Abstract
The strong adaptability of Broussonetia papyrifera (L.) Vent. to low phosphorus (P) conditions can be attributed to the large amount of root-exuded organic acids and the high efficiency of P extraction. However, microelement contents are influenced by low-P stress, and their effects on the photosynthetic capability of B. papyrifera remain unknown. In this study, we investigated the effects of low-P treatment on net photosynthetic rate (P N); chlorophyll a fluorescence (ChlF) characteristics; and Fe, Mn, Cu, and Zn contents of B. papyrifera and Morus alba L. seedlings. Results show that B. papyrifera exhibited better photosynthetic capability under moderate P deficiency (0.125, 0.063, and 0.031 mmol/L P treatments), whereas the photosynthetic capability of M. alba decreased under moderate and severe P deficiency (0.016 and 0 mmol/L P treatments). Under moderate P deficiency, the decrease in Cu and Zn contents in B. papyrifera was lower than that in M. alba. Under severe P deficiency, a considerable decrease of photosynthetic capability in B. papyrifera and M. alba was associated with low Cu and Zn contents. The P N of the two Moraceae species exhibited a better correlation with Cu and Zn contents than with Fe or Mn content. P deficiency could not only decrease cyclic photophorylation and photosynthetic efficiency, but could also affect the stability of thylakoid membrane structure and electron transport efficiency by influencing the contents of Cu or Zn, thereby affecting photosynthesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Broussonetia papyrifera (L.) Vent. and Morus alba L. are perennial tree species belonging to the family Moraceae and are characterized by higher growth rate and greater adaptability to adverse environments than other species in this family (Zhao et al. 2005). B. papyrifera is more tolerant to a low-phosphorus (P) environment (Liu et al. 2010).Unlike M. alba, B. papyrifera can acclimate to karst soil and resist alien invasion (Wu et al. 2009). A higher amount of organic acids is found in root exudates of B. papyrifera than in M. alba in low-P environments (Shahbaz et al. 2006). Root-exuded organic acids sometimes form organometallic complexes with insoluble phosphoric compounds in soil, releasing available P (Jones and Darrah 1994); the strong adaptability of B. papyrifera to low-P environments can be attributed to the high efficiency of P extraction (Zhao and Wu 2014). However, the root-exuded organic acids might also improve the bioavailability of microelements (Hoffland et al. 1992).The concentration and uptake of plant microelements increase when smaller amounts of P are applied (Racz and Haluschak 1974; Rutkowska et al. 2014). Nonetheless, the relationship between the increase in concentration of plant microelements and the adaptability of B. papyrifera remains unknown.
Inorganic P is one of the least available nutrients in the soils of several terrestrial ecosystems (Alloush et al. 2003; Li et al. 2006). P deficiency leads to a considerable decrease in cyclic photophorylation and photosynthetic efficiency (Watanabe and Yoshida 1970). Some studies have demonstrated that P deficiency induces possible photoinhibition and damage to photosystem II (PSII) (Li et al. 2004). Moreover, the uptake of microelements—such as Fe, Mn, Cu, and Zn—in plants is also related to photosynthesis. Fe is an essential micronutrient for plants (Hao et al. 2007). Bertamini et al. (2002) proved that a low photosynthetic electron transport rate in Fe-deficient grapevine leaves is mainly due to the loss of PSII activity. Mn plays an important role in the water-splitting reaction leading to oxygen evolution in photosynthesis (Sauer 1980). The most important role of Cu is associated with blocking of photosynthetic electron transport, leading to production of radicals which start peroxidative chain reactions involving membrane lipids (Fernandes and Henriques 1991). Finally, Zn deficiency causes reduction of electron use in dark reactions and decreases heat dissipation (Hajiboland et al. 2010).
A certain amount of supplemental P increases the uptake of some micronutrients, while decreasing the uptake of Zn in some plant organs (Nyoki and Ndakidemi 2014). The total amounts of Fe, Mn, Cu, and Zn absorbed by aboveground plant tissue decrease in treatments in which nutrient deficiencies are observed, but the micronutrient concentrations in tissues do not decrease (Choi and Lee 2012). Root-exuded organic acids might increase the uptake of microelements in plants, compensate for the loss of microelements under a low-P environment, and slow the decrease of microelement contents. As a result, the lower rate of decrease in microelement contents could alleviate the damage caused by microelement deficiency to plants in a low-P environment, thereby enhancing photosynthesis. Given that microelements play important roles in photosynthesis, the variations in microelement contents could be a photosynthetic adaptive mechanism of B. papyrifera to low-P stress.
Non-invasive methods such as chlorophyll a fluorescence (ChlF) have been used to observe various types of stress affecting the photosynthetic machinery (Huang et al. 2004). ChlF has been widely used to evaluate plant tolerance to environmental stresses (Gray et al. 2006). The present study examined net photosynthetic rate (P N); ChlF parameters; and Fe, Mn, Cu, and Zn contents in two Moraceae species. The influence of Fe, Mn, Cu, and Zn contents on the photosynthetic capability of B. papyrifera and M. alba was analyzed. Results of this study provide deeper understanding of the photosynthetic adaptive mechanism of B. papyrifera in a low-P environment, which can be used as a guide for selecting appropriate plant species for reforestation efforts in a heterogeneous environment.
2 Materials and methods
2.1 Plant growth and low-P treatment
Seedlings of B. papyrifera and M. alba were cultivated and treated according to Xing and Wu (2014). After 90 days of growth, the nutrient solution was replaced by a modified Hoagland solution (Hoagland and Arnon 1950) containing 6 mmol/L KNO3, 4 mmol/LCa(NO3)2, 2 mmol/L MgSO4, 2 mmol/L Fe(Na)EDTA, 2 μmol/LKCl, 50 μmol/L H3BO3, 4 μmol/L MnSO4, 4 μmol/L ZnSO4, 0.2 μmol/L CuSO4, and 0.2 μmol/L (NH4)6MO7O24. Five low-P treatments of 0.125, 0.063, 0.031, 0.016, and 0 mmol/L were simulated by varying concentration combinations of NH4H2PO4 and NH4Cl; 0.250 mmol/L P was used as the control. Determination was conducted on day 45 from the onset of treatment. Five recently-matured leaves from each of the treated seedlings were measured.
2.2 Measurement of net photosynthetic rate
Net photosynthetic rate was determined using the method in Xing and Wu (2012).
2.3 Measurement of chlorophyll a fluorescence
ChlF was determined according to the method described by Xing and Wu (2012). The maximum quantum yield of PSII (F v/F m) was calculated as (F m − F o)/F m, where F v = F m − F o. The actual photochemical quantum efficiency of PSII (Φ PSII) was calculated as (F′m − F s)/F′m.
2.4 Contents of Fe, Mn, Cu, and Zn
Five plants from each treatment group were selected and dried in an oven at 80 °C. Approximately 0.3–0.5 g of dried plant tissue was digested using the H2SO4–H2O2 digestion method (Xu 2000).The Fe, Mn, Cu, and Zn contents were determined using atomic absorption spectroscopy (PE-5100-PC, PerkinElmer, USA).
2.5 Statistical analysis
All experimental measurements consisted of five replicates. Data were analyzed using SPSS software (version 13.0, SPSS Inc). The differences between low-P treatments were assessed using the least significant difference (LSD) post hoc test at a 5 % significance level (p ≤ 0.05). Data were shown as the means ± standard errors (SE), which were determined using one-sample T test (confidence interval was 95 %). The correlation between P N and content of Fe, Mn, Cu, or Zn was analyzed using bivariate correlations.
3 Results
3.1 Effect of low-P on net photosynthetic rate
M. alba showed a significant decrease in P N from the onset of low-P treatment (Table 1). While the significant decrease of P N of B. papyrifera appeared at 0.063 mmol/L. The P N of M. alba exhibited a more significant decrease than that of B. papyrifera from 0.031 to 0.016 mmol/L. The values of P N of B. papyrifera at 0.016 and 0 mmol/L were 49 % and 41 %, respectively, of the value of the control, while the values of P N of M. alba at 0.016 and 0 mmol/L were only 38 % and 13 %, respectively, of the value of the control. The value of P N of B. papyrifera was higher than M. alba at each low-P stress level.
3.2 Effects of low-P on ChlF parameters
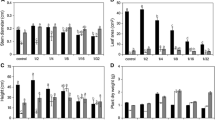
The values of initial fluorescence (F o) of B. papyrifera subjected to 0.016 and 0 mmol/L P treatments were higher than those in 0.250, 0.125, 0.063, and 0.031 mmol/L P treatments; the F o values of B. papyrifera in treatments with P nutrient levels ranging from 0.250 to 0.031 mmol/L showed no significant difference (Fig. 1a). The F o value of M. alba of the control was lower than that at other P nutrient levels; the F o values of M. alba in treatments with P nutrient levels ranging from 0.125 to 0.016 mmol/L showed no significant difference.
Effects of low-P on initial fluorescence (F o), maximum quantum yields of PSII (F v/F m) and the actual photochemical quantum efficiencies of PSII (Φ PSII). a F o; b F v/F m; c Φ PSII. Different letters appear above the error bars of the same parameter of the same plant species when subsequent values differ significantly—at P ≤ 0.05, according to one-way ANOVA and t tests
The maximum quantum yields of PSII (F v/F m) of B. papyrifera at 0.016 and 0 mmol/L P treatments were lower than those of higher P treatments; the F v/F m values of B. papyrifera in treatments with P nutrient levels ranging from 0.250 to 0.031 mmol/L showed no significant difference (Fig. 1b). The F v/F m of M. alba of the control was higher than that of other P nutrient levels; the F v/F m values of M. alba in treatments with P nutrient levels ranging from 0.125 to 0.016 mmol/L showed no significant difference.
The actual photochemical quantum efficiencies of PSII (Φ PSII) closely mimicked the pattern observed in maximum quantum yields. Φ PSII values of B. papyrifera at 0.016 and 0 mmol/L were lower than those with higher P treatments; the Φ PSII values of B. papyrifera in treatments with P nutrient levels ranging from 0.250 to 0.031 mmol/L showed no significant difference (Fig. 1c). The Φ PSII of M. alba of the control was higher than of those subjected to P deficiency; the Φ PSII values of M. alba in treatments with P nutrient levels ranging from 0.125 to 0.031 mmol/L showed no significant difference.
3.3 Effects of low-P on contents of Fe, Mn, Cu, and Zn
Fe, Mn, Cu, and Zn contents varied between plant species and P levels (Table 2). Low-P treatment was associated with lower contents of Fe and Mn at all P-deficient treatment levels in B. papyrifera, whereas the Fe and Mn contents in M. alba in treatments with P nutrient levels ranging from 0.250 to 0.031 mmol/L showed no significant difference. The micronutrient contents in M. alba in 0.016 and 0 mmol/L P treatments were lower than in higher P treatments.
Low-P treatment was consistently associated with lower contents of Cu in both B. papyrifera and M. alba (Table 2).
The contents of Zn in B. papyrifera in treatments with P nutrient levels ranging from 0.250 to 0.031 mmol/L showed no significant difference. The Zn contents of B. papyrifera at 0.016 and 0 mmol/L P treatments were lower than those higher-P treatments (Table 2).
3.4 Relationship between net photosynthetic rate and content of Fe, Mn, Cu, or Zn
The relationships between P N and the content of Cu or Zn displayed good positive correlations (Fig. 2c, d), in which the determination coefficient (R 2) ranged from 0.245 to 0.865; the R 2 of Cu or Zn was greater than that of Fe or Mn (Fig. 2a, b). In other words, an increase in Cu and Zn contents correlated with an increase of P N in both B. papyrifera and M. alba.
4 Discussion
Photosynthetic activity represents the growth potential of a plant (Walters et al. 1993). B. papyrifera exhibited better photosynthetic capability than M. alba under moderate P deficiency, whereas the photosynthetic capability of M. alba decreased significantly under moderate and severe P deficiency; the influence of low-P on photosynthesis was more severe in M. alba than in B. papyrifera. In addition, the resistances of B. papyrifera and M. alba to a low-P environment were different; the photochemical apparatus of B. papyrifera was not damaged under moderate P deficiency, whereas the increase of Fo in B. papyrifera under severe P deficiency indicated that the activity of PSII reaction centers had decreased. The response to decreased P of Fv/Fm and Φ PSII in B. papyrifera also indicated little damage to the PSII reaction centers under moderate P deficiency. However, the activity of PSII reaction centers in M. alba decreased in low-P treatments; that is, the PSII reaction centers of M. alba were damaged under low-P stress conditions. When the P concentration of nutrient solution was higher than 0.031 mmol/L, the B. papyrifera exhibited good tolerance. However, the tolerance of M. alba decreased under even moderate P deficiency.
Under moderate P deficiency, the amount of root-exuded organic acids in B. papyrifera increased more significantly than in M. alba (Wu and Zhao 2013), and according to Hoffland et al. (1992), the bioavailability of microelements around the root of B. papyrifera could be improved as a result. Due to the increase in root-exuded acids, the Cu content in B. papyrifera was higher than that in M. alba, and the Zn content in B. papyrifera exhibited a lower rate of decrease than that in M. alba. Since the most important role of Cu is associated with blocking of peroxidative chain reactions involving membrane lipids (Fernandes and Henriques 1991), higher Cu content in B. papyrifera could maintain the stability of thylakoid membrane structure more efficiently. Moreover, Zn could improve electron use in dark reactions (Hajiboland et al. 2010); therefore, the lower rate of decrease in Zn in B. papyrifera could maintain the stability of dark reactions more efficiently. By contrast, the damage caused by the decrease in Cu and Zn contents to M. alba could not be alleviated efficiently, and the photosynthesis of M. alba could not be meliorated. The results in the present study also indicate that the photosynthetic capability of B. papyrifera was relatively unaffected, whereas the photosynthetic capability of M. alba is sensitive to moderate P deficiency. Under severe P deficiency, the amount of root-exuded organic acids in M. alba increased more significantly than in B. papyrifera (Wu and Zhao 2013). Although the Cu content in M. alba exhibited a lower rate of decrease, the decrease in Zn content was significant. Under severe P deficiency, the increase in the amount of root-exuded organic acids in B. papyrifera and M. alba could not alleviate the loss of microelements. Moreover, a considerable decrease of photosynthetic capability in B. papyrifera and M. alba was associated with the lower Cu and Zn contents.
Given that the availability of Fe and Mn are higher than that of Cu or Zn in the soils of several terrestrial ecosystems(Chen et al. 2013), plant growth is more easily affected by Cu or Zn content in soil. In the present study, the applied amounts of Fe and Mn were probably enough for the growth of the two Moraceae species in low-P environments. P deficiency could not only bring about a decrease in cyclic photophorylation and photosynthetic efficiency(Watanabe and Yoshida 1970), but could also affect the stability of thylakoid membrane structure and electron transport efficiency by influencing the content of Cu or Zn, thereby affecting photosynthesis. Moreover, the P N of the two Moraceae species exhibited a better correlation with Cu and Zn contents, rather than with Fe or Mn content. P deficiency had almost no effect on PSII activity or on the water-splitting reaction in either plant species.
5 Conclusion
The responses of B. papyrifera and M. alba to low-P treatment with different Fe, Mn, Cu, and Zn contents varied in terms of photosynthetic capability. B. papyrifera exhibited better photosynthetic capability under moderate P deficiency, whereas the photosynthetic capability of M. alba decreased under moderate and severe P deficiency. Under moderate P deficiency, the decreases in Cu and Zn contents in B. papyrifera were lower than those in M. alba. Under severe P deficiency, a significant decrease of photosynthetic capability in B. papyrifera and M. alba was associated with lower Cu and Zn contents. The P N of the two Moraceae species exhibited a better correlation with Cu and Zn contents than with Fe or Mn content. Furthermore, P deficiency could not only decrease the cyclic photophorylation and photosynthetic efficiency, but also affect the stability of thylakoid membrane structure and electron transport efficiency by influencing the content of Cu or Zn, thereby affecting photosynthesis. Nevertheless, P deficiency had almost no effect on PSII activity or water-splitting.
Abbreviations
- Φ PSII :
-
Actual photochemical quantum efficiency of PSII
- B. papyrifera :
-
Broussonetia papyrifera (L.) Vent
- ChlF:
-
Chlorophyll a fluorescence
- F s :
-
Fluorescence in stable state
- F o :
-
Initial fluorescence
- LSD:
-
Least significant difference
- F m :
-
Maximum fluorescence
- F′m :
-
Maximum fluorescence in the light-adapted state
- F v/F m :
-
Maximum quantum yield of PSII
- M. alba :
-
Morus alba L
- P N :
-
Net photosynthetic rate
- P:
-
Phosphorus
- PSII:
-
Photosystem II
- SE:
-
Standard errors
- F v :
-
Variable fluorescence
References
Alloush GA, Boyer DG, Belesky DP, Halvorson JJ (2003) Phosphorus mobility in a karst landscape under pasture grazing system. Agronomie 23:593–600
Bertamini M, Muthuchelian K, Nedunchezhian N (2002) Iron deficiency induced changes on the donor side of PSII in field grown grapevine (Vitisvinifera L. cv. Pinot noir) leaves. Plant Sci 162:599–605
Chen C, Yang F, Liu HL, Yao HY, Song GX (2013) Effects and evaluation of soil trace elements after grassland converted into cropland in Guizhou karst area. Trans CSAE 29:230–237
Choi JM, Lee CW (2012) Influence of elevated phosphorus levels in nutrient solution on micronutrient uptake and deficiency symptom development in strawberry cultured with fertigation system. J Plant Nutr 35:1349–1358
Fernandes JC, Henriques FS (1991) Biochemical, physiological, and structural effects of excess copper in plants. Bot Rev 57:246–273
Gray DW, Gardon ZG, Lewis LA (2006) Simultaneous collection of rapid chlorophyll fluorescence induction kinetics, fluorescence quenching parameters, and environmental data using an automated PAM-2000/CR10X data logging system. Photosynth Res 87:295–301
Hajiboland R, Pasbani B, Amirazad H (2010) Effect of low Zn supply on growth, leaf pigments and photosynthesis in red cabbage (Brassica oleracea L. var. capitata f. rubra) plants grown under different light conditions. Iran J Plant Biol 1:25–36
Hao HL, Wei YZ, Yang XE, Feng Y, Wu CY (2007) Effects of different nitrogen fertilizer levels on Fe, Mn, Cu and Zn concentrations in shoot and grain quality in rice (Oryza sativa). Rice Sci 14:289–294
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. CalifAgricExpStnCirc 347:1–32
Hoffland E, Boogaard RVD, Nelemans J, Findenegg G (1992) Biosynthesis and root exudation of citric and malic acids in phosphate-starved rape plants. New Phytol 122:675–680
Huang ZA, Jiang DA, Yang Y, Sun JW, Jin SH (2004) Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 42:357–364
Jones DL, Darrah PR (1994) Role of root derived organic-acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Li SC, Hu CH, Gong J, Dong ST, Dong ZX (2004) Effects of low phosphorus stress on the chlorophyll fluorescence of different phosphorus use efficient maize (Zea mays L.). Acta Agro Sin 30:365–370
Li J, Liu CQ, Wang SL, Zhu ZZ, Zhou ZH, Xiao HY (2006) Vertical variation of phosphorus forms in surface sediments from Wuli Bay, Taihu Lake, China. Chin J Geochem 25:279–284
Liu CC, Liu YG, Guo K, Zheng YR, Li GQ, Yu LF, Yang R (2010) Influence of drought intensity on the response of six woody karst species subjected to successive cycles of drought and rewatering. PhysiolPlantarum 139:39–54
Nyoki D, Ndakidemi PA (2014) Influence of Bradyrhizobium japonicum and phosphorus on micronutrient uptake in Cowpea. A case study of zinc (Zn), iron (Fe), copper (Cu) and manganese (Mn). Am J Plant Sci 5:427–435
Racz GJ, Haluschak PW (1974) Effects of phosphorus concentration on Cu, Zn, Fe and Mn utilization by wheat. Can J Soil Sci 54:357–367
Rutkowska B, Szulc W, Sosulski T, Stępień W (2014) Soil micronutrient availability to crops affected by long-term inorganic and organic fertilizer applications. Plant Soil Environ 60:198–203
Sauer K (1980) A role for manganese in oxygen evolution in photosynthesis. Acc Chem Res 13:249–256
Shahbaz AM, Oki Y, Adachi T, Murata Y, Khan MHR (2006) Phosphorus starvation induced root-mediated pH changes in solublization and acquisition of sparingly soluble P sources and organic acids exudation by Brassica cultivars. Soil Sci Plant Nutr 52:623–633
Walters MB, Kruger EL, Reich PB (1993) Relative growth rate in relation to physiological and morphological traits for northern hardwood tree seedlings: species, light environment and ontogenetic considerations. Oecologia 96:219–231
Watanabe H, Yoshida S (1970) Effects of nitrogen, phosphorus, and potassium on photophosphorylation in rice in relation to the photosynthetic rate of single leaves. Soil Sci Plant Nutr 16:163–166
Wu YY, Zhao K (2013) Root-exuded malic acid versus chlorophyll fluorescence parameters in four plant species under different phosphorus levels. J Soil Sci Plant Nutr 13:604–610
Wu YY, Liu CQ, Li PP, Wang JZ, Xing D, Wang BL (2009) Photosynthetic characteristics involved in adaptability to Karst soil and alien invasion of paper mulberry (Broussonetia papyrifera (L.) Vent.) in comparison with mulberry (Morus alba L.). Photosynthetica 47:155–160
Xing DK, Wu YY (2012) Photosynthetic response of three climber plant species to osmotic stress induced by polyethylene glycol (PEG) 6000. Acta Physiol Plant 34:1659–1668
Xing DK, Wu YY (2014) Effect of phosphorus deficiency on photosynthetic inorganic carbon assimilation of three climber plant species. Bot Stud 55:1–8
Xu GH (2000) Determination of plant ash and diverse nutrient element. In: Bao SD (ed) Soil and agricultural chemistry analysis. China Agriculture Press, Beijing, pp 263–270
Zhao K, Wu YY (2014) Rhizosphere calcareous soil P-extraction at the expense of organic carbon from root-exuded organic acids induced by phosphorus deficiency in several plant species. Soil Sci Plant Nutr 60:640–650
Zhao W, Pan Y, Zhang Z, Jia S, Miao X, Huang Y (2005) Phylogeny of the genus Morus (Urticales: Moraceae) inferred from ITS and trnL-F sequences. Afr J Biotech 4:563–569
Acknowledgments
This study was supported by the project of the National Natural Science Foundation of China (No. 31301243), a project funded by the Priority Academic Program Development of Jiangsu higher education institutions (PAPD), the research foundation for introduce talents of Jiangsu university (13JDG030), the brainstorm project on social development of Guizhou Province (SY[2010]3043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xing, D., Wu, Y., Yu, R. et al. Photosynthetic capability and Fe, Mn, Cu, and Zn contents in two Moraceae species under different phosphorus levels. Acta Geochim 35, 309–315 (2016). https://doi.org/10.1007/s11631-016-0099-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-016-0099-1