Abstract
Summary
We performed a prospective study using both FRAX and computed tomography to screen for osteoporosis in men undergoing radiation for prostate cancer. We found that implementing routine computed tomography (CT)-based screening was feasible in the setting of a prospective study, but the yield of osteoporosis identification was low in this population.
Purpose
Men with prostate cancer (PCa) are at increased risk of hip fracture for multiple reasons. Estimation of hip fracture risk with the FRAX tool is currently recommended, but FRAX alone may not identify a portion of men with osteoporosis. We hypothesized that adding bone mineral density (BMD) screening using CT to FRAX is feasible and would identify more men with osteoporosis.
Methods
Men with PCa scheduled to undergo CT simulation for radiation treatment were enrolled in a single-arm prospective study. The mean attenuation of the mid-L5 vertebral body trabecular bone (L5CT) was calculated on a single slice using the radiation simulation CT scan. The 10-year risk of hip fracture was calculated using the FRAX tool. Dual energy X-ray absorptiometry (DXA) was performed for men whose L5CT measurement was less than 130 Hounsfield units (HU).
Results
A total of 98 eligible men were enrolled and underwent FRAX and CT screening. The median 10-year risk of hip fracture was 1.1% and exceeded 3% in 16 cases; the median L5CT was 162.28 HU (range 55.6–526.1 HU). DXA scan was completed in 15 men who had L5CT < 130 HU but 10-year calculated hip fracture risk < 3%, 1 of whom was found to have osteoporosis (T-score ≤ −2.5).
Conclusions
Implementing CT-based BMD screening was feasible in the setting of a prospective study for men receiving radiation for PCa, but fewer cases than anticipated of osteoporosis were identified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Men with prostate cancer are at increased risk of hip fracture for multiple reasons. Prostate cancer is classically a disease of older men. Prostate cancer patients are therefore at risk for age-related decline in bone mineral density (BMD), which can be compounded by androgen deprivation therapy (ADT), a mainstay in treatment for prostate cancer either as an adjunct to radiation therapy or in cases of advanced staged disease [1,2,3,4]. Additionally, the use of radiation therapy (RT) doubles the risk of hip fracture [4, 5]. In order to mitigate fracture risk, consensus guidelines recommend using FRAX® fracture risk assessment tool to identify patients with potential osteoporosis prior to cancer therapy, thus avoiding formal BMD assessment for all [6]. Unfortunately, the false-negative rate of FRAX alone for detecting osteoporosis may be as high as 8% in prostate cancer patients when compared with dual X-ray absorptiometry (DXA) [7, 8]. Further, the false-negative rate of FRAX without DXA may be higher for African American men than for Caucasian American men with prostate cancer [8, 9].
Incidentally obtained computed tomography (CT) scans (CT scans obtained for other reasons) have recently been recognized as a potential tool for osteoporosis screening [10]. CT attenuation of the trabecular bone in the lumbar spine strongly correlates with DXA-based BMD [11,12,13] and has a high discriminatory ability for the presence of osteoporosis [11]. For patients who have undergone CT scans for other reasons, quantitative CT analysis may also be more cost effective than routine DXA [14]. Since CT scans are an integral part of the RT planning process, we hypothesized that CT-based screening for osteoporosis could be added to FRAX to improve the detection rate of men with osteoporosis without additional scans or tests. When we retrospectively analyzed an institutional cohort of 609 men with prostate cancer, we found that that up to 11.6% of men had CT characteristics concerning for possible osteoporosis, depending on the screening threshold used [15]. We therefore performed this prospective study to confirm the feasibility of implementing routine CT-based BMD screening in men receiving RT for prostate cancer and to estimate the number of additional men with osteoporosis identified by adding CT-based screening to FRAX.
Methods and materials
Eligibility criteria and enrollment
This prospective study enrolled men 18 years or older with newly diagnosed prostate cancer, who were scheduled to undergo CT simulation for primary definitive RT planning. Up to 4 months of ADT prior to enrollment was allowed since changes in BMD are generally not detectable until 6–9 months after initiating ADT [16]. Men were excluded if they had a known history of osteoporosis, were receiving bisphosphonate therapy, or had known CT artifact which would interfere with quantitative assessment of the lumbar vertebrae. This study was approved by the institutional review board at the University of Alabama at Birmingham, and all patients provided written informed consent before participating in the study. The study schema is illustrated in Fig. 1.
Study schema. One patient was ineligible due to > 4 months of androgen suppression, and 1 patient had artifact precluding lumbar spine vertebral body trabecular bone assessment (indicated by superscript 1). Twenty-two additional men underwent DXA as part of a companion protocol, 6 in the group with 10-year hip fracture risk > 3%, and 14 in the group with L5CT ≥ 130 HU (indicated by superscript 2)
FRAX assessment
The race-specific FRAX tool without DXA was used to calculate the 10-year risk of hip fracture and major osteoporotic fracture. The risk factors utilized by FRAX were assessed by directly questioning participants and reviewing medical records. Use of ADT was considered as secondary osteoporosis (“yes” to question 10 of FRAX). Since the FRAX calculator caps the upper limit of weight at 125 kg, a value of 125 kg was utilized for patients whose weight exceeded this maximum threshold for the risk calculator. Men with 10-year risk of hip fracture exceeding 3% or 10-year risk of major osteoporotic fracture exceeding 20% were offered referral to an osteoporosis specialist.
CT acquisition and analysis
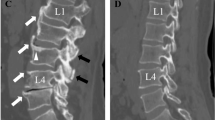
All participants underwent non-contrast pelvic CT scanning as part of the RT simulation procedure. CT scans were acquired by either a Philips Brilliance Big Bore (Koninklijke Philips N.V., Amsterdam, Netherlands) or a GE Lightspeed Big Bore (General Electric, Boston, MA, USA) scanner. CT quality assurance was performed in accordance with the American Association for Physics in Medicine Task Group No. 66 report [17]. Scans were performed in the supine position, were extended from approximately L2 to mid-femur, and used 140 kV photons. CT scans were analyzed using the Varian Eclipse software (Varian Medical Systems, Palo Alto, CA, USA) by the principal investigator (AM). The mid-plane of the L5 vertebral body was identified, the trabecular bone of the vertebral body was segmented on a single slice (Fig. 2), and the average Hounsfield units (HU) of all voxels in the region of interest was calculated. Participants with mid-L5 mean trabecular bone attenuation (L5CT) < 130 HU were referred for DXA. In cases where CT artifact interfered with accurate assessment at mid-L5, single-slice measurement at the mid-plane of another lumbar vertebral body was performed.
The mid-plane at the L5 vertebra was selected for measurement since L5 is included in prostate CT simulation protocols at most institutions, and the area under a receiver-operator curve (AUC) for predicting osteoporosis has been previously reported to be 0.82 [11]. The screening threshold of 130 HU to trigger confirmatory DXA was chosen to optimize sensitivity and specificity [11]. Our previous retrospective study had suggested that ~ 10% of the men would have L5CT < 130 HU but would have a 10-year FRAX-assessed hip fracture risk of < 3% [15].
DXA
Men with L5CT < 130 HU were referred for DXA, which was considered the standard for diagnosing osteoporosis. Men for whom more than 12 months of ADT was planned were also referred for DXA as part of a companion protocol. Failure of a participant to complete DXA despite 2 attempts to reschedule was considered a passive refusal. DXA scans were performed using a Hologic Discovery scanner (Hologic, Inc. Marlborough, MA, USA). T-scores were calculated for lumbar spine, left femoral neck, and left total hip regions using a female normative sample as recommended by the International Society for Clinical Densitometry [18]. Osteoporosis was defined as the presence of a T-score of ≤ − 2.5 in any region.
Endpoints and statistical methods
This study was designed as a pilot to establish feasibility of prospectively using CT-based osteoporosis screening for men scheduled to receive RT for prostate cancer, with an enrollment goal of 100 men. The primary clinical endpoint of this study was the prevalence of osteoporosis among men whose FRAX-based 10-year hip fracture risk was < 3%. We estimated that 10% of men would have L5CT < 130 and half would be confirmed by DXA as having osteoporosis. Assuming that the addition of CT-based screening identified 5% of men as having osteoporosis, enrollment of 100 men in this study would result in a 90% confidence interval of 2 to 10.2%. Between-group testing of means was performed using the independent sample t test (normal distribution assumed) or independent sample Mann-Whitney U test (normal distribution not assumed).
Results
Baseline characteristics and fracture risk assessment
One hundred men were enrolled between September 2017 and February 2019. One man was ineligible due to a history of > 4 months of androgen suppressing therapy prior to enrollment, and one man was found to have extensive metal artifact from prior lumbar spine surgery such that the trabecular bone attenuation was not able to be assessed at any vertebral level within the lumbar spine. The baseline characteristics for the 98 evaluable patients are described in Table 1. The median age at enrollment was 66.5 years (range 38.7–89.4 years).
Initial FRAX assessment and CT analysis
The median 10-year risk of hip fracture assessed with FRAX without BMD was 1.1% and exceeded 3% in 16 cases. No man had 10-year risk of major osteoporotic fracture that exceeded 20%. The responses to the individual elements of the FRAX calculator across the cohort are described in Table 2. The median L5CT value was 162.28 HU (range 55.6–526.1 HU), inclusive of 2 patients for whom measurement was obtained at the mid-L2 level due to metal artifact in the lower lumbar region (159.6 HU and 215.8 HU). Of the 29 men for whom L5CT was below 130 HU, 22 (75.9%) had 10-year hip fracture estimate of less than 3% using FRAX without BMD (Fig. 3). The correlation between L5CT and the 10-year risk of hip fracture was r = −0.169 (p = 0.099).
Correlation between DXA and L5CT
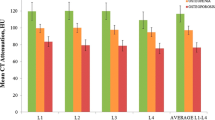
For the 37 DXA scans that were obtained as part of this study (inclusive of 22 DXA scans obtained for men scheduled to receive > 12 months of concurrent ADT as part of their prescribed treatment regimen), the correlation between L5CT and DXA T-score of the L1-L4 region was r = 0.504 (p = 0.001). The correlations between L5CT and DXA T-scores of the femoral neck and total hip were r = 0.53 (p = 0.001) and r = 0.622 (p < 0.001), respectively.
DXA results for men with L5CT < 130 but 10-year FRAX-based hip fracture risk < 3%
DXA scan was completed in 15 of the 22 men with L5CT < 130 but 10-year calculated hip fracture risk < 3% (5 active refusals, 1 passive refusals, and 1 participant developed new onset significant medical problems). Details of the 22 participants who were referred for DXA are provided in Table 3. There are no statistically significant differences in age, FRAX estimate, and L5CT between the participants who underwent DXA and those who did not (Supplemental Table 1).
DXA confirmed osteoporosis in 1 participant (L5CT = 119 HU, 10-year hip fracture estimate of 0.1%, DXA T = − 2.5 for L1–L4 region). Thus, the addition of CT-based BMD screening to FRAX in this study was associated with a 1% (90% confidence interval 0.1–4.7%) increase in the rate of osteoporosis detection.
Secondary analysis—change in FRAX fracture risk with BMD added
The 10-year risk of hip fracture was re-calculated for the 15 men who had L5CT < 130 and 10-year FRAX-based hip fracture risk < 3% and who completed DXA. The addition of femoral neck BMD to the FRAX calculator resulted in 1 participant’s 10-year hip fracture risk being revised to > 3%.
Discussion
The utility of CT scans in determining bone mineralization has long been appreciated [19]. Since the initial reports of the correlation between single-slice CT measurements and DXA-based BMD nearly a decade ago, there have been numerous applications in large cohorts showing high predictive ability for osteoporosis [11, 12, 20]. The theoretical benefits of opportunistic osteoporosis screening with CT in high-risk populations have been described in several retrospective studies [21,22,23,24], including our prior study [15], but confirmatory prospective studies had been lacking. We designed this prospective screening study of men scheduled to receive RT for prostate cancer in order to confirm the feasibility of clinically implementing CT-based osteoporosis screening and to determine preliminary estimates of the benefit of adding CT-based screening to FRAX-based risk scores. We found implementing prospective CT-based BMD screening using radiation simulation CT scans to be feasible in the clinical setting, but the overall yield for osteoporosis detection was lower than expected.
Fracture risk assessment is not commonly performed in men with prostate cancer despite being included in expert guidelines [25,26,27]. We did not encounter any barriers in implementing FRAX, and the importance of fracture risk assessment in this population was highlighted by the fact that 16 of 98 (16.3%) evaluable participants had 10-year hip fracture risk exceeding 3%, a threshold used by the National Osteoporosis Foundation to identify patients likely to benefit from pharmacologic therapy [28]. Assessment of the trabecular bone mean attenuation on a single CT slice was technically feasible for 98 of 99 (98.9%) eligible study participants, with 96 measurements performed at mid-L5 and 2 measurements performed at mid-L2. We observed that 22 (22.4%) men had L5CT < 130 HU but had FRAX 10-year hip fracture risk of < 3%, which was a higher frequency than anticipated. The reason for the higher than anticipated rate of men who met the L5CT screening threshold is not entirely clear but could be related to chance, enrollment, or other unanticipated bias, or more accurate FRAX estimation than in our prior study (in-person rather than retrospective).
Active refusal to complete the DXA was more frequent than anticipated and represents an important issue that could not have been appreciated by prior retrospective studies. In this population of men with prostate cancer, bone demineralization and fracture risk were not generally perceived by patients as important health concerns, and keeping an additional appointment for DXA was logistically challenging in the context of daily radiation treatments. No additional accrual was planned to compensate for DXA refusals, since the DXA completion rate was considered a key feasibility endpoint that would aid in developing future studies.
Given that L5CT < 130 has been previously reported to have a sensitivity of 74.1% for the presence of osteoporosis [11], we conservatively estimated that half of men with FRAX 10-year hip fracture risk < 3% but L5CT < 130 would be confirmed with DXA as having osteoporosis. In contrast to these expectations, we observed that only 1 of 12 participants who completed DXA met the osteoporosis criteria. This discrepancy between our expected and observed results is likely explained by two key factors. First, a shift from a male to a female normative sample for T-score calculation has taken place since the screening thresholds for L5CT were first described, which has resulted in male T-scores increasing by an average of 0.3 [29]. The other critical factor is that the population used to describe the screening accuracy of single-slice lumbar vertebral body trabecular bone attenuation differ from our study population. The University of Wisconsin study that established the discriminatory ability of single-slice lumbar vertebral body measurements for detecting osteoporosis retrospectively examined patients who had undergone both CT and DXA as part of routine care [11]. The study cohort consisted of predominantly women and was likely enriched by patients with elevated pre-test probability of osteoporosis. In contrast, our study consisted entirely of men who tend to have a lower pre-test probability of osteoporosis and for whom routine DXA scan is not commonly performed. As a result, the positive predictive value of L5CT < 130 HU in our study was significantly lower than previously reported.
We recognize that the small sample size and single institution design are important limitations of this study. The sample size of 100 men was chosen as an adequate sample to confirm feasibility of implementation, but it is associated with a wide confidence interval given that the outcome of interest was infrequent. We minimized the chance bias by utilizing broad enrollment criteria; however, the demographics of our study population are representative of prostate cancer in the southeastern USA, particularly race and body weight which are both associated with BMD and fracture risk. We utilized the mid-plane of L5 for single-slice measurement since this is routinely included in pelvic scan protocols. Though prior studies have shown L5 measurement to have similar predictive ability for osteoporosis, we acknowledge this as a potential weakness since measurement at L1 or L3 is more common [11]. The choice not to perform DXA for men with L5CT ≥ 130 HU may be viewed by some as a weakness, but the utility of DXA in this group was felt to be very low and is unlikely to have identified any additional men with osteoporosis. No statistical differences were identified between the group of men who completed confirmatory DXA and those who did not, but this remains a source of potential bias. The study was not designed to enroll additional participants to compensate for non-compliance with the confirmatory DXA since this was a component of feasibility. Finally, the decision to utilize DXA as the reference standard may be criticized in the setting of growing support for quantitative CT densitometry techniques to diagnose osteoporosis.
In conclusion, we performed one of the first prospective studies to assess the feasibility and yield of CT-based BMD screening. We found that implementing CT-based BMD screening methods was feasible in the setting of a prospective trial for men receiving RT for prostate cancer. The rate of non-compliance with confirmatory DXA was high and should be considered when designing future studies. The false-positive rate of CT-based osteoporosis screening in this study was higher than expected, and the overall yield of adding CT-based BMD screening was low, though this was a secondary aim and the sample size of this study was associated with a wide confidence interval. The results of this study emphasize that while CT-based BMD screening is an exciting and potentially cost-effective option to improve osteoporosis screening, prospective studies are crucial to verify the findings of retrospective cohort studies and determine how to best implement these techniques to specific patient populations.
References
Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, Pritchard KI (2008) Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol 26:5465–5476
Kiratli BJ, Srinivas S, Perkash I, Terris MK (2001) Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology 57:127–132
Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C (1999) Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol 161:1219–1222
Elliott SP, Jarosek SL, Alanee SR, Konety BR, Dusenbery KE, Virnig BA (2011) Three-dimensional external beam radiotherapy for prostate cancer increases the risk of hip fracture. Cancer 117:4557–4565
Abrahamsen B, Nielsen MF, Eskildsen P, Andersen JT, Walter S, Brixen K (2007) Fracture risk in Danish men with prostate cancer: a nationwide register study. BJU Int 100:749–754
Mohler JL, Kantoff PW, Armstrong AJ et al (2014) Prostate cancer, version 2.2014. J Natl Compr Canc Netw 12:686–718
Ensrud KE, Taylor BC, Peters KW et al (2014) Implications of expanding indications for drug treatment to prevent fracture in older men in United States: cross sectional and longitudinal analysis of prospective cohort study. BMJ 349:g4120
Adler RA, Hastings FW, Petkov VI (2010) Treatment thresholds for osteoporosis in men on androgen deprivation therapy: T-score versus FRAX. Osteoporos Int 21:647–653
Aizer AA, Wilhite TJ, Chen MH, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Trinh QD, Hu JC, Nguyen PL (2014) Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer 120:1532–1539
Lenchik L, Weaver AA, Ward RJ et al (2018) Opportunistic screening for osteoporosis using computed tomography: state of the art and argument for paradigm shift. Curr Rheumatol Rep 20:74
Pickhardt PJ, Pooler BD, Lauder T, del Rio A, Bruce RJ, Binkley N (2013) Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 158:588–595
Pickhardt PJ, Lee LJ, del Rio AM et al (2011) Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res 26:2194–2203
Lee S, Chung CK, Oh SH, Park SB (2013) Correlation between bone mineral density measured by dual-energy X-ray absorptiometry and Hounsfield units measured by diagnostic CT in lumbar spine. J Korean Neurosurg Soc 54:384–389
Pisu M, Kopperdahl DL, Lewis CE et al (2019) Cost-effectiveness of osteoporosis screening using biomechanical computed tomography for patients with a previous abdominal CT. J Bone Miner Res 34:1229–1239
McDonald AM, Jones JA, Cardan RA, Saag KS, Mayhew DL, Fiveash JB (2016) Combining computed tomography-based bone density assessment with FRAX screening in men with prostate cancer. J Clin Densitom 19:430–435
Ryan CW, Huo D, Bylow K, Demers LM, Stadler WM, Henderson TO, Vogelzang NJ (2007) Suppression of bone density loss and bone turnover in patients with hormone-sensitive prostate cancer and receiving zoledronic acid. BJU Int 100:70–75
Mutic S, Palta JR, Butker EK, Das IJ, Huq MS, Loo LN, Salter BJ, McCollough C, van Dyk J, AAPM Radiation Therapy Committee Task Group No. 66 (2003) Quality assurance for computed-tomography simulators and the computed-tomography-simulation process: report of the AAPM radiation therapy committee task group no. 66. Med Phys 30:2762–2792
Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom 16:455–466
Rüegsegger P, Elsasser U, Anliker M, Gnehm H, Kind H, Prader A (1976) Quantification of bone mineralization using computed tomography. Radiology 121:93–97
Schreiber JJ, Anderson PA, Rosas HG et al (2011) Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am 93:1057–1063
Therkildsen J, Winther S, Nissen L et al (2018) Feasibility of opportunistic screening for low thoracic bone mineral density in patients referred for routine cardiac CT. J Clin Densitom
Buckens CF, van der Graaf Y, Verkooijen HM, Mali WP, Isgum I, Mol CP, Verhaar HJ, Vliegenthart R, Oudkerk M, van Aalst C, de Koning HJ, de Jong PA (2015) Osteoporosis markers on low-dose lung cancer screening chest computed tomography scans predict all-cause mortality. Eur Radiol 25:132–139
Alacreu E, Moratal D, Arana E (2017) Opportunistic screening for osteoporosis by routine CT in Southern Europe. Osteoporos Int 28:983–990
Kim YS, Lee S, Sung YK, Lee BG (2016) Assessment of osteoporosis using pelvic diagnostic computed tomography. J Bone Miner Metab 34:457–463
Mohler JL, Armstrong AJ, Bahnson RR, D'Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Kane CJ, Kawachi MH, Kuettel M, Lee RJ, Meeks JJ, Penson DF, Plimack ER, Pow-Sang JM, Raben D, Richey S, Roach M 3rd, Rosenfeld S, Schaeffer E, Skolarus TA, Small EJ, Sonpavde G, Srinivas S, Strope SA, Tward J, Shead DA, Freedman-Cass DA (2016) Prostate cancer, version 1.2016. J Natl Compr Canc Netw 14:19–30
Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM (2006) Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology 68:126–131
Alibhai SM, Yun L, Cheung AM, Paszat L (2012) Screening for osteoporosis in men receiving androgen deprivation therapy. JAMA 307:255–256
Cosman F, de Beur SJ, LeBoff MS et al (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381
Binkley N, Adler R, Bilezikian JP (2014) Osteoporosis diagnosis in men: the T-score controversy revisited. Curr Osteoporos Rep 12:403–409
Funding
Research reported in this manuscript was supported by the National Center for Advancing, Translational Sciences of the National Institutes of Health under award number UL1TR003096. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the institutional review board at the University of Alabama at Birmingham, and all patients provided written informed consent before participating in the study.
Conflict of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
McDonald, A.M., Yang, E.S., Saag, K.G. et al. Osteoporosis screening using computed tomography for men with prostate cancer: results of a prospective study. Arch Osteoporos 15, 32 (2020). https://doi.org/10.1007/s11657-020-0711-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-020-0711-1