Abstract
Coconut is a crop of economic importance, and protocols for the propagation in vitro of coconut are already in use. However, during acclimatization, some micropropagated plants or plantlets do not survive. There are reports that show that the application of arbuscular mycorrhizal fungi (AMF) improves the development of plants. So far, there are no reports of acclimatization of coconut plantlets with or without AMF. Therefore, this study reports the evaluation of survival and growth during acclimatization of coconut plantlets (obtained by somatic embryogenesis) testing inoculation with native or commercial AMF. Survival increased from 1.19 to 1.24-fold with native AMF, but no increase occurred with commercial AMF. Growth and photosynthetic parameters were evaluated, and there were no significant changes among treatments at 180 d. However, 6 mo later, there was a significant increase in height, leaf area, and stem diameter in plantlets inoculated with commercial AMF. There were differences in the development of secondary roots when plantlets were treated with commercial AMF. The colonization with native AMF showed a greater proportion of coils and hyphae, whereas, with commercial AMF, arbuscules and hyphae were more abundant. According to this study’s results, AMF inoculation can be recommended to improve the survival and growth of micropropagated coconut plantlets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coconut palm (Cocos nucifera L.) is a crop of economic importance in several countries, contributing to increasing food security and job creation (Karandeep et al. 2019). In the last 15 yr, the demand for coconut products has increased (Prades et al. 2016), particularly for packaged water, virgin oil, milk, sugar, and fiber derivatives (Roolant 2014; Pham 2016). Unfortunately, coconut production is decreasing, mainly due to palm senescence, pests, and diseases, particularly the phytoplasma-associated disease lethal yellowing (LY) that has killed millions of palms in several countries in the Caribbean region and Africa; such diseases have also been reported in countries in Asia and the south-western Pacific (Gurr et al. 2016; Oropeza-Salín et al. 2020). Therefore, to sustain the growth of the coconut industry, it is necessary to carry out extensive replanting worldwide, which should be done with genotypes selected for LY resistance and other, traits such as high yield.
Screening of LY-resistant genotypes started in the twentieth century identifying Malayan Dwarfs and Pacific Tall ecotypes (Been 1991; Zizumbo-Villareal et al. 2008). Screening new resistant genotypes is ongoing (Castillo et al. 2022; Garavito-Guyot et al. 2022). Massive coconut production required for replanting worldwide will be difficult to achieve if plants are only produced through seed propagation. So, efficient micropropagation through somatic embryogenesis is becoming an important alternative (Kalaipandian et al. 2021). Such a process is already available; it can yield thousands of somatic embryos from a single plumule explant through the formation and multiplication of embryogenic callus, and most of these embryos convert into micropropagated plants or plantlets (Pérez-Núñez et al. 2006; Oropeza 2016). However, improvement of this micropropagation process is necessary to become more competitive, reducing production costs (Sáenz-Carbonell et al. 2016). One such improvement that is needed is increasing ex vitro survival of the plantlets after acclimatization, which facilitates their adaptation in the field (Hazarika 2006).
Previous studies that evaluated the ex vitro survival of coconut plantlets that were produced by in vitro germination of zygotic embryos reported low percentages, initially. However, after several strategies, the survival has increased significantly to 90% (Table 1S; Fuentes et al. 2005; Pech Aké et al. 2007; Talavera et al. 2005; Samosir and Adkins 2014; Sisunandar et al. 2018).
Ex vitro survival of plantlets obtained through somatic embryogenesis has been reported in the palm species Phoenix dactylifera and Elaeis guineensis, with 60 to 84 and 58 to 63% survival, respectively (Schultz 2001; Al-Khayri and Naik 2017). However, there are no previous studies of acclimatization of coconut plantlets produced through somatic embryogenesis.
During transfer to ex vitro conditions, plantlets face a transplant shock, exposure to elevated temperature and light intensity, and altered physiology and morphology (Chandra et al. 2010; Kumar and Rao 2012). A promising strategy for adaptation to the new conditions is the use of arbuscular mycorrhizal fungi (AMF) to improve the survival of plantlets produced in vitro. AMF contributes to more vigorous growth, improves the root system, increases photosynthetic efficiency, improves water-conducting capacity, improves nutrient absorption, prevents the attack of soil-borne pathogens, and relieves environmental stress (Kapoor et al. 2008; Soumare et al. 2021).
Different reports mention the importance of using mixed AMF since some AMF taxa are more associated with specific functions, allowing functional complementarity that improves their synergistic effects on plants (Crossay et al. 2019). Also, coconut has long been a naturalized species; the association of coconut with native AMF can positively affect the survival, growth, and development of the plantlets during acclimatization and, eventually, final establishment. In addition, native AMF species could be more effective symbionts than commercial species because they develop in specific conditions (Guadarrama and Ramos-Zapata 2020).
The use of AMF for in vitro–produced coconut plantlets has not been reported, but it has been studied in P. dactylifera (El Kinany et al. 2019) and Elaeis guineensis with good results (Schultz 2001). Therefore, here, for the first time, the evaluation of micropropagated coconut plantlets inoculated with native AMF, isolated from the rhizosphere of coconut palms from different sites of the coastal dune of Yucatan in Mexico, and compared with commercial AMF to determine their effect on survival and performance of the plantlets during the acclimatization stage has been reported.
Material and Methods
Experimental Site and Plant Material
The present study was carried out at Centro de Investigación Científica de Yucatán, A.C. México, in greenhouse facilities (temperature and humidity were 27.6 ± 2.5 °C and 51.7 ± 3.1%). Coconut plantlets of the Mexican Pacific Tall hybrid (MxPT1 × MxPT2 ecotypes) were obtained by micropropagation according to Pérez-Nuñez et al. (2006) and Sáenz-Carbonell et al. (2018). Briefly, the plantlet production was through somatic embryogenesis from plumule explants, involving embryogenic callus formation and its multiplication, followed by the formation of somatic embryogenic callus. Finally, the embryos germinated and developed into plantlets. At the end of the process, plantlets were selected according to a uniform height (21 ± 2.6 cm), 2 to 3 bifid leaves, and the presence of secondary roots.
Arbuscular Mycorrhizal Fungi Used
Two different AMF mixes were used. One was a commercial AMF (PHP® Endo Rhyza Mini Plug, Mexico City, Mexico) that is a mix of two species (Rhizoglomus intraradices and Acaulospora colombiana) within a vermiculite inert substrate and vegetative propagules (fragments of mycelium and mycorrhized roots). The second one was a native AMF mix with 13 species from soil associated with the coconut rhizosphere. Soil, as a source of native AMF, was collected from six coconut-growing areas in Yucatán (Lara-Pérez et al. 2020). After collection, AMF was multiplied by trap cultures using Panicum maximum as the host plant in a greenhouse for 6 mo. P. maximum seeds were disinfected with 0.5% of NaClO (Hycel, Zapopan, Mexico) for 15 min before use and planted in a substrate of soil and sterile sand (121 °C, 1 h, 3 times). The plants were fertilized (15% Hoagland solution, without phosphorous, prepared in our laboratory) once a week in the first 2 mo and then twice a week. Native AMF contained fragments of mycorrhized roots (60 to 80%).
AMF Spore Extraction and Identification
The spores were isolated from 10.0 g of a substrate according to Genderman and Nicholson (1963), by mixing with 1 L of water, and this suspension passed through a nest of four soil sieves (500, 150, 73, and 38 µm, WStyler, Mentor, OH). The material retained by the 38-µm sieve was suspended in 30 mL of water and centrifuged at 3000 rpm for 3 min (Eppendorf 5804R, Hamburg, Germany). Next, the pellet was centrifuged at 1000 rpm for 1 min in a sucrose (Zulka, Culiacan, Mexico) solution with a stepped density gradient (15% and 60% w/v) according to Walker (1997). The resulting supernatant was sieved (38 µm), and what was retained was washed with water to eliminate sucrose. Finally, the spores were counted in a stereoscopic microscope (Nikon SWZ800, Melville, NY) to 40 × . Spore numbers were estimated per each 10 g, using five replicates. Additionally, to identify the quantified spores, for native AMF species level identification, spores were mounted on slides in polyvinyl alcohol-lactic acid-glycerol (PVLG) and a mix of PVLG—Melzer’s reagent (J.T. Baker, Mexico City, Mexico) and examined under a microscope (Zeizz Primo Star, Oberkochen, Germany). The identification was based on spore color, size, form, wall structure, decoration, hyphae type, germination mode, and different subcellular structures (Błaszkowski 2012).
AMF Inoculation and Acclimatization of Coconut Plantlets
The plantlets were individually transferred from in vitro conditions into black polyethylene nursery bags with bellows (21 × 35 cm, 600 gauge) containing approximately 2.5 kg of sterilized substrate (beach sand, regional soil, and sphagnum peat moss in a 1:1:1 ratio). The native or commercial AMF were inoculated at the time of transplant (10 to 15 g containing 246 ± 15 spores). Next, the substrate mix was irrigated with 0.3 L of water 1 d before planting the plantlets. The black bags had 12 small holes at the base to drain excess water. Finally, this set-up with the plantlet was covered with a transparent polyethylene bag (400 gauge; Plastica peninsular, Mérida, México) with 32 horizontal cuts (1.5 cm long) distributed uniformly (Pech-Aké et al. 2004; Talavera et al. 2005). Plantlets were placed randomly in the greenhouse and kept for 2 wk without further irrigation under a shade mesh with ventilation in the greenhouse. Then, the transparent upper bags were withdrawn, and the plantlets were kept in a greenhouse for 6 mo that was irrigated with an automatic micro-sprinkler system every 3 d for 5 min.
Experiments Performed
There were three experiments in this study with three treatments in each experiment: (a) non-inoculated plantlets (control), (b) plantlets inoculated with native AMF, and (c) plantlets inoculated with commercial AMF. Experiment 1 started in October 2019 and was carried out to evaluate the survival rate for 6 mo after the transplant with monthly monitoring. Fifteen plantlets were used for each treatment. Experiment 2 started in October, 2020, and was carried out to evaluate the rate of survival, growth, and physiological parameters for 6 mo with monitoring at 0, 30, 90, and 180 d after transplant. Thirty plantlets were used for each treatment. Finally, experiment 3 started in April 2021 and was carried out to evaluate root growth (primary and secondary root length) and colonization roots. Three plantlets for each treatment were used, and sampling was at 0, 15, 30, 60, 90, and 180 d.
Growth Parameters
Plantlet height (in cm) was measured using a flexometer (± 1.2 mm, Truper, Torreón, Mexico). Stem diameter (in cm, taken 2.0 cm above the substrate surface level) was measured using a dial caliper (± 0.0381 mm, Fowler, Canton, MA). The number of leaves per plantlet was determined visually. The area of the youngest open leaf (in cm2) was estimated with ImageJ (version 1.52p) using leaf length as a reference.
Physiological Parameters
Measurements were taken at 12.00 to 14:00 h. The photosynthetic activity was determined at 30 °C and 60% relative humidity, 1000 µmol PPFD ·m−2·s−1 light intensity, and 400 μmol⋅mol−1 reference CO2, using a portable photosynthetic system (LICOR LI-6400XT, Lincoln, NE) (Fuentes et al. 2005). This evaluation was carried out for 60 s on the youngest expanded leaf fixed within the chamber with the adaxial surface upwards. The chlorophyll fluorescence was determined from the ratio of variable to maximum fluorescence (Fv/Fm), and the performance index (PIABS) with a fluorescence-modulated system analyzer (mPEA, Hansatech, Norfolk, UK). This evaluation was carried out on the youngest expanded leaf that was dark-adapted for 20 min, with a saturation pulse at 3000 µmol m−2 s−1, and 70% intensity (Fuentes et al. 2005; Talavera et al. 2005).
Root Growth and Colonization
To determine root growth during acclimatization, primary and secondary roots were manually counted, and the primary root length was measured for 6 mo at different times. For root colonization, samples (20 root segments of 1.0 cm) were cleared with 10% KOH (J.T. Baker) and 5% H2O2 (J.T. Baker) at 120 °C for 20 min, followed by washing with tap water, acidified with HCl (Hycel) 0.1 N for 10 min, and stained with trypan blue (0.05%, Bio Basic, Markham, Canada) at 120 °C for 20 min according to Phillips and Hayman (1970). To identify and count AMF structures, roots were mounted on a slide with PVLG. The root segments with mycorrhizal structures (arbuscules, vesicles, hyphae, coils) were estimated according to the method of McGonigle et al. (1990) with the following equation:
Statistical Analysis
Data were analyzed with repeated measures ANOVA test, followed by Dunnett’s post hoc test (p ≤ 0.05), normality using Lilliefors test, homogeneity of variances with Cochran’s and Bartlett’s tests, and percentage data transformation by arcsin √(× /100) before analyses. All statistical analyses were performed using Minitab (version 17.1).
Results
Plantlet Survival
Two experiments evaluated plantlets’ survival after transference to ex vitro conditions. In experiment 1 (Fig. 1A), the survival rate after 6 mo was 92.0 ± 0.3% for plantlets inoculated with native AMF and 77.0 ± 0.4% for both plantlets inoculated with commercial AMF; the control treatment (without inoculation) (Fig. 1A) and the difference were significant (p ≤ 0.05). In experiment 2 (Fig. 1B), the survival rate after 6 mo was 87 ± 0.2% for plantlets inoculated with native AMF: 67.0 ± 0.0% for commercial inoculum and 70.0 ± 0.0% for the control. The difference was significant between the native AMF treatment and the other two treatments, commercial AMF and control, but it was not significant between these last two treatments. In both experiments, the survival rate with the native AMF treatment was greater than with the other two treatments, 1.2 times and 1.3 times, in experiment 1 and experiment 2, respectively.
Plantlet Height and Stem Diameter
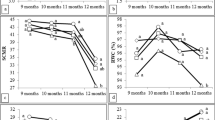
The plantlet height growth response during acclimatization to the native AMF, commercial AMF, and control treatments (Fig. 2A) showed no significant differences between them (p ≤ 0.05). The plantlet height increased from 90 d. On average, it increased from about 19.5 to 34.4 cm during the 180 d of the experiment.
Growth parameters in micropropagated Cocos nucifera L. plantlets during acclimatization under three different treatments of arbuscular mycorrhizal fungi inoculation. Each line represents the means of 30 plantlets for treatment ± DE (standard error) during 180 d under greenhouse conditions. (A) Plantlet height, (B) Stem diameter, (C) Leaf number, and (D) Leaf area. Different letters in each line show significant differences between treatments (p ≤ 0.05, Dunnett’s test).
The pattern of response for plantlet stem diameter growth was very similar in shape and quantitatively with the three treatments (Fig. 2B). It showed a slight decrease at 30 d; from then on, it increased steadily for the rest of the duration of the experiment. There were quantitative differences among treatments, particularly at 90 and 180 d, but they were not significant (p ≤ 0.05).
Number of Leaves and Leaf Area
With the different AMF treatments, the number of leaves (Fig. 2C) per plantlet did not show a significant difference. It increased on average one leaf per plantlet by day 90; but then at 180 d, it decreased. This difference must result from pruning, a regular practice that must be done regularly on these plantlets during acclimatization.
The response in the plantlet leaf area was similar in the three treatments (Fig. 2D). There was a steady increase throughout the experiment, from about 22.8 to 132.4 cm2. Differences within the first 90-d period were small and not significant (p ≤ 0.05); a greater difference was observed at 180 d with a leaf area of 145.3 cm2 for plantlets under the commercial AMF treatment, 126.9 cm2 for plantlets under the plantlet inoculated with native AMF, and 124.8 cm2 for the control.
The plantlets were kept for a longer time under shelter prior to transfer to the field. This situation provided the opportunity to evaluate plant height, stem diameter, and leaf area at 360 d (Fig. 3A). The results showed that plant height and stem diameter were significantly higher (p ≤ 0.05) in plantlets inoculated with commercial AMF compared with plantlets with the other two treatments (Fig. 3B). In addition, leaf area was significantly higher (p ≤ 0.05) in plantlets inoculated with commercial and native AMF compared to non-inoculated plants (Fig. 3B).
Growth of Cocos nucifera L. plantlets inoculated with arbuscular mycorrhizal fungi 360 d after the start of acclimatization. (A) Cocos nucifera L. plantlets with the different treatments: inoculation with native or commercial arbuscular mycorrhizal fungi, and control with no inoculation. (B) Growth evaluation on plant height, stem diameter, and leaf area of plantlets with the different treatments: inoculation with native or commercial arbuscular mycorrhizal fungi. Each bar represents the means of 30 plantlets for treatment ± DE (standard error). Different letters show significant differences between treatments (p ≤ 0.05 Dunnett’s test).
Chlorophyll Fluorescence
During acclimatization, the chlorophyll fluorescence (Fv/Fm) of the leaves ranged from 0.735 to 0.801 at the time of transplant; it decreased at 30 d 0.739, then increased to 0.753 at 90 d to remain with very little change afterward (Fig. 4A). There were differences among the treatments but none that were significant (p ≤ 0.05). The performance index (PIABS) initially ranged from 3.95 to 4.32 and increased from 4.78 to 4.96 at 180 d of acclimatization (Fig. 4B). Again, the differences between treatments were not significant (p ≤ 0.05, Dunnett’s test).
Photosynthetic parameters of Cocos nucifera L. plantlets inoculated with arbuscular mycorrhizal fungi. Each line represents the means of 30 repetitions ± DE (standard error). (A) Relation Fv/Fm (B) Performance index (PIabs). (C) Photosynthetic rate. Different letters in each line show significant differences between treatments (p ≤ 0.05 on the Dunnett’s test).
Photosynthetic Rate
Regarding the photosynthetic rate, the initial values ranged from 7.26 to 7.65 µmol CO2·m−2·s−1 for the three treatments. Then, it showed an increase at 90 d, ranging from 10.67 to 12.24 µmol CO2·m−2·s−1, with very little change at 180 d (Fig. 4C). The differences observed between treatments were not significant (p ≤ 0.05).
Root Growth
The number of primary roots of the coconut plants during acclimatization for the different treatments showed very little change from 3 to 3.5 at the beginning and from 3 to 4 at the end of the 180-d period (Fig. 5A). Therefore, this difference from the beginning to the end of the evaluation and the differences between treatments were not significant.
Rooting parameters of micropropagated Cocos nucifera L. plantlets grown with different arbuscular mycorrhizal fungi treatments. (A) Primary roots number, (B) Primary root length, (C) Secondary roots number. Each value represents the means of three independent replicates ± DE. Different letters in each line show significant differences (p ≤ 0.05) between treatments with the Dunnett’s test.
In the case of root growth, an increase was observed for primary roots in plants with the three treatments. It ranged from 3 to 4.5 cm at the beginning, and this increased from 9 to 19 cm at the end of the 180-d period (Fig. 5B). There were differences between treatments, but they were not significant.
The number of secondary roots in the plants showed no increase during the first 60 d with the three treatments. However, after 90 d, it increased significantly (p ≤ 0.05) to 109.0 ± 14.1 in plants under the commercial AMF treatment and 61.5 ± 4.13 in plants under the native AMF treatment, compared to 38.3 ± 17.5 in non-inoculated plants (Fig. 5C). The secondary root length was not considered because it had a large variability.
Identification of Species of Arbuscular Mycorrhizal Fungi
The native AMF species identified were (1) Acaulospora sp. 1, (2) Acaulospora sp. 2, (3) Acaulospora sp. 3, (4) Claroideoglomus etunicatum, (5) Dominikia aurea, (6) Entrophospora infrequens, (7) Glomus aff. Glomerulatum, (8) Glomus sp. 1, (9) Glomus sp. 2, (10) Rhizoglomus aggregatum, (11) Racocetra, (12) Sclerocystis sp. 1, and (13) Septoglomus sp. 1 (Fig. 6).
Arbuscular mycorrhizal fungi isolated from soil associated with the coconut rhizosphere. (1) Acaulospora sp. 1, (2) Acaulospora sp. 2, (3) Acaulospora sp. 3, (4) Claroideoglomus etunicatum (W.N. Becker & Gerd.) C. Walker & A. Schüßler, (5) Dominikia aurea (Oehl & Sieverd.) Błaszk., Chwat, G.A. Silva & Oehl, (6) Entrophospora infrequens (I.R. Hall) R.N. Ames & R.W. Schneid, (7) Glomus aff. glomerulatum Sieverd, (8) Glomus sp. 1, (9) Glomus sp. 2, (10) Rhizoglomus aggregatum (N.C. Schenck & G.S. Sm.) Sieverd., G.A. Silva & Oehl, (11) Racocetra gregaria (N.C. Schenck & T.H. Nicolson) Oehl, F.A. Souza & Sieverd, (12) Sclerocystis sp. 1, and (13) Septoglomus sp. 1.
Root Colonization
The mycorrhization frequency estimated in coconut plantlet roots inoculated with native AMF is shown in Fig. 7A. The colonization of native AMF started at 15 d with hyphae formation (6.8% of frequency). Then, it decreased on day 30 to 1.2% and increased again to 10.4% on day 60. At day 90, it decreased again to 2.2% and then increased to 7.9% at day 180. Vesicles appeared at 30 d, peaking at 10.6% on 60, and decreased at day 90 at nearly 0.6%. Finally, it increased to 1.2% at 180 d. Arbuscules appeared at day 60 with 3.1% frequency, decreased to nearly 0% at day 90, then increasing again to 1.8% at 180 d. In the case of coils, they appeared on day 30 at 2.4%, then decreased to 0.6% on day 60, only to increase again to 10.2% on day 180.
The frequency of mycorrhization in plantlet roots inoculated with commercial AMF is shown in Fig. 7B. Colonization started with the appearance of hyphae with a frequency of 8.4% on day 15. It decreased to about 5.9% on day 30. Then, it more than doubled to 18.3% at day 90 and slightly decreased to 16.1% at day 180. Vesicles and coils were nearly non-existent (≤ 1%) throughout the 180 d. Arbuscules appeared at day 60 with a frequency of 12.1% and decreased to 4.1% at day 90. It increased afterward to 9% at 180 d. In contrast, the plantlets without AMF did not show mycorrhizal colonization.
Discussion
Acclimatization of micropropagated plantlets is the last stage before they are ready for establishment in the field, and it is essential to ensure their survival under ex vitro conditions. In the case of coconut micropropagated plantlets, there are no reports in the literature on their acclimatization. However, there are reports for micropropagated plantlets of other palm species with survival of 84% for P. dactylifera (El Kinany et al. 2019) and 55% for E. guineensis (Schultz 2001). However, it is also found that these responses could be improved by up to 100% when the plantlets were inoculated with AMF during acclimatization. So, the present study reported the effect of native and commercial AMF on the performance and survival during the acclimatization of coconut plantlets obtained by somatic embryogenesis. Two experiments were carried out in which survival was evaluated. In the first one, the survival percentage was 77%, and when plantlets were inoculated with native AMF, survival increased 1.19-fold. In the second experiment, survival was lower (67%), but again when treated with native AMF, it increased 1.24-fold.
The reduced survival in the second experiment might have been because the experiment was carried out during the pandemic, and the acclimatization conditions could not be kept optimal, particularly regarding irrigation frequency. Whereas for the first experiment, conditions were optimal. Therefore, even under suboptimal conditions, native AMF treatment promotes survival. However, in both experiments, survival increased only with native AMF but not with commercial AMF. Although there are no reports in the literature testing comparatively the use of native and commercial AMF mixes on plantlets, a report on cassava (Manihot esculenta Crantz) plantlets (Azcón-Aguilar et al. 1997) showed that the survival of AMF species Glomus deserticola increased from 75 to over 90% during acclimatization, similarly to the present results.
Growth and physiological parameters were evaluated in parts above the ground. In the case of growth parameters, very similar patterns were observed for the three treatments with no significant differences. However, height, stem diameter, and leaf area were slightly higher in plantlets treated with commercial AMF. So, for these parameters, there was an additional evaluation at 360 d when plantlets were growing in the nursery before being transferred to the field; there were significantly larger values in plantlets treated with AMF (commercial or native) than in those not treated. Similarly, in a study with coconut seedlings treated with AMF, the volume and dry weight of primary, secondary, tertiary, and quaternary roots increased after 5 to 7 mo in the nursery, and leaf production rate and stem girth were significantly higher after 12 to 18 mo in the field (Senarathne and Ilangamudali 2018). When growth was evaluated in roots, there was no significant difference in the number and length of primary roots between treatments. However, the number of secondary roots at day 90 and day 180 was significantly larger in plantlets treated with commercial AMF than with the other treatments. Senarathne and Ilangamudali (2018) also reported greater growth of roots of coconut seedlings after 5 to 7 mo of applying a commercial AMF treatment. Also, El Kinany et al. (2019) observed that after 12 mo of growth, the number of roots of date palms was significantly improved following AMF and compost application. Regarding measurements of photosynthetic parameters during acclimatization, there were no significant variations when plantlets were treated with AMF. However, no negative effects were observed, indicating that the plants were not stressed (Fv/Fm) and that the performance index values (PIABS) were typical. Similar results were observed in date palms after 3 to 4 mo of growth under acclimatization conditions when treated with Rhizoglomus irregulare and a native consortium (Anli et al. 2020a, 2020b). In the case of photosynthesis, although it was low at the beginning, it was expected as plantlets were coming from an in vitro environment.
In a third experiment, root colonization was evaluated when plantlets were treated with commercial or native AMF, and there were differences. The root of all plantlets sampled was colonized, and there were two colonization patterns. According to the mycorrhizal structures found, it could be assumed that the coconut root morphology showed intermedia-type colonization between the Arum and the Paris types, similar to other palm species (Brahea armata, Chamaerops humilis L., Phoenix canariensis, Phoenix dactylifera L.; Dreyer et al. 2010). The patterns differed in amount and type of structures, but there was also an earlier increase in all structures with native AMF. Furthermore, within this period of 60 d, there was a drop in the percentage of surviving plantlets that was about 10% or lower with native AMF, but it was 20% or higher with commercial AMF or no AMF treatment. So, treating the plantlets with native AMF helped reduce plantlet loss during acclimatization, an effect that could be associated with the pattern of colonization during the first 60 d of acclimatization. This difference in survival associated with native AMF could be because native AMF species are adapted to local climatic and soil conditions and, therefore, could be more likely to survive and spread after transplantation than non-native AMF (Davidson et al. 2016). Several species of mono- and dicotyledonous plants have been reported to experience an increase in their survival when treated with AMF (native or commercial) with an average of 1.31- and 1.50-fold (see Table 2S) and 1.26-fold in plantlets inoculated with native AMF.
These differences could result from having different fungi species mixtures in both the native AMF mix and the commercial AMF mix. The commercial AMF contains G. intraradices and E. colombiana; these species have contributed to improve plant height and stem diameter in different hosts (Vázquez-Hernández et al. 2011; Vafadar et al. 2014). Native AMF contains mainly Glomus and Acaulospora genera, which are the most common and abundant species, especially in the Neotropical region of Mexico where there is a generic codominance (Polo-Marcial et al. 2021) both in environments natural and transformed by man with an ability to adapt to a wide range of environmental and soil conditions and thus confer a more beneficial effect to hosts (Estrada et al. 2013; Nobre et al. 2018). The use of native AMF mixes confers fungal-plant compatibility and functional complementarity, which is essential for the symbiotic efficiency of the host plants (Goetten et al. 2016; Crossay et al. 2019).
The observed differences in the colonization patterns could be related to the role of the structures involved in the colonization process. In this process, the hyphal network is a key element in the interconnection of plant roots in the soil (Baslam et al. 2014; da Silva et al. 2021). In the case of arbuscules, they are considered the main site of symbiotic exchange with the host plant (Brundrett et al. 1996), mainly inorganic phosphorous, which is taken up by AMF hyphae and transferred to intraradical fungal structures (Wipf et al. 2019). It is also proposed that coils represent a large surface area of an intracellular interface similar to an arbuscule; in the absence of arbuscules, the plant must use coils in a similar way to arbuscules, and they have a longer life than the arbuscules (Brundrett and Kendrick 1988; Brundrett and Kendrick 1990; Brundrett et al. 1996; Jakobsen et al. 2003; Fedderman et al. 2010; Smith and Smith 2011). In addition, vesicles are fundamental in the generation of propagules (Willis et al. 2013). Klironomos and Hart (2003) provided evidence suggesting that vesicles alone are infective, suggesting that vesicles can favor the successful colonization of more roots. All the responses reported here in coconut plantlets treated with AMF must be the result of the AMF-plant interaction; and, as reported by Bahadur et al. (2019), these AMF-mediated responses in plants include induction of genes and concomitant induction of metabolic and physiological pathways.
Conclusions
The present study showed that using native AMF increased the survival of coconut plantlets produced in vitro through somatic embryogenesis. Also, commercial AMF improved the plant height, leaf area, stem diameter, and secondary root. Then, considering this differential effect of the native and commercial AMF mixes tested, it will be very important to test both AMF treatments applied together simultaneously, or sequentially, then applying native AMF first and commercial AMF second, to evaluate if the effects are additive. In addition, it will be necessary to evaluate the performance and survival of the acclimatized and AMF-treated plants treated after establishing them in the field. Finally, another area of research that should be considered is the study of the underlying mechanisms of the interaction of AMF with coconut plantlets to further understand it. The results of both types of research, basic and applied, will be useful to establish the basis for the improvement of the use of AMF for acclimatization and field performance of micropropagated plants.
References
Al-Khayri JM, Naik PM (2017) Date palm micropropagation: advances and applications. Ciênc Agrotec 41:347–358
Anli M, Symanczik S, El Abbassi A, Ait-El-Mokhtara M, Boutasknita A, Ben-Laouane R, Baslam M, Mäder P, Hafidi M, Meddich A (2020a) Use of arbuscular mycorrhizal fungus Rhizoglomus irregulare and compost to improve growth and physiological responses of Phoenix dactylifera ‘Boufgouss.’ Plant Biosyst Int J Plant Biol 155:763–771
Anli M, Baslam M, Tahiri A, Raklami A, Symanczik S, Boutasknit A, Ait-El-Mokhtar M, Ben-Laouane R, Ben-Laouane R, Ait Rahou Y et al (2020b) Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front Plant Sci 11:1–21
Azcón-Aguilar C, Cantos M, Troncoso A, Barea JM (1997) Beneficial effect of arbuscular mycorrhizas on acclimatization of micropropagated cassava plantlets. Sci Hortic 72:63–71
Bahadur A, Batool A, Nasir F, Jiang S, Mingsen, Q, Zhang, Q, ... Feng, H (2019) Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int J Mol Sci 20:4199
Błaszkowski J (2012) Glomeromycota, Publisher: W Szafer Institute of Botany, Polish Academy of Sciences, Kraków, Poland, pp. 303, https://www.cabdirect.org/cabdirect/abstract/20123352848
Baslam M, Qaddoury A, Goicoechea N (2014) Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 28:161–172
Been BO (1991) Observations on field resistance to lethal yellowing in coconut varieties and hybrids in Jamaica. Oléagineux 36:9–11
Brundrett MC, Kendrick WB (1988) The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Can J Bot 66:1153–1173
Brundrett MC, Kendrick WB (1990) The roots and mycorrhizae of herbaceous woodland plants. II. Structural aspects of morphology. New Phytol 114:469–479
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. Australian Centre for International Agricultural Research, Canberra. https://doi.org/10.13140/2.1.4880.5444
Castillo R, Ortiz CF, Narvaez M, Fernández M, Torres N, Enriquez R, Vázquez M, Luis-Pantoja M, Paredes-Tomas C, Wayne M, Oropeza C (2022) Resistance trials of plants of coconut varieties naturally exposed to palm lethal yellowing phytoplasmas in Tabasco and Yucatán. Phytopathogenic Mollicutes 12:77
Chandra S, Bandopadhyay R, Kumar V, Chandra R (2010) Acclimatization of tissue cultured seedlings: from laboratory to land. Biotechnol Lett 32:1199–1205
Crossay T, Majorel C, Redecker D, Gensous S, Medevielle V, Durrieu G, Cavaloc Y, Amir H (2019) Is a mixture of arbuscular mycorrhizal fungi better for plant growth than single-species inoculants? Mycorrhiza 29:325–339
da Silva MR, Vasconcelos SS, Viana-Junior AB, Castellani DC, Kato OR (2021) Oil palm (Elaeis guineensis) shows higher mycorrhizal colonization when planted in agroforestry than in monoculture. Agrofor Syst 95:731–740
Davidson BE, Novak SJ, Serpe MD (2016) Consequences of inoculation with native arbuscular mycorrhizal fungi for root colonization and survival of Artemisia tridentata ssp. wyomingensis seedlings after transplanting. Mycorrhiza 26:595–608
Dreyer B, Morte A, López JÁ, Honrubia M (2010) Comparative study of mycorrhizal susceptibility and anatomy of four palm species. Mycorrhiza 20:103–115
El Kinany S, Achbani E, Faggroud M, Ouahmane L, El Hilalia R, Haggoud A, Bouamria R (2019) Effect of organic fertilizer and commercial arbuscular mycorrhizal fungi on the growth of micropropagated date palm cv. Feggouss J Saudi Soc Agric 18:411–417
Estrada B, Aroca R, Barea JM, Ruiz-Lozano JM (2013) Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. J Plant Sci 201–202:42–51
Feddermann N, Finlay R, Boller T, Elfstrand M (2010) Functional diversity in arbuscular mycorrhiza–the role of gene expression, phosphorous nutrition and symbiotic efficiency. Fungal Ecol 3:1–8
Fuentes G, Talavera C, Oropeza C, Desjardins Y, Santamaria JM (2005) Exogenous sucrose can decrease in vitro photosynthesis but improve field survival and growth of coconut (Cocos nucifera L.) in vitro plantlets. In Vitro Cell Dev Biol - Plant 41:69–76
Garavito-Guyot A, Rivallan R, Bocs S, Baudouin L, Ndede EY (2022) Genomic screening for tolerance of coconut populations differentially exposed to coconut’s lethal yellowing in Ghana using genotyping by sequencing. Phytopathogenic Mollicutes 12:70
Gerdemann JW, Nicolson YH (1963) Spores of mycorrhiza endogone species extracted from soil by wet sieving and decanting. Trans Brit Mycol Soc 46:235–244
Goetten LC, Moretto G, Stürmer SL (2016) Influence of arbuscular mycorrhizal fungi inoculum produced on-farm and phosphorus on growth and nutrition of native woody plant species from Brazil. Acta Bot Bras 30:9–16
Guadarrama MPC, Ramos-Zapata JA (2020) Importancia de las micorrizas como estrategia de restauración en la duna costera de Yucatán. Bioagrociencias 13:38–47
Gurr GM, Johnson AC, Ash GJ, Wilson BAL, Ero MM, Pilotti CA, Dewhurst CF, You MS (2016) Coconut lethal yellowing diseases: a phytoplasma threat to palms of global economic and social significance. Front Plant Sci 7:1521
Hazarika BN (2006) Morpho-physiological disorders in in vitro culture of plants. Sci Hortic 108:105–120
Jakobsen I, Smith SE, Smith FA (2003) Function and diversity of arbuscular mycorrhizae in carbon and mineral nutrition. In: van der Heijden, MGA, Sanders, IR (eds) Mycorrhizal Ecology, Ecological Studies, Springer, Berlin, Heidelberg, pp 75–92
Kalaipandian S, Mu Z, Kong EYY, Biddle J, Cave R, Bazrafshan A, Wijayabandara K, Beveridge CF, Nguyen Q, Adkins SW (2021) Cloning coconut via somatic embryogenesis: a review of the current status and future prospects. Plants 10:2050
Kapoor R, Sharma D, Bhatnagar AK (2008) Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci Hortic 116:227–239
Karandeep K, Navnidhi C, Poorva S, Garg MK, Anil P (2019) Coconut meal: nutraceutical importance and food industry application. Foods Raw Mater 7:419–427
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Kumar K, Rao IU (2012) Morphophysiologicals problems in acclimatization of micropropagated plants in-ex vitro conditions-a reviews. J Ornam Hortic 2:271–283
Lara-Pérez LA, Oros-Ortega I, Córdova-Lara I, Estrada-Medina H, O’Connor-Sánchez A, Góngora-Castillo E, Sáenz-Carbonell L (2020) Seasonal shifts of arbuscular mycorrhizal fungi in Cocos nucifera roots in Yucatan, Mexico. Mycorrhiza 30:269–283
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Nobre CP, da Costa MG, Goto BT, Gehring C (2018) Arbuscular mycorrhizal fungi associated with the babassu palm (Attalea speciosa) in the eastern periphery of Amazonia, Brazil. Acta Amazon 48:321–329
Oropeza C (2016) Coconut micropropagation in Mexico using plumule and floral explants. CORD 32:6–6
Oropeza-Salín C, Sáenz L, Narvaez M, Nic-Matos G, Córdova I, Myrie W, Ortíz FC, Ramos E (2020) Dealing with lethal yellowing and related diseases in coconut. In: Adkins S, Foale M, Bourdeix R, Nguyen Q, Biddle J (eds) Coconut biotechnology: towards the sustainability of the ‘Tree of Life.’ Springer, Cham, pp 169–197
Pech Aké A, Maust B, Orozco-Segovia A, Oropeza C (2007) The effect of gibberellic acid on the in vitro germination of coconut zygotic embryos and their conversion into plantlets. In Vitro Cell Dev Biol - Plant 43:247–253
Pech Aké AE, Souza R, Maust B, Santamaria JM, Oropeza C (2004) Enhanced aerobic respiration improves in vitro coconut embryo germination and culture. In Vitro Cell Dev Biol - Plant 40:90–94
Pérez-Núñez MT, Chan JL, Sáenz L, González T, Verdeil JL, Oropeza C (2006) Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. In Vitro Cell Dev Biol - Plant 42:37–43
Pham LJ (2016) Coconut (Cocos nucifera). In: Thomas A McKeon, Douglas G Hayes, David F Hildebrand, Randall J Weselake (eds) Industrial oil crops, Elsevier, Amsterdam, pp 231–242
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158-IN18
Polo-Marcial MH, Lara-Pérez LA, Goto BT, Margarito-Vista X, Andrade-Torres A (2021) Glomeromycota in Mexico, a country with very high richness. Sydowia 74:33–63
Prades A, Salum UN, Pioch D (2016) New era for the coconut sector. What prospects for research? Oilseeds Fats Crops Lipids 23:1–4
Roolant L (2014) Why coconut water is now a one billion industry. Available https://transferwise.com/blog/2014-05/why-coconut-water-is-now-a-1-billion-industry/. Cited 02 Nov 2019
Sáenz L, Chan JL, Narváez M, Oropeza C (2018) Protocol for the micropropagation of coconut from plumule explants. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Plant cell culture protocols. Springer, New York, pp 161–170
Sáenz-Carbonell L, Montero-Cortés M, Pérez-Nuñez T, Azpeitia-Morales A, Andrade-Torres A, Córdova-Lara I, Chan-Rodríguez JL, Sandoval-Cancino G, Rivera-Solis G, Oropeza-Salín C (2016) Somatic Embryogenesis in Cocos nucifera L. In: Loyola-Vargas V, Ochoa-Alejo N (eds) Somatic embryogenesis: fundamental aspects and applications. Springer, Cham, pp 297–318
Samosir Y, Adkins S (2014) Improving acclimatization through the photoautotrophic culture of coconut (Cocos nucifera) seedlings: an in vitro system for the efficient exchange of germplasm. In Vitro Cell Dev - Plant 50:493–501
Schultz C (2001) Effect of (vesicular-) arbuscular mycorrhiza on survival and post vitro development of micropropagated oil palms (Elaeis guineensis Jacq.) [phD. dissertation], University of Göttingen, pp 152
Senarathne SHS, Ilangamudali IMPS (2018) Effect of arbuscular mycorrhizal fungi based biofertilizer on coconut seedlings growth in nursery. CORD 34:12
Sisunandar A, Husin A, Julianto T, Yuniaty A, Rival A, Adkins SW (2018) Ex vitro rooting using a mini growth chamber increases root induction and accelerates acclimatization of Kopyor coconut (Cocos nucifera L.) embryo culture-derived seedlings. In Vitro Cell Dev Biol - Plant 54:508–517
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250
Soumare A, Diehdhiou AG, Arora NK, Tawfeeq Al-Ani LK, Ngom M, Fall S, Hafidi M, Ouhdouch Y, Kouisni L et al (2021) Potential role and utilization of plant growth promoting microbes in plant tissue culture. Front Microbiol 12:615
Talavera C, Contreras F, Espadas F, Fuentes G, Santamaría JM (2005) Cultivating in vitro coconut palms (Cocos nucifera) under glasshouse conditions with natural light, improves in vitro photosynthesis nursery survival and growth. Plant Cell Tiss Org Cult 83:287–292
Vafadar F, Amooaghaie R, Otroshy M (2014) Effects of plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J Plant Interact 9:128–136
Vázquez-Hernández MV, Arévalo-Galarza L, Jaen-Contreras D, Escamilla-García JL, Mora-Aguilera A, Hernández-Castro E, Cibrián-Tovar J, Téliz-Ortiz D (2011) Effect of Glomus mosseae and Entrophospora colombiana on plant growth, production, and fruit quality of ‘Maradol’ papaya (Carica papaya L.). Sci Hortic 128:255–260
Walker C, Redecker D, Thierfelder H, Walker C, Werner D (1997) Restriction analysis of PCR-amplified internal transcribed spacers of ribosomal DNA as a tool for species identification in different genera of the order Glomales. Appl Environ Microbiol 63:1756–1761
Willis A, Rodrigues BF, Harris PJ (2013) The ecology of arbuscular mycorrhizal fungi. Crit Rev Plant Sci 32:1–20
Wipf D, Krajinski F, van Tuinen D, Recorbet G, Courty PE (2019) Trading on the arbuscular mycorrhiza market: from arbuscules to common mycorrhizal networks. New Phytol 223:1127–1142
Zizumbo-Villareal D, Colunga-García M, Fernández-Barrera M, Torres-Hernández N, Oropeza-Salín C (2008) Mortality of Mexican coconut germplasm due to lethal yellowing. Plant Genet Resour Newsl 156:23–33
Acknowledgements
The authors thank Ph.D. Hassan Polo Marcial for his assistance during the taxonomic identification presented in this work.
Funding
This research was supported by CONACYT with the project (No. 2018–2021, FORDECYT 296195), the CONACYT scholarship No. 733853.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gómez-Falcón, N., Sáenz-Carbonell, L.A., Andrade-Torres, A. et al. Arbuscular mycorrhizal fungi increase the survival and growth of micropropagated coconut (Cocos nucifera L.) plantlets. In Vitro Cell.Dev.Biol.-Plant 59, 401–412 (2023). https://doi.org/10.1007/s11627-023-10345-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10345-5