Abstract
In this study, adventitious organogenesis from mature tissues of ‘Verna 51’ and ‘Fino 49’ lemon cultivars has been studied, as well as their relation with endogenous cytokinins and ethylene production. Thus, nodal explants in which buds were completely removed, obtained from in vitro shoot cultures of both lemon cultivars, were cultured in regeneration media, and the regeneration rate and the explants that regenerated were recorded. Also, after regeneration, endogenous cytokinin content was analyzed, as well as the ethylene emitted by the explants of both cultivars. After 8 wk in the culture medium, the explants of ‘Fino 49’ were more regenerative than those of ‘Verna 51’. Likewise, the endogenous content of cytokinins was globally higher in ‘Verna 51’, even if the levels of isopentenyladenine and isopentenyladenosine were higher in ‘Fino 49’. Additionally, ‘Verna 51’ explants had higher concentrations of zeatin than ‘Fino 49’, and the same content of N6-benzyladenine and N6-benzyladenosine. In like manner, the ethylene level emitted by the explants was higher in ‘Verna 51’. According to these results, the amount of endogenous cytokinins as well as the ethylene production by the explant plays an important role in the organogenesis of mature explants of C. limon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a genus, Citrus includes a large number of fruit tree species with high economic and nutritional values. From an economic point of view, lemon (Citrus limon [L.] Burm. f.) is the third most important citrus species in the world, having a special relevance in the Mediterranean basin. Thus, lemon growers demand improved and more productive cultivars that comprise a source of innovation in a very conservative market. Notwithstanding this, difficulties associated to the complex reproductive biology of citrus (namely, high level of heterozygosity, long juvenile periods, and poliembryony) (Deng and Duan 2006) have triggered the interest in biotechnological alternatives, such as genetic transformation, where a protocol for de novo regeneration is mandatory.
The regeneration potential of citrus varies with the genotype and explant type, but better results have usually been obtained from regeneration protocols involving the use of immature explants (Sajeva et al. 2008). Nevertheless, there are very few authors that have reported regeneration in lemon from this kind of tissues, mainly epicotyls (Carimi et al. 1994; Sajeva et al. 2008; Kasprzyk-Pawelec et al. 2015; Dutt et al. 2020), which is of scientific utility, but not useful from an agronomic point of view. Juvenile tissues could be different from the cultivar they come from and exhibit juvenile traits, which is undesirable for propagation, production, and genetic improvement: these tissues require several years of maturation before they can be evaluated for their horticultural traits (Navarro-García et al. 2016b). Here lies the importance of using mature tissues to ensure the presence of the rest of the characteristics we would like to preserve. In spite of this, recalcitrance linked with lemon is greater in adult tissues. In fact, only Navarro-García et al. (2016a; 2016b) have published a regeneration protocol in lemon from adult tissues. Therefore, there is an urge to boost the de novo regeneration potential of these tissues and unblind the inner mechanisms that trigger somatic regeneration.
It is well known that cytokinins are responsible for many processes related to the growth and development of the plant and the adventitious shoot regeneration in in vitro plants. Actually, cytokinins are key in tissue culture, inasmuch as their exogenous application triggers inner regeneration (Hnatuszko-Konka et al. 2021). The addition of exogenous cytokinins has an effect on the endogenous cytokinins of plants, activating a crosstalk between them that can end up inducing or inhibiting organogenesis (Zhang et al. 2010). Thus, some authors have tried to study endogenous changes in the concentration of cytokinins during de novo regeneration (Pérez-Jiménez et al. 2014a). However, to our knowledge, this has never been done before in citrus.
The interplay between hormonal signaling and stress signaling is key for defining cell fate and differentiation (Ikeuchi et al. 2019). Here, ethylene, its increment produced by stress, and its impossibility to be diluted in tissue culture due to the use of closed vessels, has made this hormone a target of study due to its effect on regeneration. Thus, some authors concluded that ethylene accumulation during the culture has a direct effect over organogenesis and must be regulated to optimize adventitious regeneration protocols (Arigita et al. 2003; Navarro-García et al. 2016a).
There are many factors controlling and affecting plant regeneration. However, cytokinins and the production of ethylene seem to be a major source of concern. Hence, in this study, the regeneration capacity of two lemon cultivars has been studied, as well as their relation with the endogenous cytokinin content along with the emission of ethylene during the process.
Material and Methods
Plant Material
Adult explants were derived from in vitro shoot cultures of Citrus limon, cultivars ‘Fino 49’ (F49) and ‘Verna 51’ (V51), that were previously developed in our laboratory (Pérez-Tornero et al. 2010). Explant preparation was carried out from healthy, green (not lignified), thick, elongated shoots using the standard procedures as described before (Tallón et al. 2013). After removing the leaves and pre-existing buds by running a sharp scalpel parallel to the stem, nodal explants were cut transversally into thin segments (5–10 mm). Nodal segments from the full explant, except the basal lignified segments, were used. Explants were placed horizontally in contact with the regeneration medium selected from previous experiments (Navarro-García et al. 2016b), which consisted on macronutrients, micronutrients, and vitamins of Murashige and Skoog medium (Murashige and Skoog 1962), 30 g l−1 of sucrose, 25 mg l−1 of phloroglucinol, 0.1 mg l−1 of indolebutyric acid (IBA), 6 g l−1 of agar (Pronadisa, Madrid, Spain), 2 mg l−1 of N6-benzyladenine (BA), and 1 mg l−1 of gibberellic acid (GA) added by filtration after medium sterilization. After adding plant growth regulators and adjusting the pH to 5.7, medium was sterilized in an autoclave at 121 °C for 21 min, and then dispensed aseptically (25 ml per sterile dish) into plastic Petri dishes measuring 9 cm in diameter and 1.5 cm in depth. Explants were incubated at 25 ± 1 °C in darkness for 2 or 4 wk, for F49 or V51 respectively, before being exposed to light with a 16-h photoperiod. Nodal explants were transferred to fresh medium every 4 wk.
For each cultivar, 5 replicates (Petri dishes) were prepared and each of them included at least 10 nodal segments. After 8 wk of incubation, the number of nodal explants forming adventitious buds and the number of buds formed per responsive explant were recorded. The assessment of nodal explants was carried out with a stereomicroscope. Adventitious buds that regenerated within the first 2 wk were considered developed from preexisting meristems and eliminated. The experiment was repeated twice.
Cytokinins Determination
The concentration of the main cytokinins in the nodal segments of F49 and V51 was analyzed using high-performance liquid chromatography (HPLC)/mass spectrometry (MS) (HPLC/MS) as described by Bacaicoa and García-Mina 2009 after the experiment (2009). The following cytokinins were studied: zeatin (Z), BA, N6-benzyladenosine (BAR), isopentenyladenine (iP), and isopentenyladenosine (iPR). The extraction and purification of the different plant regulators were carried out using the method described by Dobrev and Kamínek (2002) with some variations.
Frozen plant tissue (0.25 g; previously ground in a mortar to get a powder with liquid nitrogen) was homogenized with 5 ml of precooled (− 20 °C) methanol:water:formic acid (15:4:1, v/v/v). Deuterium-labeled CKs internal standards [2H5]-zeatin (D-Z), [2H7] N6-benzyladenine (D-BA), [2H7] N6-benzyladenosine (D-BAR), [2H6] N6-isopentenyladenine (D-iP), and [2H6] N6-isopentenyladenosine (D-iPR) from Olchemim were added to the extraction medium. After overnight extraction at –20 °C, solids were separated by centrifugation at 12,000 rpm for 10 min at 4 °C and were re-extracted for 1 h with an additional 4 ml of extraction mixture. Supernatants were passed through a Strata C18-E cartridge (3 cm3, 200 mg, 8B-S001-FBJ; Phenomenex) preconditioned with 4 ml of methanol followed by 2 ml of extraction medium. After evaporation at 40 °C to aqueous phase using an Eppendorf Vacufuge Plus Vacuum Concentrator, 2 ml of 1 M formic acid was added and applied to an Oasis MCX column (3 cm3, 60 mg, 186,000,254; Waters) preconditioned with 4 ml of methanol and 2 ml of 1 M formic acid. The column was washed successively with 2 ml of 1 M formic acid, 2 ml of methanol, and 2 ml of 0.35 M NH4OH, and the bases, ribosides, and glucosides of the CKs were eluted with 2 ml of 0.35 M NH4OH in 60% (v/v) methanol. This eluted fraction was evaporated to dryness in the evaporator and redissolved in 250 ml of methanol:0.05% formic acid (40:60, v/v). The solution was centrifuged at 8000 rpm for 5 min before being injected to the LC/MS/MS system.
The separation and analysis of samples were performed with an HPLC/MS system consisting of an Agilent 1290 Infinity II Series HPLC (Agilent Technologies, Santa Clara, CA) equipped with an Automated Multisampler module and a High-Speed Binary Pump, and connected to an Agilent 6550 Q-TOF Mass Spectrometer (Agilent Technologies) using an Agilent Jet Stream Dual electrospray (AJS-Dual ESI) interface. Experimental parameters for HPLC and Q-TOF were set in MassHunter Workstation Data Acquisition software (Agilent Technologies, Rev. B.08.00).
Standards or samples (20 μl) were thermostatted at 4 °C and injected onto a Zorbax Eclipse Plus C18 (2.1 × 100 mm, 1.8 µm) HPLC column, at a flow rate of 0.4 ml/min. Column was equilibrated at 40 °C. Solvents A (MilliQ water with 0.08% acetic acid) and B (acetonitrile with 0.08% acetic acid) were used for compound separation.
The mass spectrometer was operated in the positive mode. The nebulizer gas pressure was set to 30 psi, whereas the drying gas flow was set to 16 l min−1 at a temperature of 150 °C, and the sheath gas flow was set to 11 l min−1 at a temperature of 300 °C. The capillary spray, nozzle, fragmentor, and octopole 1 RF Vpp voltages were 4000 V, 500 V, 360 V, and 750 V, respectively. Profile data in the 50–500 m z−1 range were acquired for MS scans in 2 GHz extended dynamic range mode with 3 spectra s−1, 333.3 ms/spectrum, and 2722 transients/spectrum. Reference mass at 121.0509 was used for mass correction during the analysis. Data analysis was performed with MassHunter Qualitative Analysis Navigator software (Agilent Technologies, Rev. B.08.00).
Ethylene Determination
The ethylene level that was accumulated during the culture, for each lemon cultivar, was determined using the protocol previously described by Navarro-García et al. (2016a). Nodal segments preparation, medium sterilization, and culture conditions were as described previously.
To discard the amount of ethylene synthesized during the explant preparation, due to the stress produced by wounding, explants were cultured in Petri dishes and maintained in darkness for 1 wk in the regeneration medium before placing them in the glass tubes.
To measure ethylene production during the in vitro culture, explants were transferred to glass tubes (150 × 25 mm) containing 25 ml of the same medium used for regeneration and closed with silicone caps to avoid ethylene loss. The volume of gas inside the tube was similar to the volume of gas inside the Petri dishes. Tubes were kept in darkness during explant culture.
Ethylene accumulation inside of the tube was determined by taking a 0.5 ml sample from the headspace atmosphere of the glass tube, by means of a hypodermic syringe, 1 day (Ethylene I) and 7 days (Ethylene II) after the culture into the glass tube. The ethylene concentration accumulated in the sample was quantified by gas chromatograph (Hewlett–Packard 5890A model, Wilmington, DE), using a flame ionization detector (FID) and a stainless-steel column (3 m long and 3.5-mm internal diameter), filled with alumina (80/100 mesh). The temperature of the column was 90 °C, and the temperature of the injector and detector was 150 °C. Ethylene was identified by its retention time, which corresponds to 1.44 min, in the used conditions, compared with the chromatograms obtained with 10 ppm of an ethylene standard in nitrogen.
As in the regeneration experiment, five replicates (glass tubes) were prepared for each treatment, each of them containing at least 10 nodal segments. The full study was carried out twice.
Statistical Analysis
Data were first tested for homogeneity of variance and normality of distribution. Significance was determined by analysis of variance (ANOVA), and the significance (P < 0.05) of any differences between mean values was tested by Duncan’s new multiple range test, using Statgraphics Centurion® XVI (StatPoint Technologies Inc., The Plains, VA).
Results
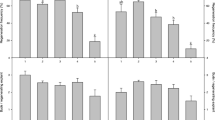
After the experiment, explants of both cultivars had induced de novo buds that further developed into new shoots (Fig. 1). The analysis of the results showed significant differences by genotype (P < 0.001) in both regenerating explant percentage and bud formation rate. F49 induced a higher regeneration than V51. Thus, significant differences were found in the percentage of regenerated explants, with 56% of the explants of F49 regenerating de novo buds against 30% of the explants in V51; almost twice as many explants (Fig. 2). Also, the bud formation rate was significantly higher in F49; over 1 bud per responsive explant was obtained in F49, decreasing to nearly 0.4 in V51. Therefore, rate was almost twofold in F49 (Fig. 2).
Regenerating explants percentage and de novo bud formation rate (number of buds formed per responsive explant) in nodal explants of lemon cv. ‘Fino 49’ and ‘Verna 51’ after being cultured in the regeneration medium. Data represent average ± SD values. Bars with different lowercase letters indicate a significant difference by LSD test (P ≤ 0.05).
Non-significant differences were detected on the endogenous BA and BAR concentration by genotype (P > 0.05), with similar results in both cultivars (Fig. 3). The endogenous amount of BA was around 0.15 ng ml−1 in both cultivars, while the BAR level was around 90 ng ml−1. However, genotype had a significant effect on the endogenous total cytokinins, Z, iP, and iPR levels (P < 0.001). The amount of iP and iPR detected in F49 was significantly higher than that of V51 (Fig. 3); the endogenous level of iP was almost 4 times the amount detected in V51 (over 2 ng ml−1), and iPR concentration was 20% higher in F49 than in V51. On the other hand, opposite results were obtained in Z and the total amount of cytokinins, since in both cases the endogenous amounts detected were higher in V51 than in F49 (Fig. 3). Z ranged from 800 ng ml−1 in F49 to 1200 ng ml−1 in V51, while the total level of cytokinins was about 300 ng ml−1 higher in V51 than in F49.
Endogenous content of N6-benzylaminopurine (BA), N.6-benzyladenosine (BAR), isopentenyladenine (iP), isopentenyladenosine (iPR), zeatin (Z), and total cytokinins (Cks) in explants of lemon cv. ‘Fino 49’ and ‘Verna 51’ after the culture in the regeneration medium. Data represent average ± SD values. Bars with different lowercase letters indicate a significant difference by LSD test (P ≤ 0.05).
When ethylene results were analyzed, no significant effect (P > 0.05) of genotypes was observed on the ethylene level emitted the first d of culture. However, after 7 d of culture, the cultivar had a significant effect on the ethylene level (P < 0.001). Ethylene concentration produced by V51 was 32% higher than in F49 (Fig. 4).
Discussion
Plant regeneration is a critical step in any protocol for plant transformation or mutation breeding by tissue culture, and depends on many factors, such as genotype, culture medium, plant growth regulators, type of explant or light conditions (Tallón et al. 2013; Ikeuchi et al. 2019). This has been evidenced in previous studies in lemon, where differences in the response to regeneration depended on culture media and conditions (Navarro-García et al. 2016a, b). In this experiment, F49 nodal segments showed a higher response to adventitious regeneration induction than V51 when both were cultured in the same conditions and media; therefore, differences in the results would be caused by the genotype, which would implement a different activation of mechanisms and molecules that would end up defining their responses. These differences could revolve around cytokinins, as they are well known as adventitious shoot regeneration inductors.
The addition of cytokinins to the culture media stimulates shoot proliferation and regeneration, and BA is the cytokinin that is most widely used in woody plants for this aim. Moreover, apart from being a synthetic cytokinin, BA is present in plants as an endogenous cytokinin. Plant tissues generally absorb and metabolize BA from the culture medium readily, modulating the endogenous concentration of other cytokinins (Zhang et al. 2010). This could be due to the common interaction between BA and the cytokinin receptor CRE1, which involves an impressive phytohormone crosstalk (Inoue et al. 2001).
Our results show that the endogenous contents of BA and BAR were similar in both cultivars, which may imply a homogenous uptake of the synthetic BA by the explants, both in F49 and in V51. However, the interaction between BA and the rest of the studied cytokinins varied according to the genotype, and different levels of iP, iPR, Z, and total amount of cytokinins were found in the two cultivars. Then, F49, which obtained the highest regeneration rate, showed higher levels of iP and iPR than V51, and lower levels of Z and total amount of cytokinins. Zhang et al. (2010) described a decrease in Z, and He et al. (2020) observed an increase in iPR, both due to the action of BA and related with an upturn of the regeneration process, which is in line with our results. However, although a part of this induction can be explained by the addition of BA to the culture media, the final result seems to depend on the genotype, which probably regulates the metabolic processes and their action over other processes, such as other cytokinins.
It is also noteworthy that, although cytokinins boost organogenesis by means of their action, this does not imply that a higher concentration of them necessarily results in a higher regeneration rate. In fact, in this experiment, V51 showed a higher content in total cytokinins than F49, along with a lower regeneration percentage. Previously, other authors found lower levels of cytokinins in genotypes that had the most pronounced reaction to the adventitious shoot regeneration induction than in those that regenerated less (Zhang et al. 2010; Pérez-Jiménez et al. 2014b). Also an inhibition of plant regeneration at high concentrations of cytokinins was observed (He et al. 2020). Therefore, it has been suggested that there must be a threshold of cytokinins where organogenesis falls and it is not favored any longer. It seems that this is not a matter of quantity, but accuracy.
Ethylene accumulation in glass vessels is believed to affect adventitious shoot regeneration in tissue cultured plants, relating high levels of ethylene with a lower regeneration (Arigita et al. 2003; Pérez-Jiménez et al. 2014b; Navarro-García et al. 2016a). In this study, the lower levels of ethylene in F49 after 7 days of culture could be related to a higher capacity to produce adventitious shoot proliferation in this experiment. Previous experiments in lemon with different ethylene modulators showed an increase in regeneration capacity when ethylene action or production was diminished (Navarro-García et al. 2016a), which agrees with other studies (Pérez-Jiménez et al. 2014a). However, mechanisms that underlie behind the interaction between ethylene and regeneration are unknown, and some authors have found the opposite relationship (Yasmin et al. 2014). Ethylene production is the result of stress in the plant, but not every process that causes stress produces the same response in this sense. Given the controversial results obtained by many authors concerning ethylene levels and regeneration, the possibility that the real modulator of the organogenetic process was the origin of the stress behind the ethylene production instead of the ethylene itself should be taken into consideration.
Conclusions
In this study, lemon regeneration in V51 and F49 has been studied, as well as their relation with endogenous cytokinins and ethylene production. The control of the changes produced in the endogenous cytokinins, along with the ethylene level inside the plate, could be key to regulate the de novo regeneration in lemon. However, the control of this aspect seems to be related to the genotype. Therefore, the possibility of controlling it by modulating the exogenous parameters is not clear. The amount of endogenous cytokinins, as well as the ethylene production by the explant, seems to be related to the organogenetic capacity of the explants. However, the mechanisms underlying this process are unknown.
References
Arigita L, Sánchez-Tamés R, González A (2003) 1-Methylcyclopropene and ethylene as regulators of in vitro organogenesis in kiwi explants. Plant Growth Regul 40:59–64
Bacaicoa E, García-Mina JM (2009) Iron-efficiency in different cucumber cultivars: the importance of the optimizing the use of foliar iron. J Am Soc Hortic Sci 134
Carimi F, De Pasquale F, Crescimanno FG (1994) Somatic embryogenesis from styles of lemon (Citrus limon). Plant Cell Tiss Organ Cult 37:209–211
Dobrev PI, Kamínek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr 950:21–29
Dutt M, Mahmoud LM, Grosser J (2020) Antioxidants, osmoprotectants and plasmolysis treatments enhance the Agrobacterium-mediated gene transfer and regeneration of difficult to transform lemon cultivars. HortSci 55(9)
He G, Yang P, Tang Y, Cao Y, Qi X, Ming J (2020) Mechanism of exogenous cytokinins inducing bulbil formation in Lilium lancifolium in vitro. Plant Cell Rep 39:861–872
Hnatuszko-Konka K, Gerszberg A, Weremczuk-Jeżyna I, Grzegorczyk-Karolak I (2021) Cytokinin signaling and de novo shoot organogenesis. Genes 12(2):265
Ikeuchi M, Favero DS, Sakamoto Y, Iwase A, Coleman D, Rymen B, Sugimoto K (2019) Molecular mechanisms of plant regeneration. Annu Rev Plant Biol 29(70):377–406
Inoue T, Higuchi M, Hashimoto Y, Sekl M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409:1060–1063
Kasprzyk-Pawelec A, Pietrusiewicz J, Szczuka E (2015) In vitro regeneration induced in leaf explants of Citrus limon L. Burm cv. ‘Primofiore.’ Acta Sci Pol Hortorum Cultus 14(4):143–153
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Navarro-García N, Martínez-Romero D, Pérez-Tornero O (2016) Assessment of the impact of ethylene and ethylene modulators in Citrus limon organogénesis. Plant Cell Tiss Organ Cult 127:405–415
Navarro-García N, Morte A, Pérez-Tornero O (2016) In vitro adventitious organogenesis and histological characterization from mature nodal explants of Citrus limon. In Vitro Cell Dev Biol-Plant 52(2):161–173
Pérez-Jiménez M, Cantero-Navarro E, Pérez-Alfocea F, Cos-Terrer J (2014) Endogenous hormones response to cytokinins with regard to organogenesis in explants of peach (Prunus persica L. Batsch) cultivars and rootstocks (P. persica x Prunus dulcis). Plant Physiol Biochem 84:197–202
Pérez-Jiménez M, Cantero-Navarro E, Pérez-Alfocea F, Cos-Terrer J (2014) Relationship between endogenous hormonal content and somatic organogenesis in callus of peach (Prunus persica L. Batsch) cultivars and Prunus persica x Prunus dulcis rootstocks. J Plant Physiol 171(8):619–24
Pérez-Tornero O, Tallón C, Porras I (2010) An efficient protocol for micropropagation of lemon from mature nodal segments. Plant Cell Tiss Organ Cult 100:263–271
Sajeva M, Carra A, de Pasquale F, Carimi F (2008) Somatic embryogenesis and plant regeneration from pistil transverse thin cell layers of lemon (Citrus limon). Plant Biosyst 142(2):199–203
Tallón CI, Porras I, Pérez-Tornero O (2013) High efficiency in vitro organogenesis from mature tissue explants of Citrus macrophylla and C. aurantium. In Vitro Cell Dev Biol Plant 49:145–155
Yasmin S, Mensuali-Sodi A, Perata P, Pucciariello C (2014) Ethylene influences in vitro regeneration frequency in the FR13A rice harbouring the SUB1A gene. Plant Growth Regul 72:97–103
Zhang F, Horgan KJ, Reynolds PHS, Jameson PE (2010) 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiata buds in vitro. Tree Physiol 30(4):514–526
Acknowledgements
The authors want to thank Fernando Córdoba for his technical assistance in the laboratory.
Funding
This work was supported by the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Pérez-Jiménez, M., Celdrán-Sánchez, V. & Pérez-Tornero, O. Role of endogenous cytokinins and ethylene in adventitious shoot regeneration in lemon (Citrus limon). In Vitro Cell.Dev.Biol.-Plant 58, 787–793 (2022). https://doi.org/10.1007/s11627-022-10286-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-022-10286-5