamin D total III assay was also valid and reliable [43]. In addition, 25(OH)D levels were not available in all patients, which might alter the true prevalence of vitamin D deficiency in our study. Finally, although the developed model demonstrated good internal validity, external validation is mandatory to evaluate the generalizability of the tool.
In conclusion, specific causes of osteoporosis were demonstrated in 26.5% of apparent healthy elderly individuals presented with fragility fractures. The most common contributing factor for secondary osteoporosis was vitamin D deficiency, followed by GIO. We identified the patient’s age, BMI, corrected calcium level, phosphate level, TSH level, and FRAX-H calculated by FRAX® as potential predictors for secondary osteoporosis. Subsequently, a diagnostic model for screening secondary osteoporosis was constructed using these simple clinical and biochemical parameters. This diagnostic screening tool may provide clinicians with guidance for further advanced investigations for secondary causes of osteoporosis.
Data availability
All data generated or analyzed during this study are included in this published article and supplementary online resource.
References
Adler RA (2014) Osteoporosis in men: a review. Bone Res 2:14001
Ebeling PR, Nguyen HH, Aleksova J, Vincent AJ, Wong P, Milat F (2022) Secondary osteoporosis. Endocr Rev 43(2):240–313
Walker-Bone K (2012) Recognizing and treating secondary osteoporosis. Nat Rev Rheumatol 8(8):480–492
Mirza F, Canalis E (2015) Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol 173(3):R131–R151
Gregson CL, Armstrong DJ, Bowden J, Cooper C, Edwards J, Gittoes NJL et al (2022) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 17(1):58
Songpatanasilp T, Sritara C, Kittisomprayoonkul W, Chaiumnuay S, Nimitphong H, Charatcharoenwitthaya N et al (2016) Thai Osteoporosis Foundation (TOPF) position statements on management of osteoporosis. Osteoporos Sarcopenia 2:191–207
Charatcharoenwitthaya N, Jaisamrarn U, Songpatanasilp T, Kuptniratsaikul V, Unnanuntana A, Sritara C et al (2023) Summary of the Thai Osteoporosis Foundation (TOPF) Clinical Practice Guideline on the diagnosis and management of osteoporosis 2021. Osteoporos Sarcopenia 9(2):45–52
National Institute for Health and Care Excellence: Guidelines (2017) Osteoporosis: assessing the risk of fragility fracture. London: National Institute for Health and Care Excellence (NICE) Copyright © NICE 2019
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A et al (2020) American Association of Clinical Endocrinologists/American College Of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract 26(Suppl 1):1–46
Kanis JA, Cooper C, Rizzoli R, Reginster JY (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30(1):3–44
Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR et al (2012) Treatment failure in osteoporosis. Osteoporos Int 23(12):2769–2774
Díez-Pérez A, Olmos JM, Nogués X, Sosa M, Díaz-Curiel M, Pérez-Castrillón JL et al (2012) Risk factors for prediction of inadequate response to antiresorptives. J Bone Miner Res 27(4):817–824
Fink HA, Litwack-Harrison S, Taylor BC, Bauer DC, Orwoll ES, Lee CG et al (2016) Clinical utility of routine laboratory testing to identify possible secondary causes in older men with osteoporosis: the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int 27(1):331–338
Johnson K, Suriyaarachchi P, Kakkat M, Boersma D, Gunawardene P, Demontiero O et al (2015) Yield and cost-effectiveness of laboratory testing to identify metabolic contributors to falls and fractures in older persons. Arch Osteoporos 10:226
Tannenbaum C, Clark J, Schwartzman K, Wallenstein S, Lapinski R, Meier D et al (2002) Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 87(10):4431–4437
Mendis AS, Ganda K, Seibel MJ (2017) Barriers to secondary fracture prevention in primary care. Osteoporos Int 28(10):2913–2919
Lee GE, Muffly S, Golladay GJ (2019) Management of fragility hip fractures: our institutional experience. Geriatr Orthop Surg Rehabil 10:2151459319828618
Gani L, Reddy SK, Alsuwaigh R, Khoo J, King TFJ (2017) High prevalence of missed opportunities for secondary fracture prevention in a regional general hospital setting in Singapore. Arch Osteoporos 12(1):60
Nguyen K, Chen X, Hughes T, Hofflich H, Woods GN, McCowen KC (2021) Surprisingly few women with severe osteoporosis by bone densitometry undergo workup for secondary causes - a retrospective evaluation. Acta Endocrinol (Buchar) 17(4):537–542
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgström F et al (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23(9):2257–2276
Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, Eastell R et al (2018) Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 29(12):2585–2596
Sheu A, Diamond T (2016) Secondary osteoporosis. Aust Prescr 39(3):85–87
Pludowski P, Takacs I, Boyanov M, Belaya Z, Diaconu CC, Mokhort T et al (2022) Clinical practice in the prevention, diagnosis and treatment of vitamin D deficiency: a Central and Eastern European Expert Consensus Statement. Nutrients 14(7):1483
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR et al (2021) Consensus on diagnosis and management of Cushing's disease: a guideline update. Lancet Diabetes Endocrinol 9(12):847–875
Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C et al (2014) Guidelines for the Management of Asymptomatic Primary Hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metabol 99(10):3561–3569
Ketteler M, Block GA, Evenepoel P et al (2017) Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int 92:26–36
Mazziotti G, Canalis E, Giustina A (2010) Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med 123(10):877–884
Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA (2010) Premature menopause or early menopause: long-term health consequences. Maturitas 65(2):161–166
Collins GS, Reitsma JB, Altman DG, Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 162(1):55–63
Royston P, Sauerbrei W (2005) Building multivariable regression models with continuous covariates in clinical epidemiology--with an emphasis on fractional polynomials. Methods Inf Med 44(4):561–571
Giustina A, Bouillon R, Binkley N, Sempos C, Adler RA, Bollerslev J et al (2020) Controversies in vitamin D: a statement from the Third International Conference. JBMR Plus 4(12):e10417
Chailurkit LO, Kruavit A, Rajatanavin R (2011) Vitamin D status and bone health in healthy Thai elderly women. Nutrition 27(2):160–164
Kruavit A, Chailurkit LO, Thakkinstian A, Sriphrapradang C, Rajatanavin R (2012) Prevalence of vitamin D insufficiency and low bone mineral density in elderly Thai nursing home residents. BMC Geriatr 12:49
Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S et al (2010) Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 25(2):305–312
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19(4):385–397
Subramaniam S, Ima-Nirwana S, Chin KY (2018) Performance of osteoporosis self-assessment tool (OST) in predicting osteoporosis-a review. Int J Environ Res Public Health 15(7)
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA et al (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71(1):31–38
Williams GR, Bassett JHD (2018) Thyroid diseases and bone health. J Endocrinol Invest 41(1):99–109
Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR et al (2015) Subclinical thyroid dysfunction and fracture risk: a meta-analysis. Jama 313(20):2055–2065
Zhu S, Pang Y, Xu J, Chen X, Zhang C, Wu B et al (2022) Endocrine regulation on bone by thyroid. Front Endocrinol (Lausanne) 13:873820
Biondi B, Cappola AR (2022) Subclinical hypothyroidism in older individuals. Lancet Diabetes Endocrinol 10(2):129–141
Favresse J, Schiettecatte J, Wolff F, Cotton F, Elsen M, Eucher C et al (2022) Two-site evaluation of the Roche Elecsys vitamin D total III assay. Clin Chem Lab Med 60(10):1598–1606
Funding
This study is granted by the Faculty of Medicine Research Fund, Chiang Mai University (grant number 084/2565).
Author information
Authors and Affiliations
Contributions
Conceptualization, P.P., M.P. and N.A.; methodology, P.P., M.P., W.M., and T.K.; software, N.A.; validation, M.P. and W.M.; formal analysis, N.A.; investigation, P.P.; resources, T.K.; data curation, P.P. and N.A.; writing—original draft preparation, P.P., M.P., and N.A.; writing—review and editing, M.P., N.A., W.M., and T.K; visualization, M.P., W.M.; supervision, M.P., W.M., and T.K.; project administration, P.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Pitchaporn Phudphong, Mattabhorn Phimphilai, Worapaka Manosroi, Nath Adulkasem, and Tawika Kaewchur declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Phudphong, P., Phimphilai, M., Manosroi, W. et al. A diagnostic predictive model for secondary osteoporosis in patients with fragility fracture: a retrospective cohort study in a tertiary care hospital. Arch Osteoporos 18, 114 (2023). https://doi.org/10.1007/s11657-023-01321-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-023-01321-y