Abstract
Summary
Our research shows that the newborns of vitamin D–deficient mothers have higher serum alkaline phosphatase (ALP) activity compared with those of vitamin D–non-deficient mothers, which is likely related to increased bone turnover rather than just being a marker for bone formation. This has a potential negative impact on fetal bone development and subsequent skeletal growth.
Purpose/introduction
Low maternal serum 25-hydroxy vitamin D (25(OH)D) level during pregnancy contributes to vitamin D deficiency in infants at birth, which is associated with multiple potential adverse effects on fetal skeletal mineralization and growth. We studied the relationship between maternal 25(OH)D level and newborn serum alkaline phosphatase activity (ALP) at term.
Methods
In this prospective cross-sectional hospital-based study, venous blood samples of healthy pregnant mothers were drawn to measure 25(OH)D levels within 6 h of delivery. Cord blood samples were examined for calcium, phosphorus levels, and ALP activity immediately after birth. In addition, we also recorded the newborns’ anthropometric measurements.
Results
Seventy-two percent (n = 108/150) of mothers in our study were vitamin D–deficient (serum 25(OH)2D < 25 nmol/l). In a multivariate logistic regression model, young maternal age (odds ratio (OR) = 0.94, 95% CI 0.88–0.99, p = 0.04) and increased weight (OR = 1.03, 95% CI 1.01–1.07, p = 0.02) as well as decreased milk intake (OR = 0.31, 95% CI 0.13–0.74, p = 0.009) were all significantly associated with maternal vitamin D deficiency. ALP activity was significantly higher in newborns of vitamin D–deficient compared with vitamin D–non-deficient mothers (median = 176 (IQR = 139–221) and 156 (IQR = 132–182), respectively, p = 0.04). A significant inverse correlation (Pearson’s coefficient = − 0.18, p = 0.03) was observed between maternal 25(OH)D levels and babies’ ALP activities. This association persisted in a multivariate logistic regression model (OR = 3.46, 95% CI 1.18–10.18, p = 0.024).
Conclusions
Our findings indicate that newborns of vitamin D–deficient mothers have higher serum ALP activity than those of non-deficient mothers, which might be related to increased bone turnover rather than just being a marker for bone formation. This could have a potential negative impact on fetal bone development and subsequent skeletal growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At discovery, in the early twentieth century, vitamin D was identified as an anti-rachitic factor and, lately, was also found to play a role beyond calcium and bone metabolism such as in fetal growth, regulation of cellular differentiation, apoptosis, and immune system development [1, 2]. In many parts of the world, vitamin D deficiency (VDD) in pregnancy is fairly common [3]. Observational studies have consistently shown that serum 25-hydroxy vitamin D (25(OH)D) level in pregnant women contributes to VDD in infants at birth [3,4,5]. This is associated with multiple potential adverse outcomes in mothers and infants [6,7,8]. Although the synthesis and metabolism of vitamin D in the non-pregnant state is well known, its metabolism during pregnancy is not fully understood [3]. The status of maternal 25(OH)D level during pregnancy is a determining factor for infant’s stores of vitamin D and its skeletal mineralization and growth [6,7,8,9]. In general, cord blood 25(OH) vitamin D concentrations are approximately 60–89% of the maternal value [10, 11]. It is, therefore, important for maternal vitamin D stores to be at appropriate level to meet the fetal demand in that period.
The fetal growth is regulated by multiple factors including maternal nutrition. In some literature, maternal 25(OH)D level early in pregnancy can be associated with risk of low birth weight and small-for-gestational age [12].
During fetal life, both calcium and phosphorus are required for bone development. Calcium, phosphorus, and magnesium are transported actively across the placenta from mother to fetus, with bulk of materno-fetal transfer occurring in the third trimester of pregnancy [13]. Vitamin D deficiency in fetal life may have permanent effect on body physiology and metabolism [7, 8]. However, there are gaps in the understanding of whether and how vitamin D influences fetal bone development and whether markers of bone turnover in cord blood could predict bone mineral content. After the first trimester, 1,25(OH)2 vitamin D that does not cross the placenta increases by 100–200% in the mother and fetus without any correlation with fetal calcium level [10, 11]. The fetal mineral homeostasis starts in utero with influence of several factors like parathyroid hormone (PTH) and PTH-related protein (PTHrP), which are produced by fetal parathyroid glands and the placenta, respectively, to maintain serum calcium concentration [14,15,16]. However, the transported calcium across the placenta leads to PTH suppression, with a further decrease in the hormone level towards the end of gestation; hence, PTH is not a reliable marker for a bone turnover in the newborn [17].
Neonatal rickets has been reported in infants born to mothers with severe vitamin D deficiency [18,19,20]. Raised serum alkaline phosphatase (ALP) activity, which reflects osteoblastic activity and increased bone turnover, is a well-known marker of rickets. ALP level, as a bone biomarker, does increase during pregnancy regardless of vitamin D status and attains higher values in umbilical cord blood than maternal blood, possibly reflecting higher rates of bone turnover [21, 22]. ALP activity is also known to have inversed association with bone mineral content [23, 24].
The aim of this study is to determine the effect of maternal vitamin D status on fetal bone metabolism, mainly represented by the newborns cord blood alkaline phosphatase activity. We also looked at the correlation between maternal 25(OH)D level and the growth parameters of the newborns in our cohort.
Methods
This is a cross-sectional study conducted at King Abdulaziz Medical City, Riyadh, which is one of the two largest tertiary centers in Riyadh, Saudi Arabia, which serves a catchment area of East Riyadh with a target population of 1.5 million, mainly represented by the National Guard employees. The annual number of deliveries in the hospital is approximately 7000. In this study, we recruited healthy pregnant Saudi women attending this center over a month period (November 2011). Riyadh, the capital city of Saudi Arabia, lies at longitude 46.4 E and latitude 24.7 N. It is likely to have low ALP activity in November since babies are born at the end of fall and early wintertime. The aim of the study was to assess the following: (1) factors associated with maternal vitamin D deficiency and (2) the effect of maternal 25(OH)D level on newborns’ alkaline phosphatase activity as well as the newborns’ anthropometric parameters.
King Abdullah International Medical Research Centre (KAIMRC), Riyadh, Saudi Arabia, ethically approved the study and an informed consent was obtained from every participant in the study. For inclusion into the study, all newborns and mothers had to be healthy (i.e., no chronic illness such as diabetes mellitus and they were not severely obese). Furthermore, all pregnant women were non-smokers and not under medications that may affect their 25(OH)D level. A consent form and a detailed questionnaire were distributed among recruited mothers. Collected data included the following: age, weight and height at the time of delivery, multi-parity status, gestational age (< or ≥ 37 weeks), sunscreen application, vitamin D supplements, sun exposure per week (1 h, 2 h, or 3 or more h), fortified milk (400 IU/l of vitamin D) intake (1, 2, 3, or 4 cups/day; 1 cup = 180 ml), vitamin D supplements, and level of education (no formal education, primary-secondary education, or tertiary education).
Venous and cord blood samples were drawn and collected in serum separator tubes (SST) (Becton Dickinson (BD) Company (NJ, USA)) from all women within 6 h in postpartum period. Blood samples were centrifuged at 3000 rpm and serum were separated in secondary tubes and analyzed immediately; otherwise, samples were stored in the freezer with − 70 °C. Vitamin 25(OH)D level was analyzed by using immunoassay technique Chemiluminescent Microparticle Immunoassay (CMIA) (LIAISON® XL, DiaSorin Company; VC, Italy). This method measured both two forms of vitamins D2 and D3 and reported as total vitamin D. In order to reduce the limitation of the immunoassay variability, we have ensured that the used method included and checked against the standard reference materials in the assay, which was manufactured by the National Institute of Standards and Technology (NIST). In addition, our laboratory has participated in an external proficiency program from the College of American Pathologist (CAP) to monitor and compare the performance of the vitamin D assay accuracy and precision.
The alkaline phosphatase enzyme catalyzed the substrate p-nitrophenyl phosphate (p-NPP) and the formed yellow products were directly proportional to the alkaline phosphatase enzyme activity and were measured by the colorimetric method using ARCHITECT c Systems (ABBOTT Company; IL, USA). In addition, calcium and phosphate assays were measured by the colorimetric methods using ARCHITECT c Systems (ABBOTT Company; IL, USA).
Most commonly, and as in ESPGHAN and NASPGHAN consensus statement (2013), vitamin D deficiency is defined as 25(OH)D with a serum concentration < 50 nmol/l and severe vitamin D deficiency at < 25 nmol/l. The immunoassay manufacturer’s definition used in this study stated that deficiency is at < 25 nmol/l, insufficiency 25–75 nmol/l, sufficiency 75–250 nmol/l, and toxicity > 250 nmol/l. We aimed to include only such patients with marked vitamin D deficiency in order to demonstrate any apparent statistical relationships between vitamin D status and newborn bone markers of metabolic bone disease, namely, alkaline phosphatase (ALP) and calcium and phosphate levels in the cord blood.
All newborns were full-term healthy babies apart from four babies who were between 33 and 36 gestational weeks in age who did not require active resuscitation or intubation. All babies who were very unwell and required intubation or had hepatic problems were excluded from the study. The cord blood samples for calcium, phosphorus levels, and ALP activity were taken immediately after birth for all babies. Newborns’ physical examination and anthropometric measurements (birth weight, birth length, and head circumference) were taken at birth. Birth weight was measured to the nearest 10 g using an infant digital scale (weight scale, seca, model 727). Birth length and the head circumference were measured to the nearest 0.1 cm using a paper tape measure.
Statistical analysis
Data were summarized as medians (inter-quartile range (IQR)) or percentages. Continuous variables were compared using the Mann-Whitney test and categorical variables using the χ2 test. Logistic regression models were fitted to determine factors associated with (1) vitamin D deficiency (25-hydroxyvitamin D level < 25 nmol/l) and (2) increased ALP activity (> 75th percentile; 212 mg/dl). Factors significant in univariate analysis were included in a final model. All tests were two-sided, and a p < 0.05 was considered significant.
Results
The study included 150 mothers. Seventy-two percent (n = 108/150) had 25(OH)D level < 25 nmol/1 (vitamin D–deficient). The non-deficient mothers (n = 42/150) had 25(OH)D level 25–75 nmol/l. Multi-parity status, education level, sunscreen use, intake of vitamin D supplement, and sun exposure was not different in vitamin D–deficient and vitamin D–non-deficient mothers. However, vitamin D–deficient mothers were significantly younger and had increased weight and BMI and less milk intake (Table 1). Further analysis by logistic regression models had confirmed these findings (Table 2). In the multivariate logistic regression analysis model, variables independently associated with mothers with vitamin D deficiency were young age (odds ratio (OR) = 0.94; 95% CI 0.88–0.99, p = 0.04)), weight (OR = 1.03; 95% CI 1.01–1.07; p = 0.02), and milk intake (OR = 0.31, 95% CI 0.13–0.74; p = 0.009).
Increased 25(OH)D levels were observed with increased milk intake (Fig. 1).
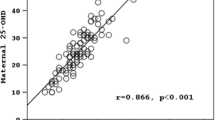
Newborns to mothers in the two groups had similar gender, weight, length, head circumference, and serum calcium and phosphorus levels (Table 3). From a clinical point, all ALP values in our data fall within normal range of ALP activity. However, statistically, ALP activity was significantly higher in newborns of vitamin D–deficient mothers (median (IQR) = 176 (139–221)) compared with newborns of non-vitamin D–deficient mothers (median (IQR = 156 (132–182)), p = 0.04) (Table 3). An inverse relationship was observed between newborn ALP activity and their maternal vitamin D levels (correlation coefficient r = − 0.18, p = 0.03) (Fig. 2).
In the final multivariate logistic regression model for factors associated with newborns higher ALP activity, vitamin D–deficient mothers (OR = 3.46; 95% CI 1.18–10.18; p = 0.03) and gestational weeks < 37 (OR = 11.76; 95% CI 2.11–65.53, p = 0.005) were the factors independently associated with higher activity of ALP.
Discussion
Two-thirds of pregnant mothers in our study were vitamin D–deficient. Cord blood serum ALP activity was higher in infants born to vitamin D–deficient mother. Furthermore, we found an inverse correlation between maternal serum 25OHD and cord blood ALP activity. While we cannot infer causality from this association, it is likely that intrauterine infant bone mineralization might be impaired in infants born to vitamin D–deficient mothers. Infants’ growth can also be affected as shown in previous literature, although this was not observed in our data. Being young and obese with low milk intake put pregnant ladies at a higher risk of vitamin D deficiency.
We know that severe maternal vitamin D deficiency causes neonatal rickets and raised cord blood ALP activity is a marker of subclinical rickets [18,19,20]. Furthermore, possible “tracking” of impaired mineralization was previously demonstrated by low bone mass density and impaired bone geometry in later childhood [23,24,25].
In this study, we focused on ALP activity in cord blood as a useful marker of fetal bone turn over. Two-thirds of pregnant women in our study had vitamin D deficiency. In addition, the ALP activity in the newborns of vitamin D–sufficient and vitamin D–deficient mothers was within the normal range; however, the range of ALP activity was significantly higher in the newborns of vitamin D–deficient mothers. This could be explained by more bone activity for bone formation. There are some studies that observed low maternal 25(OH)D level associated with hypocalcaemia in cord blood of the newborns or during the first week of life [13, 26,27,28]. In a study in Iran, for the newborns with severe vitamin D deficiency (< 12.5 ng/ml) and maternal vitamin D deficiency, serum ALP activity was higher (331.4 ± 95 compared with 606.9 ± 270.3 (p = 0.001) [29]. However, the cutoff level of vitamin D deficiency used in this study was very low and not the commonly used in clinical practice. To our knowledge, the data on the effect of maternal vitamin D status on the newborns’ ALP is scarce. In Greece, they reported no association between ALP in vitamin D–deficient mothers (range 133–187 IU/l) and their newborn ALP activity (range of 116–175 IU/l) (p value = 0.76) [30]. But they did not directly compare maternal 25(OH)D level and newborn ALP activity.
We found that women who were drinking less milk are significantly more deficient in 25(OH)D levels. Some of these women in both groups were even on vitamin D supplements, although many studies have shown that women who received vitamin D supplements had higher levels of 25(OH)D compared with those who did not [31,32,33]. However, Koski et al. compared the restricted and non-restricted milk takers (1 cup = 250 ml, restricted < 1 cup and non-restricted > 1cup) and found that mothers with restricted milk intake gave birth to lower birth weight babies [34]. In our study, the low 25(OH)D level in deficient ladies could be explained by lifestyle (avoidance of sun exposure) in addition to limited intake of milk. Moreover, raw camel milk consumption, which is unfortified with vitamin D, is not uncommon in Saudi Arabia. Limited sunlight exposure is due to excessive heat, hence, a tendency to live outdoors in the evening times. Inadequate sunscreen application, poor compliance to treatment, or not using enough doses of vitamin D to meet the demands of pregnant women could also be additional factors. Vitamin D deficiency is of multifactorial etiology despite launching vitamin D support programs in some countries [35]. It continued to be a serious problem worldwide, in most of pregnant ladies and their infants in these countries [35, 36].
Our result showed that vitamin D–deficient women are younger and heavier with significantly higher BMI compared with vitamin D–sufficient women. We observed that there was no difference in either groups in the parity status, education level, and sunlight exposure and sunscreen application. In 78 Turkish women younger and older than 25 years, no significant correlation was found between maternal vitamin D levels and the age and parity of mothers [37]. Our study showed heavier women had low vitamin D level, compared with a study done in Greece, which found no correlation between maternal weight gaining (median gaining was 13 kg) during pregnancy and their 25(OH)D level [30]. This was supported by a study in Iran with similar findings [29]. In our study, heavier women had lower 25(OH)D levels that could be influenced by natural weight gaining during pregnancy and increased demand of vitamin D supplements. Moreover, it is well known that fat cells absorb 25(OH)D reducing its serum level [38]. Nevertheless, this could be protective to 25(OH)D from degradation extending its half-life [38].
In our study, we did not find correlation between maternal 25(OH)D level and anthropometric birth parameters and calcium and phosphate levels. Maternal vitamin D status during pregnancy may program skeletal development and body composition in the offspring by influencing the interaction between osteoblasts and adipocytes [7]. Low maternal serum 25-OHD is associated with shorter duration of gestation and, consequently, reduced growth of long bones in newborns [39]. Maternal vitamin D status early in pregnancy was associated with risk of low birth weight and small-for-gestational age infants in one study, whereas another study found this relation only among white women [12]. Polymorphisms in vitamin D receptor gene may contribute to vitamin D–related disparities in fetal growth [8]. During the fetal period, other hormones, such as PTH-related protein, prolactin, or placental lactogen, may contribute to enhanced calcium metabolism [14,15,16]. In Irani pregnant women and their newborns (total numbers = 552), their study also showed no correlation of maternal 25(OH)D level and birth parameters [29]. However, a positive correlation between the level of ionized calcium in deficient mother and crown-head length of the newborns was found in thirty Pakistani women [40]. In Australia, they reported no association between maternal 25(OH)D level and birth length of the newborns [41]. That was also supported by a recent report from Pakistan by Hossain et al. [42]. Mannion et al. however found an association between vitamin D intake during pregnancy and birth weight. They found the birth weight of newborn increased by 11 g for every additional 40 IU of maternal vitamin D intake [34]. In another UK study, they also reported an association between low maternal 25(OH)D level during pregnancy and intrauterine long bone growth as early as 19 weeks of gestation [43]. Nevertheless, in a UK study, of a longer duration, they found no statistically significant associations between maternal 25(OH)D levels and any growth measurement of children at birth, at 9 months of age, or even at age of 9 years [44]. In addition, two earlier data showed no difference in the birth length of babies to women who took vitamin D supplement during pregnancy compared with women who did not take it [32, 45]. But these studies showed those babies who their mothers were on vitamin D supplement had larger head circumference. Mallet et al. showed no difference of weight at birth of infants from women who received vitamin D supplement compared with women who did not [33]. Most of the studies support our findings of no association between maternal 25(OH)D level and birth parameters of newborns.
Improving mother’s milk-derived calcium intake would be highly recommended especially with the sun avoidance practice and traditional dressings with veils in these communities. It should have a protective effect through reducing maternal plasma PTH, which in turn reduces catabolism of 25OHD. However, this is a speculation, as plasma PTH levels were not measured in maternal bloods in our cohort. Although, the population treated in our institute constitutes of different tribes of the Saudi population, a limitation in our study is that it is a single-center study and immunoassay, which is not as reliable as the nowadays LCMSMS standard method, was used for the measurement of vitamin D at the time of the study. However, we did our early validation study in the lab and we have compared our immunoassay results with LCMSMS analyzer and the correlation was accepted.
Conclusion
Vitamin D deficiency is prevalent among pregnant women in Saudi Arabia especially in the overweight and young age groups. We emphasize on the importance of maternal 25(OH)D level for a normal bone formation in the newborns. The newborns of vitamin D–deficient Saudi mothers are likely to be at a higher risk of metabolic bone disease having significantly higher alkaline phosphatase activity.
References
Dawodu A, Wagner CL (2007) Mother-child vitamin D deficiency: an international perspective. Arch Dis Child 92(9):737–740
Pela I (2012) How much vitamin D for children? Clin Cases Miner Bone Metab 9:112–117
Dawodu A, Wagner CL (2012) Prevention of vitamin D deficiency in mothers and infants worldwide – a paradigm shift. Paediatr Int Child Health 32(1):3–13
Dawodu A, Akinbi H (2013) Vitamin D nutrition in pregnancy: current opinion. Int J Women's Health 5:333–343
Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW (2012) Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients 4(3):208–230
Lucas RM, Ponsonby AL, Pasco JA, Morley R (2008) Future health implications of prenatal and early-life vitamin D status. Nutr Rev 66(12):710–720
Thorne-Lyman A, Fawzi WW (2012) Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol 26(Suppl 1):75–90
Dror DK (2011) Vitamin D status during pregnancy: maternal, fetal, and postnatal outcomes. Curr Opin Obstet Gynecol 23(6):422–426. https://doi.org/10.1097/GCO.0b013e32834cb791
Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, wt al (2010). Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab 95(4):1749–1757. https://doi.org/10.1210/jc.2009-1391
Dror KD, King JC, Eb F, van Loan MD, Gertz ER, Allen LH (2012) Evidence of associations between feto-maternal vitamin D status. Cord Parathyroid Hormone and Bone-Specific Alkaline Phosphatase, and newborn Whole Body Bone Mineral Content, Nutrients 4(2):68–77
Markestad T, Aksnes L, Ulstein M, Aarskog D (1984) 25-Hydroxyvitamin D and 1,25-dihydroxyvitamin D of D2 and D3 origin in maternal and umbilical cord serum after vitamin D2 supplementation in human pregnancy. Am J Clin Nutr 40(5):1057–1063
Burris HH, Rifas-Shiman SL, Camargo CA Jr et al (2012) Plasma 25-hydroxyvitamin D during pregnancy and small-for-gestational age in black and white infants. Ann Epidemiol 22(8):581–586
Ritchie LD, Fung EB, Halloran BP et al (1998) A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr 67:693–701
Kovacs CS (2014) Bone metabolism in the fetus and neonate. Paediatr Nephrol 9(5):793–803. https://doi.org/10.1007/s00467-013-2461-4
Kovacs CS (2011) Bone development in the fetus and neonate: role of the calciotropic hormones. Curr Osteoporos Rep 9(4):274–283. https://doi.org/10.1007/s11914-011-0073-0
Okonofua F, Menon RK, Houlder S et al (1987) Calcium, vitamin D and parathyroid hormone relationships in pregnant Caucasian and Asian women and their neonates. Ann Clin Biochem 24:22–28
Jones G., Dwyer T., Hynes K.L., Parameswaran V., Greenaway T. m (2005). Vitamin D insufficiency in adolescent males in Southern Tasmania: prevalence, determinants, and relationship to bone turnover marker, Osteoporos Int 16:636-664.
Moncrieff M, Fadahunsi TO (1974) Congenital rickets due to maternal vitamin D deficiency. Arch Dis Child 49:810–811
Park W, Paust H, Kaufmann HJ, Offermann G (1987) Osteomalacia of the mother–rickets of the newborn. Eur J Pediatr 146(3):292–293
Mittal M, Kumar A, Ramji S, Narula S, Thirupuram S (1990) Congenital rickets. Indian Pediatr 27(8):857–859
Calero JA, Munoz MT, Argente J, Traba ML, Mendez-Davila C, Garcia-Moreno C, De la Piedra CA (1999) Variation in bone alkaline phosphatase levels that correlates positively with bone loss and normal levels of aminoterminal propeptide of collagen l in girls with anorexia nervosa. Clin Chim Acta 285:121–129
Lucas A, Brooke OG, Baker BA, Bishop N, Morley R (1989) High alkaline phosphatase activity and growth in preterm neonates. Arch Dis Child 64:902–909
Ikeuchi K, Umesaki N (2009) Factors affecting bone mineral density of young women and predictive factors of low bone mineral density. Clin Exp Obstet Gynecol 36:87–90
Crofton PM, Shrivasatava A, Wade JC, Stephen R, Kelnar CC, Lyon AJ, McIntosh N (1999) Bone and collagen markers in preterm infants: relationship with growth and bone mineral content over the first 10 weeks of life. Pediar Res 16:581–587
Javaid MK, Crozier SR, Harvey NC et al (2006) Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367:36–43
Dawodu A, Agarwal A, Patel M, Ezimokhai M (1997) Serum 25-hydroxyvitamin D and calcium homeostasis in the United Arab Emirates mothers and neonates: a preliminary report. Middle E Paediatr 2:9–12
Ardawi MS, Nasrat HA & BA’Aqueel HS (1997). Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol 137:402–409.
Paunier L, Lacourt G, Pilloud P, Schlaeppi P, Sizonenko PC (1978) 25-Hydroxyvitamin D and calcium levels in maternal, cord and infant serum in relation to maternal vitamin D intake. Helv Paediatr Acta 33:95–103
Zhila maghbooli, Arsh Hossein-Nezhad, Ali R Shfaei (2007). Vitamin D status in mothers and their newborns in Iran. BMC pregnancy and childhood 7:1 dol: https://doi.org/10.1186/1471-7-1.
Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E, Tsagris V, Costalos C, Antsaklis A (2006) Low vitamin D status in mother-newborn pairs in Greece. Calcif Tissue Int 78(6):337–342
Marya RK, Rathee S, Lata V, Mudgil S (1981) Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Investig 12:155–161
Brooke OG, Brown IRF, Bone CDM, Carter ND, Cleeve HJW (1980) Vitamin D supplements in pregnant Asian women: effects on calcium and fetal growth. Br Med J 1:751–754
Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H (1986).Vitamin D supplementation in pregnancy: a controlled trial of two methods.obsteteric and Gynecology 68:300-4.
Mannion C.A., Gray-Donald K., Koski K. G (2006). Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ 174:1273–1277. doi: https://doi.org/10.1503/cmaj.1041388.
Holick MF, Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080–1086. 2
Özdemir AA, Gündemir YE, Küçük M, Sarıcı DY, Elgörmüş Y, Çağ Y, Bilek G (2018) Vitamin D deficiency in pregnant women and their infants. J Clin Res Pediatr Endocrinol 10(1):44–50
Buyru N, Tezol A, Yosunkaya-Fenerci E, Dalay N (2003) Vitamin D receptor gene polymorphisms in breast cancer. Exp Mol Med 31 35(6):550–555
Abboud M, Gordon-Thomson C, Hoy AJ, Balaban S, Rybchyn MS, Cole L, Su Y, Brennan-Speranza TC, Fraser DR, Mason RS (2014) Uptake of 25-hydroxyvitamin D by muscle and fat cells. J Steroid Biochem Mol Biol 144 Pt A:232–236. https://doi.org/10.1016/j.jsbmb.2013.10.020 Epub 2013 Nov 1
Congdon P, Horsman A, Kirby PA, Dibble J, Bashir T (1983) Mineral content of the forearms of babies born to Asian and white mothers. BMJ 286:1234–1235
De-Regil LM, Palacios C, Ansary A, Kulier R, Peña-Rosas JP (2012) Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev 15:CD008873
Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME (2009) Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol 70(3):372–377
Hossain N, Kanani FH, Ramzan S, Kausar R, Ayaz S, Khanani R, Pal L (2014) Obstetric and neonatal outcomes of maternal vitamin D supplementation: results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J Clin Endocrinol Metab 99(7):2448–2455
Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N et al (2010) Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res 25(1):14–19. https://doi.org/10.1359/jbmr.090701
Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S (2009) Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol 70:685–690
Marya RK, Rathee S, Dua V, Sangwan K (1988) Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res 88:488–492
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
King Abdullah International Medical Research Centre (KAIMRC), Riyadh, Saudi Arabia, ethically approved the study and an informed consent was obtained from every participant in the study.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Alwan, I., Al Badi, M., Badri, M. et al. Higher serum alkaline phosphatase activity in infants born to vitamin D–deficient mothers. Arch Osteoporos 14, 102 (2019). https://doi.org/10.1007/s11657-019-0651-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-019-0651-9