Abstract
The construction of cascade reservoirs on the Lancang River (the upper Mekong) has an important influence on the distribution and accumulation of heavy metals. Heavy metal contents in porewater provide vital information about their bioavailability, studies on this aspect are rare until now. In this study, sediment cores were collected from four adjacent cascade reservoirs in the upper Mekong River to study the distribution, potential sources, diffusive fluxes and toxicity of heavy metals in porewater. The findings indicated that the average contents of Mn, Fe, As, Ni, Cu, Zn, Cd, and Pb in the sediment porewater were 6442, 644, 11.50, 2.62, 1.23, 3.95, 0.031, and 0.24 µg/L, respectively; these contents varied as the sediment depth increased. Correlation analysis and principal component analysis showed that Cu, Zn, Cd and Pb were mainly associated with anthropogenic sources, As, Mn and Fe were primarily affected by natural inputs, and Ni was affected by a combination of natural and anthropogenic effects. The diffusive fluxes of Mn, Fe, As, Ni, Cu, Zn, Cd, and Pb in the cascade reservoirs of the Lancang River were 919 – 35,022, 2.12 – 2881, 0.17 – 750, 0.71 – 7.70, 2.30 – 31.18, (-3.35) – 6.40, 0.06 – 0.54, and (-0.52) – 4.08 µg/(m2 day), respectively. The results of toxic units suggested that the contamination and toxicity of heavy metals in porewater were not serious. Overall, in the cascade reservoirs, the content and toxicity of heavy metals in porewater of the upstream reservoirs were higher than that of the downstream reservoirs. The operation of the cascade reservoirs enabled greater accumulation of contaminants in sediments of the upstream reservoirs. This research gives strong support for the prevention of heavy metal contamination and the sustainability of water resources under the running condition of cascade reservoirs on such a large international river (the Lancang-Mekong River).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is a serious threat to aquatic ecology and has become a worldwide concern due to its toxicity, non-biodegradability, and bio-accumulation (Xu et al., 2019; Zeng et al., 2020). Most of the heavy metals discharged into aquatic environment are adsorbed on suspended particulates and eventually deposit in the sediments (Palma et al., 2015). However, when physical or biochemical conditions change, these metals in sediments may be re-dissolved into the porewater from where they can enter the overlying water column through diffusion (Blasco et al., 2000; Sullivan and Taylor, 2003). This subsequent secondary pollution will result in water quality degradation and pose a serious threat to the ecosystem (Wang et al., 2016; Li et al., 2020).
Recently, researchers came to realized that the heavy metal properties of contaminated sediment cannot directly reflect the bioavailability and toxicity characteristics of sediment (Tang et al., 2015, 2016; Lei et al., 2016), whereas the biogeochemical processes and bioavailabilities of toxic metals at sediment–water interfaces were strongly influenced by metal distributions and mobilities in porewater (Zhu et al., 2016). Moreover, porewater composition may be the most sensitive indicator of the type and extent of the reaction between contaminated sediment and the aqueous phase that contacts it (Wu et al., 2016). Thus, metal concentrations in porewater have been showed to be an effective predictor of toxic effect (Tang et al., 2016; Cleveland et al., 2017).
On the main stream of the Lancang River (the upper Mekong River), a chain of six cascade hydroelectric dams had been constructed as of 2016 (Fan et al., 2015; Shi et al., 2020), and another 17 dams in the river will be completed over the next few decades (Chen et al., 2019). There is no doubt that the cascade dams on the Lancang River could trap a portion of sediment delivered downstream (Lu and Siew, 2006; Wang et al., 2012). Recent researches estimated that the trapping efficiency of the existed cascade dam reservoirs in the Lancang River might reach to 74–94% (Liu et al., 2015; Binh et al., 2020). Consequently, large amount of sediment has been stored in these dam reservoirs (Fan et al., 2015). In addition, the sediments retained in these reservoirs were finer and rich in clay minerals compared with the downstream sediments (Guo et al., 2020). Therefore, it provides great convenience for heavy metals to accumulate in these reservoirs. Several researches have reported heavy metal contamination in sediments of these reservoirs in the Lancang River (Wang et al., 2012; Zhao et al., 2013; Li et al., 2019). However, until now, few studies have focused on the fluxes of heavy metal released from sediments, and their concentration and toxicity in porewater in the cascade reservoirs. This information is necessary to understand the sources of heavy metals in reservoir water and their toxic risks to the environment and aquatic organisms.
Therefore, in this study, four cascade reservoirs in the Lancang River were selected to (1) investigate heavy metal distribution in porewater profiles, (2) distinguish their potential sources in porewater, (3) examine the diffusive fluxes of metals at the sediment–water interface (SWI), and (4) evaluate the toxicity of heavy metals in the interstitial water.
Materials and methods
Study area
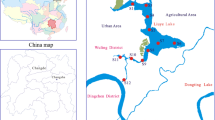
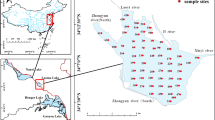
The Lancang River is the upper reach of the Mekong River which is one of the world’s famous international rivers. It is a large river in China with a length of 2153 km, ranking the 5th among all rivers in China. Originating from the northern foot of Tanggula Mountain in the south of Qinghai Province, the Lancang River flows through Tibet Plateau and Yunnan Province before flowing to foreign countries (Myanmar, Laos, Thailand, Cambodia and Vietnam) and becoming the Mekong River. The basin in China covers an area of 168,000 km2 (Guan et al., 1984). The river head is 5244 m above sea level (Fan et al., 2015). With the large descending elevation (1780 m) in Yunnan Province, the Lancang River produces plentiful hydraulic resources there, which is conducive to hydropower cascade development (Liu et al., 2015; Chen et al., 2019). The studied four cascade reservoirs are located in the middle and lower reaches of the Lancang River, which are the Manwan (MW) Reservoir, Dachaoshan (DCS) Reservoir, Nuozhadu (NZD) Reservoir, and Jinghong (JH) Reservoir, respectively (Fig. 1). The detailed information about these reservoirs was displayed in Table S1.
Sampling and analytical methods
Sediment cores were collected using a 100 cm long gravity corer with 6 cm internal diameter in April 2017, and the sampling sites are displayed in Fig. 1. The overlying water was collected near the SWI with a syringe and a silicone tube. Then, cores were sliced at 1-cm interval in the field, stored in sealed sterile centrifuge tubes and kept refrigerated at 4 °C in dark during transport to the laboratory. To obtain porewater, sediments were centrifuged at a speed of 4000 r/min for 30 min (Bufflap and Allen, 1995; Cleveland et al., 2017). The overlying water and porewater were filtered through cellulose membranes (0.45 μm), acidified to 2% HNO3, and stored at 4 °C until analysis.
The concentrations of Mn, Fe, As, Ni, Cu, Zn, Cd, and Pb were measured by inductively coupled plasma-mass spectrometry (ICP-MS, Agilent, 7700x, USA). Major cations were analyzed by inductively coupled plasma-optical emission spectroscopy (ICP-OES, iCAP6500, Thermo Scientific, Germany) (Zhao et al., 2020).
Diffusive fluxes
The diffusive fluxes of heavy metals across the SWI were estimated using Fick’s first law (Eq. (1)) (Berner, 1984).
where \(J\) is the metal flux and \(\varphi\) is the porosity of surface sediment and can be calculated by the dry weight and the wet weight of the sediment (Tang et al., 2016). \({\left(dC/dZ\right)}_{z=0}\) is the metal concentration gradient at the SWI. \({D}_{s}\) is the sediment diffusion coefficient of metals which is calculated as the following equations (Ullman and Sandstrom, 1987):
where \({D}_{0}\) is metal diffusion coefficient in free solution and values of \({D}_{0}\) for each metal were adopted from Li and Gregory (1974).
Porewater toxicity analysis
To examine the toxicity level of metals in porewater, the interstitial water criteria toxic units (IWCTU) was adopted (Liu et al., 1999; Lourino-Cabana et al., 2011):
where \({\left[{M}_{e}\right]}_{i, w}\) is the heavy metal concentration in porewate, and \({\mathrm{FCV}}_{{M}_{e}}\) represents the final chronic value of the metal by hardness. The calculation method for \({\mathrm{FCV}}_{{M}_{e}}\) was shown in Table S2. If the \({\mathrm{IWCTU}}_{{M}_{e}}\) is greater than 1, it indicates a risk of toxicity to aquatic organisms (Zhu et al., 2016). The NI (Nemeraw index) was calculated to reflect porewater quality. The NI was categorized into five levels: no impact (NI < 1), slight impact (1 < NI < 2), moderate impact (2 < NI < 3), strong impact (3 < NI < 5), and serious impact (NI > 5) (Tang et al., 2016).
Statistical analysis
Correlation analysis and principal component analysis were performed to check the significant relationships among heavy metals and identify their potential sources for this study. The independent sample t test was applied for two-group comparisons. The above analyses were conducted using SPSS 21.0.
Results and discussion
Distribution of heavy metals in porewater
Heavy metal concentrations in overlying water and porewater exhibited obvious spatial variation (Fig. 2; Table S4). The porewater in the MW Reservoir contained the highest average concentrations of Cu and Cd, and the porewater in the DCS Reservoir contained the highest average concentrations of Ni, Zn and Pb, whereas the mean concentrations of most of the heavy metals in porewater were lowest in the JH Reservoir (the lower reaches). Due to the existence of the cascade reservoirs, particles containing organic matter were preferentially deposited in the upstream reservoirs (Fig. S1). Organic matter usually has a strong affinity for heavy metals, resulting in a stronger capacity of upstream reservoir sediments to retain these metals (Perez-Esteban et al., 2014). In addition, heavy metal concentrations in porewater followed the order of Mn > Fe > As > Zn > Ni > Cu > Pb > Cd at each of the four reservoirs. Due to dilution and settling processes, the lowest concentrations of heavy metals in the overlying water were usually found in the downstream regions (Varol, 2019). However, the highest contents of Ni and Pb in the overlying water were observed in the lower reaches (the JH Reservoir) indicating significant local inputs existed.
The distribution of heavy metals in porewater varied vertically (Fig. 2). The porewater profiles of Fe and As showed a certain degree of similarity at each sampling site. Moreover, significant positive correlations existed between Fe and As in porewater from the MW Reservoir (r2 = 0.60, p < 0.01), the DCS Reservoir (r2 = 0.56, p < 0.01), the NZD Reservoir (r2 = 0.65, p < 0.001), and the JH Reservoir (r2 = 0.89, p < 0.001). These results suggested that the solubility and migration of arsenic were highly controlled by Fe oxyhydroxides. Furthermore, the poor relationship between As and Mn in porewater of these reservoirs indicated that Fe oxyhydroxides played a more important role than Mn oxyhydroxides in controlling As solubility which was consistent with results of other researches (Couture et al., 2010a, 2010b; Toevs et al., 2008). Previous studies revealed that Fe could be a good indicator to reflect sediment redox (oxic, sub-oxic, or anoxic conditions) (Campanha et al., 2012; Lei et al., 2016). In the JH Reservoir, the contents of Fe and As in interstitial water generally increased with depth before reaching peaks and then decreased. Their lower contents in the top layers might represent the oxic zone where As was absorbed or co-precipitated with Fe oxyhydroxides (Nikolaidis et al., 2004; Carraro et al., 2015). The peaks of Fe and As in the porewater profile might indicate the sub-oxic zone where partial Fe oxyhydroxides containing As reductively dissolved and released them to porewater causing the elevated concentrations of Fe and As (Keimowitz et al., 2005; Couture et al., 2010a). Similar phenomena have also been discovered by other researchers (Couture et al., 2010a; Deng et al., 2014; Sun et al., 2016). In general, the distribution patterns of most metals (Ni, Cu, Zn, Cd, and Pb) in the interstitial water were not significantly regular, although some enrichment or deficiency existed at certain discrete layers.
Overall, the mean concentrations of Mn, Fe, As, Ni, Cu, Zn, Cd, and Pb in the sediment porewater from the cascade reservoirs of the Lancang River were 6442, 644, 11.50, 2.62, 1.23, 3.95, 0.031, and 0.24 µg/L, respectively. The mean concentrations of Mn, Fe, As, Ni, Cu, Zn, Cd, and Pb in the overlying water from the cascade reservoirs of Lancang River were 19.18, 4.05, 4.10, 0.09, 0.42, 4.10, 0.021, and 0.10 µg/L, respectively. The contents of all the studied metals in overlying water and porewater met the Chinese Surface Water Environmental Quality Standard, except that the concentrations of Mn and Fe in porewater were higher than the standard (100 and 300 µg/L for Mn and Fe, respectively; China EPA, 2002).
Source identification
Correlation analysis and principal component analysis were conducted to explore the potential sources of studied metals in porewater (Chatterjee et al., 2007; Bai et al., 2016).
Correlation analysis
The CA was carried out to identify relationships among the eight metals (Fig. 3). Generally, significantly positive correlations among metals might reflect their similar sources, controlling factors, and transport behavior (Zeng et al., 2020). In this study, a significantly positive correlation was found between Cu and Cd (R = 0.76, p < 0.01), suggesting that they possibly shared the same source. Positive correlations existed among Mn, Fe, and As (p < 0.01), but they were relatively weakly correlated with other metals. Moreover, negative correlations were observed between Cu and Mn, Zn and Mn, Cd and Mn, Pb and Mn, and Cu and Fe, signifying that Mn, Fe, and As might originate from a different source relative to other metals. Cu, Zn, Cd, and Pb exhibited positive correlations among them (Fig. 3).
Principal component analysis
The PCA was used to further explore the possible sources of the selected metals in porewater. The PCA identified three principal components (PCs) with eigenvalues exceeding 1, explaining 67.2% of the total variance (Table 1). PC1 (Cu, Cd, Pb and Zn), PC2 (As, Fe and Mn), and PC3 (Ni) account for 30.91%, 20.60%, and 15.68% of the total variance, respectively. Fe and Mn were commonly applied as the geochemical reference elements (Guan et al., 2018; Sun et al., 2018; Varol, 2019) and were strongly correlated with As (p < 0.01) (Fig. 3), indicating that PC2 was primarily affected by natural inputs. Cu, Zn, Cd and Pb had high loadings on PC1 and were positively correlated with each other. Combined with the negative correlations between Cu and Mn, Zn and Mn, Cd and Mn, Pb and Mn, and Cu and Fe (Fig. 3), it could be inferred that PC1 is mainly associated with anthropogenic sources. This could also be supported by former researches (Geng et al., 2015; Zhu et al., 2016; Zeng et al., 2020); namely, Cu, Zn, Cd, and Pb were the typical anthropogenic pollutants from agricultural runoff and industrial sewage. Ni was the main component of PC3. Considering the positive correlations of Ni with Mn, Fe, Zn, and Pb (Fig. 3), and the low concentrations of Ni in porewater, thus, PC3 was defined as being affected by a combination of natural and anthropogenic effects.
Diffusive fluxes of metals at the SWI
The diffusive fluxes of the selected metals showed significant variations in the four reservoirs (Table 2). Heavy metal fluxes at the SWI can effectively indicate whether sediment is a source or sink for pollutants in the aquatic systems (Lei et al., 2016; Tang et al., 2016). The diffusive fluxes of Mn, Fe, As, Ni, Cu, Zn, Cd, and Pb in the cascade reservoirs of the Lancang River were 919–35,022 µg/(m2 day), 2.12–2881 µg/(m2 day), 0.17–750 µg/(m2 day), 0.71–7.70 µg/(m2 day), 2.30–31.18 µg/(m2 day), (− 3.35)–6.40 µg/(m2 day), 0.06–0.54 µg/(m2 day), and (− 0.52)–4.08 µg/(m2 day), respectively. The fluxes of all metals in the four reservoirs were positive (with the exception of Zn from the ZND and the JH, and Pb from the JH), suggesting export from sediment to overlying water and that sediment was generally the source of heavy metals. Human activities and the weak hydrodynamic conditions after impoundment caused the accumulation of heavy metals in sediment. Among these metals, the diffusive flux of Mn was highest, followed by Fe and As, which might negatively affect the quality of overlying water and pose health risks. The fluxes of Mn, Fe, and As in the NZD Reservoir were considerably higher than those in other reservoirs, which was ascribed to their peak concentrations in the top layer of porewater caused by the anoxic environment at the SWI of the NZD Reservoir. The highest fluxes of Cu, Zn, Cd, and Pb were found in the MW Reservoir signifying the greatest endogenous release of these metals in the upper reaches of the cascade reservoirs, whereas the lowest fluxes of all the studied metals except for Cu were observed in the JH Reservoir which was the last one of the cascade reservoirs. In addition, the negative fluxes of Zn and Pb in the JH Reservoir represented that these two metals diffused from overlying water to sediment. Combining the results of 3.1, it could be inferred that although there was Pb pollution in the JH Reservoir, sediment was able to scavenge some Pb in the overlying water of the JH Reservoir.
The fluxes of metals in this study were comparable with those observed elsewhere (Table 2). The fluxes of Mn in the cascade reservoirs of the Lancang River (except that in the JH Reservoir) were much higher than those reported in other places (such as the Chaohu Lake, the Lake Hope, and the Daya Bay). Fe and As fluxes in this study were similar to other regions (e.g. the Aha Reservoir, the Shahe Reservoir, the Chaohu Lake, the Dianchi Lake, and the Lake Hope), except their extremely high fluxes in the NZD Reservoir. Diffusive fluxes of Ni, Zn, and Pb were compared with all the areas listed. However, the fluxes of Cu and Cd in this study were higher than all of the other regions (except for the Taihu Lake) which were polluted by heavy metals in different degree, suggesting that Cu and Cd from anthropogenic sources should be paid more attention in this region. Actually, the diffusion fluxes might be overestimated because dissolved heavy metals in porewater would be partially absorbed by Fe/Mn oxyhydroxides at the SWI in the process of upward diffusion (Deng et al., 2014; Tang et al., 2016).
Toxic units
Heavy metal concentration in sediment porewater can reflect their bioavailability and the changing trend of metals in overlying water (Burgess et al., 2013; Ji et al., 2018). Therefore, it has replaced sediment as an effective predictor of toxic effects (Tang et al., 2016). The IWCTU was applied to analyze the toxicity level of a single metal in porewater, and the NI was used to evaluate the combined effects of metals. Results showed that the IWCTU values of each metal (Ni, Cu, Zn, Cd, and Pb) were less than 1 and the NI values were also low (0.05–0.10) in all the four reservoirs from the Lancang River (Table 3). These results indicated that these metals in porewater showed no toxicity risks for biota in the study areas. However, this method does not include assessments for Mn, Fe, and As. Considering the strong release fluxes and high concentrations of Mn, Fe, and As in porewater, especially in the NZD Reservoir, their potential risks in porewater should be taken seriously. It is interesting to note that the values of \({\sum \mathrm{IWCTU}}_{i}\) and NI in porewater from the upstream reservoirs (the MW Reservoir and the DCS Reservoir) were higher than those from the downstream reservoirs (the NZD Reservoir and the JH Reservoir; p < 0.01). It suggested that the operation of the cascade reservoirs enabled greater accumulation of contaminants in sediments of upstream reservoirs, which made the porewater of upstream reservoirs exhibit relatively stronger toxicity.
The results of toxic units suggested that the heavy metal contamination and toxicity in porewater were not serious. However, Deng et al. (2017) reported that Cd was moderately polluted in the sediment of the MW Reservoir and the DCS Reservoir, and Wang et al. (2012) indicated that Cd, Cu, Pb, and Zn were slightly enriched in sediment from the MW reservoir and could cause adverse effect. This seeming contradiction revealed that the risks of these metals releasing from the sediment to the water column were pretty low, and high total contents of heavy metals in contaminated sediment might not result in a severe consequence. This was further supported by the fact that contents of Ni, Cu, Zn, Cd and Pb in porewater were all below the USEPA chronic water quality criteria of 8.2, 3.1, 81, 8.8, and 8.1 μg/L, respectively (USEPA 2009). Generally, these metals showed relatively low diffusive fluxes from the sediment porewater to overlying water in most sites, suggesting that the release risk of these elements from sediment in the study area was not high. The mobility and transformation processes might be controlled by many factors, such as hydrological conditions, hardness, pH, redox potential, and the mineralization of organic matter (Lourino-Cabana et al., 2011; Lei et al., 2016). Therefore, porewater is very essential to comprehensively evaluate heavy metal pollution in sediments of aquatic ecosystem.
Conclusion
Despite its irreplaceable roles in the cycling of trace metals in aquatic ecosystems, porewater was little studied. Thus, the contents, diffusive fluxes, potential sources, and toxicity of metals in porewater were examined in the cascade reservoirs of the Lancang River. The concentrations of most of the heavy metals in porewater were lowest in the downstream reservoir. With the exception of Mn and Fe in porewater, the contents of heavy metals in overlying water and porewater met the Chinese Surface Water Environmental Quality Standard. Anthropogenic input was the main source of Cu, Zn, Cd, and Pb in sediment porewater, while As, Fe, and Mn were primarily affected by natural processes, and Ni was associated with mixed sources. Almost all the metals had positive diffusive fluxes from the interstitial water to the overlying water column in the four reservoirs suggesting that porewater was a direct source of heavy metals to the overlying water. According to the results of toxic units, the contamination and toxicity of Ni, Cu, Zn, Cd, and Pb in porewater were not serious. Considering the strong release fluxes and high concentrations of Mn, Fe, and As in porewater, their potential risks should be given a concern. This study and subsequent research would contribute to the prevention of heavy metal pollution and provide further powerful support for the sustainable development planning of the Lancang-Mekong water resources.
Data availability
Data and material is available for research purpose and for reference.
References
Bai JH, Jia J, Zhang GL, Zhao QQ, Lu QQ, Cui BS, Liu XH (2016) Spatial and temporal dynamics of heavy metal pollution and source identification in sediment cores from the short-term flooding riparian wetlands in a Chinese delta. Environ Pollut 219:379–388. https://doi.org/10.1016/j.envpol.2016.05.016
Bao LI, Shiming D, Chengxin FAN, Xiuling BAI, Hongbin YIN (2008) Estimation of diffusion fluxes on trace heavy metals at sediment-water interface in Fubao Bay of Dianchi Lake. Environ Chem 27:800–804 ((In Chinese with English abstract))
Berner RA (1984) Sedimentary pyrite formation: An update. Geochim Cosmochim Acta 48:605–615. https://doi.org/10.1016/0016-7037(84)90089-9
Binh DV, Kantoush S, Sumi T (2020) Changes to long-term discharge and sediment loads in the Vietnamese Mekong Delta caused by upstream dams. Geomorphology 353:14. https://doi.org/10.1016/j.geomorph.2019.107011
Blasco J, Saenz V, Gomez-Parra A (2000) Heavy metal fluxes at the sediment-water interface of three coastal ecosystems from south-west of the Iberian Peninsula. Sci Total Environ 247:189–199. https://doi.org/10.1016/s0048-9697(99)00490-8
Bufflap SE, Allen HE (1995) Sediment pore-water collection methods for trace-metal analysis: a review. Water Res 29:165–177. https://doi.org/10.1016/0043-1354(94)e0105-f
Burgess RM, Berry WJ, Mount DR, Di Toro DM (2013) Mechanistic sediment quality guidelines based on contaminant bioavailability: equilibrium partitioning sediment benchmarks. Environ Toxicol Chem 32:102–114. https://doi.org/10.1002/etc.2025
Campanha MB, Moreira AB, Bisinoti MC (2012) Metal fluxes at the sediment-water interface in rivers in the Turvo/Grande drainage basin, So Paulo State, Brazil. J Soils Sediments 12:1508–1516. https://doi.org/10.1007/s11368-012-0591-0
Carraro A, Fabbri P, Giaretta A, Peruzzo L, Tateo F, Tellini F (2015) Effects of redox conditions on the control of arsenic mobility in shallow alluvial aquifers on the Venetian Plain (Italy). Sci Total Environ 532:581–594. https://doi.org/10.1016/j.scitotenv.2015.06.003
Chatterjee M, Silva EV, Sarkar SK, Sella SM, Bhattacharya A, Satpathy KK, Prasad MVR, Chakraborty S, Bhattacharya BD (2007) Distribution and possible source of trace elements in the sediment cores of a tropical macrotidal estuary and their ecotoxicological significance. Environ Int 33:346–356. https://doi.org/10.1016/j.envint.2006.11.013
Chen J, Wang PF, Wang C, Wang X, Miao LZ, Liu S, Yuan QS (2019) Dam construction alters function and community composition of diazotrophs in riparian soils across an environmental gradient. Soil Biol Biochem 132:14–23. https://doi.org/10.1016/j.soilbio.2019.01.020
China, EPA (Environmental Protection Agency), 2002. Environmental Quality Standards for Surface Water (GB 3838–2002).
Cleveland D, Brumbaugh WG, MacDonald DD (2017) A comparison of four porewater sampling methods for metal mixtures and dissolved organic carbon and the implications for sediment toxicity evaluations. Environ Toxicol Chem 36:2906–2915. https://doi.org/10.1002/etc.3884
Couture RM, Gobeil C, Tessier A (2010a) Arsenic, iron and sulfur co-diagenesis in lake sediments. Geochim Cosmochim Acta 74:1238–1255. https://doi.org/10.1016/j.gca.2009.11.028
Couture, R.M., Shafei, B., Van Cappellen, P., Tessier, A., Gobeil, C., 2010b. Non-Steady State Modeling of Arsenic Diagenesis in Lake Sediments. Environmental Science & Technology 44, 197–203. https://doi-org.yorku.80599.net/https://doi.org/10.1021/es902077q
Deng L, Liu SL, Zhao QH, Yang JJ, Wang C, Liu Q (2017) Variation and accumulation of sediments and associated heavy metals along cascade dams in the Mekong River, China. Environ Eng Manag J 16:2075–2087
Deng TL, Wu Y, Yu XP, Guo YF, Chen YW, Belzile N (2014) Seasonal variations of arsenic at the sediment-water interface of Poyang Lake, China. Appl Geochem 47:170–176. https://doi.org/10.1016/j.apgeochem.2014.06.002
Fan H, He DM, Wang HL (2015) Environmental consequences of damming the mainstream Lancang-Mekong River: a review. Earth-Sci Rev 146:77–91. https://doi.org/10.1016/j.earscirev.2015.03.007
Geng JJ, Wang YP, Luo HJ (2015) Distribution, sources, and fluxes of heavy metals in the Pearl River Delta, South China. Mar Pollut Bull 101:914–921. https://doi.org/10.1016/j.marpolbul.2015.10.066
Guan JN, Wang J, Pan H, Yang C, Qu J, Lu N, Yuan X (2018) Heavy metals in Yinma River sediment in a major Phaeozems zone, Northeast China: distribution, chemical fraction, contamination assessment and source apportionment. Sci Rep 8:11. https://doi.org/10.1038/s41598-018-30197-z
Guan, Z.H., Chen, C.Y., Ou, Y.X., Fan, Y.Q., Zhang, Y.S., Cheng, Z.M., et al. (1984). Tibetan rivers and lakes (in Chinese). Beijing: Science
Guo XJ, Zhu XS, Yang ZJ, Ma J, Xiao SB, Ji DB, Liu DF (2020) Impacts of cascade reservoirs on the longitudinal variability of fine sediment characteristics: a case study of the Lancang and Nu Rivers. J Hydrol 581:10. https://doi.org/10.1016/j.jhydrol.2019.124343
Ji HB, Li HX, Zhang Y, Ding HJ, Gao Y, Xing YX (2018) Distribution and risk assessment of heavy metals in overlying water, porewater, and sediments of Yongding River in a coal mine brownfield. J Soils Sediments 18:624–639. https://doi.org/10.1007/s11368-017-1833-y
Keimowitz AR, Zheng Y, Chillrud SN, Mailloux B, Jung HB, Stute M, Simpson HJ (2005) Arsenic redistribution between sediments and water near a highly contaminated source. Environ Sci Technol 39:8606–8613. https://doi.org/10.1021/es050727t
Lei P, Zhang H, Shan BQ, Zhang BZ (2016) Distribution, diffusive fluxes, and toxicity of heavy metals and PAHs in pore water profiles from the northern bays of Taihu Lake. Environ Sci Pollut Res 23:22072–22083. https://doi.org/10.1007/s11356-016-7467-6
Li J, Cheng D, Zhao A, Dong S, You X (2019) The characteristics and the assessment of heavy metal and nutrient pollution in sediments of cascading hydropower dams in Lancang River. Acta Sci Circum 39:2791–2799 ((In Chinese with English abstract))
Li YH, Gregory S (1974) Diffusion of ions in sea-water and in deep-sea sediments. Geochim Cosmochim Acta 38:703–714
Li YY, Gao B, Xu DY, Peng WQ, Liu XB, Qu XD, Zhang M (2020) Hydrodynamic impact on trace metals in sediments in the cascade reservoirs, North China. Sci Total Environ 716:9. https://doi.org/10.1016/j.scitotenv.2020.136914
Liu Q, Liu SL, Zhao HD, Deng L, Wang C, Zhao QH, Dong SK (2015) The phosphorus speciations in the sediments up- and down-stream of cascade dams along the middle Lancang River. Chemosphere 120:653–659. https://doi.org/10.1016/j.chemosphere.2014.10.012
Liu WX, Wang ZJ, Wen XH, Tang HX (1999) The application of preliminary sediment quality criteria to metal contamination in the Le An River. Environ Pollut 105:355–366. https://doi.org/10.1016/s0269-7491(99)00041-x
Lopez DL, Gierlowski-Kordesch E, Hollenkamp C (2010) Geochemical mobility and bioavailability of heavy metals in a lake affected by acid mine drainage: Lake Hope, Vinton County, Ohio. Water Air Soil Pollut 213:27–45. https://doi.org/10.1007/s11270-010-0364-6
Lourino-Cabana B, Lesven L, Charriau A, Billon G, Ouddane B, Boughriet A (2011) Potential risks of metal toxicity in contaminated sediments of Deule river in Northern France. J Hazard Mater 186:2129–2137. https://doi.org/10.1016/j.jhazmat.2010.12.124
Lu XX, Siew RY (2006) Water discharge and sediment flux changes over the past decades in the Lower Mekong River: possible impacts of the Chinese dams. Hydrol Earth Syst Sci 10:181–195. https://doi.org/10.5194/hess-10-181-2006
Ni ZX, Zhang L, Yu S, Jiang ZJ, Zhang JP, Wu YC, Zhao CY, Liu SL, Zhou CH, Huang XP (2017) The porewater nutrient and heavy metal characteristics in sediment cores and their benthic fluxes in Daya Bay, South China. Mar Pollut Bull 124:547–554. https://doi.org/10.1016/j.marpolbul.2017.07.069
Nikolaidis NP, Dobbs GM, Chen J, Lackovic JA (2004) Arsenic mobility in contaminated lake sediments. Environ Pollut 129:479–487. https://doi.org/10.1016/j.envpol.2003.11.005
Palma P, Ledo L, Alvarenga P (2015) Assessment of trace element pollution and its environmental risk to freshwater sediments influenced by anthropogenic contributions: The case study of Alqueva reservoir (Guadiana Basin). CATENA 128:174–184. https://doi.org/10.1016/j.catena.2015.02.002
Perez-Esteban J, Escolastico C, Masaguer A, Vargas C, Moliner A (2014) Soluble organic carbon and pH of organic amendments affect metal mobility and chemical speciation in mine soils. Chemosphere 103:164–171. https://doi.org/10.1016/j.chemosphere.2013.11.055
Shi WQ, Chen QW, Zhang JY, Liu DS, Yi QT, Chen YC, Ma HH, Hu LM (2020) Nitrous oxide emissions from cascade hydropower reservoirs in the upper Mekong River. Water Res 173:8. https://doi.org/10.1016/j.watres.2020.115582
Sullivan P, Taylor KG (2003) Sediment and porewater geochemistry in a metal contaminated estuary, Dulas Bay, Anglesey. Environ Geochem Health 25:115–122. https://doi.org/10.1023/a:1021233923423
Sun Q, Ding SM, Wang Y, Xu L, Wang D, Chen J, Zhang CS (2016) In-situ characterization and assessment of arsenic mobility in lake sediments. Environ Pollut 214:314–323. https://doi.org/10.1016/j.envpol.2016.04.039
Sun XS, Fan DJ, Liu M, Tian Y, Pang Y, Liao HJ (2018) Source identification, geochemical normalization and influence factors of heavy metals in Yangtze River Estuary sediment. Environ Pollut 241:938–949. https://doi.org/10.1016/j.envpol.2018.05.050
Tang WZ, Duan SH, Shan BQ, Zhang H, Zhang WQ, Zhao Y, Zhang C (2016) Concentrations, diffusive fluxes and toxicity of heavy metals in pore water of the Fuyang River, Haihe Basin. Ecotoxicol Environ Saf 127:80–86. https://doi.org/10.1016/j.ecoenv.2016.01.013
Tang WZ, Zhang H, Zhang WQ, Shan BQ, Zhu XL, Song ZX (2015) Dynamics of heavy metals and phosphorus in the pore water of estuarine sediments following agricultural intensification in Chao Lake Valley. Environ Sci Pollut Res 22:7948–7953. https://doi.org/10.1007/s11356-014-3945-x
Toevs, G., Morra, M.J., Winowiecki, L., Strawn, D., Polizzotto, M.L., Fendorf, S., 2008. Depositional influences on porewater arsenic in sediments of a mining-contaminated freshwater lake. Environmental Science & Technology 42, 6823–6829. https://yorku.80599.nethttps://doi.org/10.1021/es800937t.
Ullman WJ, Sandstrom MW (1987) Dissloved nutrient fluxes from the nearshore sediments of Bowling Green Bay, central Great-Barrier-Reef Lagoon (Australia). Estuar Coast Shelf Sci 24:289–303. https://doi.org/10.1016/0272-7714(87)90051-5
United States Environmental Protection Agency (USEPA) (2009) National recommended water quality criteria. US Environmental Protection Agency, Washington, DC
Varol M (2019) Arsenic and trace metals in a large reservoir: seasonal and spatial variations, source identification and risk assessment for both residential and recreational users. Chemosphere 228:1–8. https://doi.org/10.1016/j.chemosphere.2019.04.126
Wang C, Liu SL, Zhao QH, Deng L, Dong SK (2012) Spatial variation and contamination assessment of heavy metals in sediments in the Manwan Reservoir, Lancang River. Ecotoxicol Environ Saf 82:32–39. https://doi.org/10.1016/j.ecoenv.2012.05.006
Wang C, Yao Y, Wang PF, Hou J, Qian J, Yuan Y, Fan XL (2016) In situ high-resolution evaluation of labile arsenic and mercury in sediment of a large shallow lake. Sci Total Environ 541:83–91. https://doi.org/10.1016/j.scitotenv.2015.09.037
Wen SF, Shan BQ, Zhang H (2012) Metals in sediment/pore water in Chaohu Lake: distribution, trends and flux. J Environ Sci 24:2041–2050. https://doi.org/10.1016/s1001-0742(11)61065-6
Wu ZH, Jiao LX, Wang SR, Xu YZ (2016) Multi-metals measured at sediment-water interface (SWI) by diffusive gradients in thin films (DGT) technique for geochemical research. Arch Environ Contam Toxicol 70:429–437. https://doi.org/10.1007/s00244-015-0184-1
Xiao X, Long J, Zhang R, Chen J, Zou Y, Wu Q, Wu J (2019) Diffusion fluxes of heavy metals at the sediment-water interface during summer and winter from Aha Reservoir, Guiyang. Chin J Ecol 38:1508–1519
Xu DY, Gao B, Peng WQ, Qu XD, Zhang M, Wang JK (2019) Novel insights into Pb source apportionment in sediments from two cascade reservoirs, North China. Sci Total Environ 689:1030–1036. https://doi.org/10.1016/j.scitotenv.2019.06.368
Yuan S, Zhang W, Zheng B (2014) Heavy metal contaminant distribution features and the diffusion flux estimation in the sediments of Shahe Reservoir, Beijing. J Saf Environ 14:244–249 ((In Chinese with English abstract))
Zeng J, Han GL, Yang KH (2020) Assessment and sources of heavy metals in suspended particulate matter in a tropical catchment, northeast Thailand. J Clean Prod 265:121898. https://doi.org/10.1016/j.jclepro.2020.121898
Zhao QH, Liu SL, Deng L, Dong SK, Wang C (2013) Longitudinal distribution of heavy metals in sediments of a canyon reservoir in Southwest China due to dam construction. Environ Monit Assess 185:6101–6110. https://doi.org/10.1007/s10661-012-3010-5
Zhao, Z.J., Li, S.H., Xue, L.L, Liao, J., Zhao, J.J., Wu, M., Wang, M.G., Sun, J., Zheng, Y., Yang, Q., 2020. Effects of dam construction on arsenic mobility and transport in two large rivers in Tibet, China. Science of The Total Environment, 140406 https://doi.org/10.1016/j.scitotenv.2020.140406
Zhu XL, Shan BQ, Tang WZ, Li SS, Rong N (2016) Distributions, fluxes, and toxicities of heavy metals in sediment pore water from tributaries of the Ziya River system, northern China. Environ Sci Pollut Res 23:5516–5526. https://doi.org/10.1007/s11356-015-5709-7
Funding
This work was supported by National Natural Science Foundation of China (Nos. 41673137, 41273146) and State Key Laboratory of Environmental Geochemistry (SKLEG2019713).
Author information
Authors and Affiliations
Contributions
Zhenjie Zhao: conceptualization, resources, formal analysis, writing — original draft, writing — review and editing; Shehong Li: conceptualization, supervision, writing — original draft, writing — review and editing; Shilu Wang: resources; Jie Liao: formal analysis; Weiqi Lu: resources; Di Tan: resources; Dan Yang: resources.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Christian Gagnon
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Z., Li, S., Wang, S. et al. Heavy metal characteristics in porewater profiles, their benthic fluxes, and toxicity in cascade reservoirs of the Lancang River, China. Environ Sci Pollut Res 29, 36013–36022 (2022). https://doi.org/10.1007/s11356-022-18652-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18652-x