Abstract
A high-efficiency regeneration and genetic transformation system is indispensable for generating desirable traits in important trees such as Eucalyptus. However, lower regeneration efficiency is common for most varieties because of the recalcitrance of this genus. Here, a stable and highly efficient in vitro organogenesis protocol and Agrobacterium-mediated genetic transformation system of Eucalyptus were developed, and transgenic plants were obtained. In this protocol, the preferred explants were the top and middle stem internodes from in vitro micro-shoots of the E. urophylla × E. tereticornis hybrid. Modified Woody Plant Medium (mWPM) containing 0.025 mg·L-1 thidiazuron (TDZ) and 0.10 mg·L-1 indole-3-butyric acid (IBA) was used to induce multiple adventitious buds that allowed 85.6% shoot formation. The binary vector pBI121 carrying the neomycin phosphotransferase II (nptII) and β-glucuronidase (uidA) genes was applied for transformation. The preferred internodes were precultured for 0 to 3 d and infected with A. tumefaciens strain GV3101 grown to a bacterial density of 0.5 (OD600). Then, they were transferred to a co-culture medium supplemented with 50 μM acetosyringone (AS) and co-cultured for 2 d in the dark. The transgenic adventitious buds formed in regeneration medium, which was replaced by the same medium with a 2-wk subculture interval through kanamycin selection. Using the aforementioned method, transgenic plantlets can be obtained within 3 mo with a transformation frequency of 3.8%, which was verified by polymerase chain reaction amplification (PCR) and histochemical analysis of GUS activity. The constructed genetic transformation system will lay a foundation for mining gene functions and further molecular breeding of Eucalyptus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucalyptus originated in Australia and is cultivated worldwide as a multipurpose woody tree owing to its fast growth, great adaptability, and high wood quality. The timber from Eucalyptus is extensively used for pulp, energy, charcoal, lumber, and furniture. In particular, it is regarded as a commercial hardwood tree for the wood and paper industry. However, biotic (such as insect pests) and abiotic (such as climatic extremes like frost) stress factors cause damage to field plants leading to major economic losses. Hence, it is of great interest to cultivate trees with stress resistance.
Transgenic technology provides a way to solve these problems by transferring genes of interest into the plant to gain a desirable genotype. Moreover, the development of gene-editing technology also supplies a method to increase growth and endow resistance to improve biotic and abiotic tolerance. These methodologies rely on the construction of an efficient and stable genetic transformation system. In addition, Eucalyptus reverse genetic approaches to identify genes related to fast growth and high-quality wood formation in Eucalyptus also rely on a genetic transformation system.
An efficient regeneration system is a prerequisite for a highly efficient and stable genetic transformation system. Organogenesis is the most common way to obtain regenerated plants, which often use leaves (Lainé and David 1994; Mendonça et al. 2013), stems (Ouyang et al. 2020), cotyledons (Bandyopadhyay et al. 1999; Shwe and Leung 2020), and hypocotyls (Li et al. 2015; Oberschelp et al. 2015) as explants. Somatic embryogenesis has also succeeded in inducing adventitious buds in E. citriodora (Muralidharan and Mascarenhas 1987), E. globulus (Andrade et al. 2011), E. camaldulensis (Prakash and Gurumurthi 2010), and E. saligna × E. maidenii hybrid (Corredoira et al. 2015). Furthermore, multiple strategies have been chosen for the genetic transformation of eucalyptus, including electroporation, biolistics, and Agrobacterium-mediated transformations (Teulières et al. 1991; Manders et al. 1992; Rochange et al. 1995; Serrano et al. 1996; Ho et al. 1998; Moralejo et al. 1998; Sartoretto et al. 2002; Aggarwal et al. 2011; Ahad et al. 2014). Several reports have described genetically modified (GM) eucalyptus tree with improvement of cellulose and lignin biosynthesis or modification, salinity and cold tolerance, herbicide resistance, and biotic factor stress such as insects and diseases (Harcourt et al. 2000; Valério et al. 2003; Dibax et al. 2010; Navarro et al. 2011; Matsunaga et al. 2012; Ouyang et al. 2012a, b; Yu et al. 2013; de la Torre et al. 2014; García et al. 2014; Oguchi et al. 2014). Recently, a study showed that the overexpression of the FLOWERING LOCUS T (FT) from Arabidopsis thaliana in an E. grandis × E. urophylla hybrid (SP7) induced precocious flowering and normal reproductive development (Klocko et al. 2016).
Although many reports have succeeded in establishing transformation protocols with different Eucalyptus species and obtaining transgenic Eucalyptus plantlets in recent years, it is still difficult to break through the difficulties of regeneration for some elite cultivated clones, which seriously hinders the establishment of genetic transformation systems and further transgenic breeding. In addition, a large number of protocols have used seedling-derived explants, which are less attractive than cloned material owing to their similar genetic background. The shoots originated from seedling explants showed variations from the mother plant and other seedlings explants, which was unsuitable for propagation and further genetic transformation (Pena and Seguin 2001). Due to low transformation efficiency and regeneration capacity, the development of transgenic Eucalyptus has been delayed in comparison with other woody plants. Hence, it is critical to develop a high-efficiency genetic transformation protocol in Eucalyptus using clonal materials.
Interspecific hybrid of E. urophylla × E. tereticornis is an important and economic hardwood timber species for pulp and has been extensively cultivated in South China. Thus, there is urgent need to develop effective and efficient genetic transformation protocol for this hybrid species to acquire superior traits such as cold resistance, insect resistance, herbicide resistance, and high wood quantity and quality. Here, a high-frequency protocol of adventitious bud regeneration was established using stem internodes obtained from micropropagated plantlets as explants. Based on this, a stable and effective Agrobacterium-mediated genetic transformation protocol of E. urophylla × E. tereticornis hybrid was optimized, given the stable genomic T-DNA insertion and transgene expression. This study will be helpful for analyzing the function of eucalypts genes and the development of GM E. urophylla × E. tereticornis hybrid plants with superior traits in the foreseeable future.
Materials and methods
Plant material
Tillers of E. urophylla × E. tereticornis clone YL02 were cut off and divided into segments, treated with 75% ethanol for 1 min, sterilized with 0.1% mercuric chloride (HgCl2) for 5 min, rinsed three times with sterile water, and transferred to a sterile 350-mL culture bottle (Dingguo, Beijing, China). The axillary buds were germinated and maintained on modified Murashige and Skoog (MS; Murashige and Skoog 1962) medium (mMS) supplemented with 0.5 mg·L-1 6-benzylaminopurine (BAP) and 0.1 mg·L-1 α-naphthaleneacetic acid (NAA) under a 16-h photoperiod (100 μmol·m-2·s-1) at 25 ± 2°C. Fresh and similar propagation medium was replaced every 20 d. The 2- to 3-cm buds were selected for transfer to adventitious root-inducing medium (1/2 MS supplemented with 0.1 mg·L-1 NAA). All plant regulators and MS medium were purchased from Duchefa Biochemie (Haarlem, The Netherlands). Then, the leaves and stem internodes from shoots cultured for 1 mo were used as explants. All media contained 30 g·L-1 sucrose and 7 g·L-1 agar (Dingguo, Beijing, China), and the pH was adjusted to 5.8. Then, they were transferred to an autoclave sterilizer for sterilization for 20 min at 121 °C.
Development of an in vitro regeneration protocol for clone YL02
In vitro callus induction and adventitious bud regeneration
The leaves and internode segments were obtained from micropropagation plantlets and cultured in 90 × 15 mm sterile Petri dishes (Dingguo, Beijing, China) with liquid mWPM to induce adventitious bud regeneration. To optimize the regeneration medium for obtaining a high frequency of adventitious bud induction, the effects of various concentrations of thidiazuron (TDZ, 0.005, 0.0075, 0.010, 0.025, 0.050, and 0.075 mg·L-1) and indole-3-butyric acid (IBA, 0.05, 0.10, 0.20, 0.40, and 0.80 mg·L-1) on callus and adventitious bud induction were investigated. In addition, the position of internode segments was also checked in this study. After an incubation of 8 wk, the leaves and internode segments were transferred to mMS medium supplemented with 0.5 mg·L-1 BAP and 0.1 mg·L-1 NAA for shoot elongation. All plant regulators and WPM medium were purchased from Duchefa Biochemie (Haarlem, The Netherlands). Each treatment involved 180 explants, and each experiment was performed in at least three replicates. The regeneration rate of adventitious buds and the number of regenerated buds per explant were calculated to determine the optimal regeneration medium, while growth parameters were also observed daily. All plant materials were cultured under a 16-h photoperiod (100 μmol·m-2·s-1) at 25 ± 2 °C.
In vitro rooting and acclimatization
Individual buds of 3 cm were cut off and transferred to 1/2 MS medium supplemented with 0.1 mg·L-1 NAA to induce adventitious roots. Subsequently, shoots with roots were transplanted into potting soil and maintained in a greenhouse.
Determination of the critical concentration of kanamycin for selection
To confirm the critical concentration of kanamycin in selecting the transformed plant process, the sensitivity toward kanamycin was tested on shoot and root organogenesis by cultivation on optimum medium containing 0, 50, 70, 90, 110, and 130 mg·L-1 kanamycin (Duchefa Biochemie). During the adventitious bud induction periods, fresh and similar media were replaced every 2 wk. After 8 wk, the occurrence frequency of adventitious buds was recorded. The frequency of adventitious root induction was statistically analyzed after culture for 4 wk. Each treatment involved 120 explants, and all experiments were performed in at least three replicates.
Agrobacterium-mediated genetic transformation
Plasmid and Agrobacterium strains
Three strains of A. tumefaciens (GV3101, LBA4404 and EHA105), which represent different types of opines such as nopaline, octopine, and succinamopine, were used to infect explants. The vector pBI121 containing the selectable marker nptII gene and reporter uidA gene was applied to construct a genetic transformation system. Agrobacterium were cultured in liquid Luria–Bertani (LB, Duchefa Biochemie) medium with 50 mg·L-1 kanamycin and 20 mg·L-1 rifampicin (Duchefa Biochemie) and grown for 48 h at 28 °C and 150 rpm with agitation in incubator shaker ISRDD3 (Crystal, Dallas, TX) until the OD600 was approximately 0.6. The Agrobacterium cells were centrifuged for 10 min at 4000 rpm in a Beckman Allegra X-30R centrifuge (Beckman Coulter, Brea, CA), and precipitated cells were resuspended in an equal volume of liquid adventitious bud induction medium.
Optimization of genetic transformation system
The top and medium internode segments were cut from plantlets that were induced to root for 1 month and were precultured for 0 to 15 d. Then, the explants were immersed in Agrobacterium suspension for 15 min with different ultrasonic treatment times (0, 10, 20, 30, 40, and 50 s). After infection, explants were removed from the bacterial suspension and blotted with sterile filter paper. Then, different volumes of additive sterile water (0, 100, 200, 300, 400, and 500 μL) were added to eliminate the excess bacterial cells and medium. The internode segments were transferred to optimum adventitious bud-inducing medium with 10, 50, 100, and 200 μM acetosyringone (AS, Sigma-Aldrich, St. Louis, MO) for co-cultivation for 2 to 4 d at 25 °C in darkness. Then, the infected explants were transferred to fresh optimum adventitious bud regeneration medium containing 200 mg·L-1 cefotaxime (Duchefa Biochemie) and 130 mg·L-1 kanamycin (Selection Medium I, SM I). Fresh SM I was altered every 14 d until the resistant adventitious buds regenerated. They were transferred to mMS medium supplemented with 0.5 mg·L-1 BAP, 0.1 mg·L-1 NAA, 200 mg·L-1 cefotaxime, and 130 mg·L-1 kanamycin (Selection Medium II, SM II) for shoot elongation. Adventitious roots were induced when resistant shoots were transferred to 1/2 MS medium supplemented with 0.1 mg·L-1 NAA, 200 mg·L-1 cefotaxime, and 110 mg·L-1 kanamycin. The transient transformation efficiency was tested by GUS histochemical assay when explants were cultured in SM I for 3 d. The frequency of adventitious bud induction was collected after 8 wk of culture, whereas phenotypic characteristics were observed and recorded.
GUS histochemical assay
Histochemical GUS staining in the putatively transgenic explants, calluses, and plantlets was performed as previously described (Jefferson et al. 1987). The plant materials were immersed at 37 °C for 24 h in a reagent that contained 2 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-Gluc), 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, 0.1 M Na-phosphate buffer (pH = 7.0), and 0.1% (v/v) Triton X-100. Following incubation, the GUS assay solution was poured away and replaced with 70% ethanol to remove chlorophyll.
DNA extraction and polymerase chain reaction (PCR) analyses
Genomic DNA was extracted from the control and putative transgenic plantlets by using the modified CTAB method and tested by PCR amplification using primers specific to the nptII gene (F: TTCTCCCAATCAGGCTTG; R: GCTATGGCTGGAAGGAAA) and uidA gene (F: GTCGCGCAAGACTGTAACCA; R: CGGCGAAATTCCAT-ACCTG). Q5 High-fidelity DNA polymerase (NEB, Ipswich, MA) was used to amplify PCR products. The size of uidA products was 1081 bp, and PCR analysis was performed according to the following parameters. First, there was 98 °C denaturation for 3 min. Second, 35 cycles were followed with 98 °C denaturation for 10 s, 65 °C annealing for 15 s, and 72 °C extension for 20 s. Third, final extension was set at 72 °C for 2 min. The size of nptII product was 567 bp and the same analysis was performed except for an annealing temperature setting at 63 °C. The amplified products were electrophoresed on 1.2% agarose gels in 1 × TAE (Tris-acetate-EDTA) buffer.
Statistical analysis
All experiments were performed in at least three replicates, and each treatment involved 120 explants at least. Data were transformed by the following formula before analyzed using one-way ANOVA followed by Duncan’s multiple range test. Different letters on graphs indicate a significant difference between means (P = 0.05).
Results
Establishment of an efficient regeneration protocol of E. urophylla × E. tereticornis
Whether leaves or stem internodes were used as explants, adventitious buds were successfully induced after culturing in mWPM supplemented with TDZ for 2 mo. The regeneration rate of adventitious buds was highest when TDZ was 0.025 mg·L-1 (Fig. 1A,B). However, the regeneration rate of stem internodes was higher than that of leaves overall. Hence, internode segments were chosen as explants for the following study. Based on the optimal TDZ concentration, the effect of different concentrations of IBA was evaluated in the establishment of the regeneration system. The results showed that 0.10 mg·L-1 IBA combined with 0.025 mg·L-1 TDZ was suitable for inducing adventitious buds (Fig. 1C). In our research, we also found that the condition of internode segments could affect the frequency of regeneration; therefore, we tested the regeneration capacity of the internode segments of different positions (top, middle, and bottom). As shown in Fig. 1D, the frequency of adventitious bud induction reached 85.6% in mWPM supplemented with 0.025 mg·L-1 TDZ and 0.10 mg·L-1 IBA by using the upper internode segments as explants.
Adventitious bud induction rate of Eucalyptus urophylla × Eucalyptus tereticornis clone YL02 after 8 wk of culture on regeneration medium. (A) Effects of thidiazuron (TDZ) concentration on adventitious bud induction using stems as explants. (B) Effects of TDZ concentration on adventitious bud induction using leaves as explants. (C) Effects of indole-3-butyric acid (IBA) concentration combined with 0.025 mg·L-1 TDZ on adventitious bud induction using stem explants. (D) Effects of the position of stem segments on adventitious bud induction. Numbers in the bar graph represent the data as counts. Numbers before “/” represent the number of regenerated explants. Numbers after “/” represent the number of total explants. The means and standard errors were calculated from triplicate repeats. Duncan’s multiple range test was used at P = 0.05, and the same letters show no significant differences.
When stem internodes were cultured on adventitious bud induction medium, the two ends of internode segments began to expand and displayed dumbbells after 6 d (Fig. 2A). Then, dark red and compact calluses were observed after culture for 15 d (Fig. 2B). By 21 d, the calluses continued to swell and further developed into fewer and obscure buds (Fig. 2C). The obscure buds propagated, and a dome-like regenerating structure appeared after 42 d (Fig. 2D). By 56 d, multiple adventitious buds were visible, and more than 5 adventitious buds regenerated on each explant (Fig. 2E).
The complete regeneration process is shown in Fig. 3. The upper stem internodes were cut and cultured in mWPM supplemented with 0.025 mg·L-1 TDZ and 0.10 mg·L-1 IBA (Fig. 3A). After 2 mo of culturing, multiple adventitious buds were generated (Fig. 3B). They continued to grow by elongation when transferred to mMS medium containing 0.5 mg·L-1 BAP and 0.1 mg·L-1 NAA (Fig. 3C). Individual buds began to grow roots in 1/2 MS medium supplemented with 0.1 mg·L-1 NAA (Fig. 3D).
Regeneration of Eucalyptus urophylla × Eucalyptus tereticornis clone YL02 from stem internodes. (A) Stem internode segments from in vitro propagation plantlets. (B) Callus and multiple adventitious bud induction after 2 mo of culture on modified Woody Plant Medium (mWPM) containing 0.025 mg·L-1 thidiazuron (TDZ) and 0.10 mg·L-1 indole-3-butyric acid (IBA). (C) Elongation of adventitious buds on modified Murashige and Skoog (mMS) supplemented with 0.5 mg·L-1 6-benzylaminopurine (BAP) and 0.1 mg·L-1 α-naphthaleneacetic acid (NAA). (D) Rooting of regenerated plantlets cultured on 1/2 Murashige and Skoog (MS) with 0.1 mg·L-1 NAA.
Determination of the critical concentration of kanamycin for selection
Cefotaxime sodium is widely used to eliminate the overgrowth of Agrobacterium after co-culture. The frequency and morphology of the regenerated shoots and roots remained stable under 250 mg·L-1 cefotaxime treatment (data not shown). Cefotaxime (200 mg·L-1) was applied in the selection medium to remove the remaining Agrobacterium effectively and to lessen the cost. Furthermore, kanamycin was specifically used to select successfully transformed cells with the nptII gene. To determine the appropriate concentration of kanamycin for screening transgenic plants, the inhibitory effects of kanamycin on adventitious buds and root induction were investigated. We noted that the effect of kanamycin at low concentrations on shoot organogenesis was not obvious (Fig. 4A). However, the capacity of regeneration for explants declined sharply with increasing kanamycin. Almost all internode segments browned and died when 130 mg·L-1 kanamycin was added. Hence, 130 mg·L-1 kanamycin was determined for screening putative transgenic plants. Similarly, adventitious root occurrence was totally inhibited when buds were cultured in root-inducing medium containing 130 mg·L-1 kanamycin (Fig. 4B). However, the difference did not differ statistically from 110 mg·L-1 kanamycin in adventitious root induction. Thus, 110 mg·L-1 kanamycin was used for adventitious root induction.
Effect of kanamycin on adventitious bud and root induction of Eucalyptus urophylla × Eucalyptus tereticornis clone YL02. (A) Regeneration percentage of adventitious buds from stem explants cultured for 8 wk. (B) Regeneration percentage of adventitious roots from individual buds cultured for 4 wk. The means and standard errors were calculated from triple repeats. Duncan’s multiple range test was used at P = 0.05, and the same letters show no significant differences.
Setup of the genetic transformation protocol
The effect of the A. tumefaciens strain was tested in this study owing to its essential role in genetic transformation. Our results showed that a higher frequency of GV3101-infected calluses yielded GUS activity (39.4%) than LBA4404 (20.3%) and EHA105 (35.2%). GV3101 was selected for further optimization of transformation efficiency due to its higher reproductive speed, although the difference between GV3101 and EHA105 was not significant (Fig. 5A). Moreover, we found that additional AS in the co-culture medium significantly affected transient GUS activity (Fig. 5B). The maximum transient GUS activity (50.0%) was obtained when the co-culture medium was supplemented with 50 μM AS. Prolonging the preculture period did not promote foreign gene transfer and expression. Explants cultured less than 3 d before inoculation had a higher expression frequency than explants cultured for a longer time (Fig. 5C). As shown in Fig. 5D, there was no significant difference in the frequency of stained GUS between 48, 72, and 96 h of co-culture. The duration of co-culture was set as 48 h to remove the remnant Agrobacterium more easily. Other factors (ultrasonic treatment during infection and additive sterile water after infection) seemed to be unhelpful in increasing the transformation efficiency (Fig. 5E,F).
Factors that affect the transient transformation efficiency of Eucalyptus urophylla × Eucalyptus tereticornis clone YL02. (A) Agrobacterium strain. (B) Concentration of acetosyringone (AS) supplemented in co-culture medium. (C) Preculture period. (D) Duration of co-cultivation. (E) Duration of sonication. (F) Volumes of additive sterile water. The means and standard errors were calculated from triplicate repeats. Duncan’s multiple range test was used at P = 0.05, and the same letters show no significant differences.
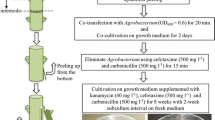
Consequently, an optimal genetic transformation protocol of YL02 was established based on GUS-staining analysis. The upper stem internodes were infected with A. tumefaciens strain GV3101 when the OD600 reached 0.5. Then, the infected explants were transferred to co-culture medium supplemented with 50 μM AS and cultivated at 25 °C for 48 h. After co-cultivation, stem internodes were transferred to SM I and cultured for 4 to 8 wk until kanamycin-resistant calluses formed (Fig. 6A–C). Then, adventitious buds gradually appeared on selection medium after 12 wk (Fig. 6D), and resistant shoots gradually elongated when transferred to SM II. Finally, the elongated shoots were cut and transferred to root-inducing medium, and putative transgenic plants were obtained.
Regeneration of Eucalyptus urophylla × Eucalyptus tereticornis transgenic plantlets and GUS activity (blue color) expression in the presence of 130 mg·L-1 kanamycin. Kanamycin-resistant calluses and shoots formed when cultured for (A) 2 wk, (B) 4 wk, (C) 8 wk, and (D) 12 wk. (E–F) Histochemical staining for GUS activity in kanamycin-resistant calluses and buds at the corresponding times.
Assessment of transgenic plants by GUS staining and PCR analysis
To further verify the insertion of T-DNA from A. tumefaciens into the genome of putative transgenic plants, genomic DNA was extracted and amplified specific to the nptII and uidA genes by using PCR. The results showed that the uidA and nptII genes were present in the genomes of several lines (Fig. 7). Moreover, the regenerated calluses and shoots that showed kanamycin resistance were also analyzed by GUS staining to describe the expression of the uidA gene. The kanamycin-resistant calluses and multiple adventitious buds were stained distinctly blue (Fig. 6E–H), whereas blue staining was not detected in the control. In total, 23 independent kanamycin-resistant plants were obtained (11.0%) from 210 infected explants, and 8 plants had target genes with a transformation efficiency of 3.8%.
PCR amplification of the uidA gene (1.0 kb) and nptII gene (0.6 kb) in selected Eucalyptus urophylla × Eucalyptus tereticornis transformed plantlets. Lane M: DNA marker; lane +: positive control, plasmid pBI121 as template; lane -: negative control, genomic DNA from a nontransformed plantlet as template; lane W: negative control, ddH2O as template; and lanes 1–9: genomic DNA from plants of 23 putatively transformed lines carrying the uidA gene and nptII gene.
Discussion
Eucalyptus has become one of the most planted hardwood trees worldwide and serves as an important source of paper and wood. The potential of biotechnology in Eucalyptus development has been recognized, and various research groups have developed much work in the last two decades. However, there are only a few reports available focusing on the production of transgenic Eucalyptus because of the absence of a desirable genetic transformation protocol. A stable and efficient genetic transformation system is essential for molecular breeding and gene function identification in Eucalyptus. Here, our study described an efficient and reproducible organogenesis and A. tumefacien-mediated transformation protocol with a 3.8% transformation frequency under various optimal factors in E. urophylla × E. tereticornis clone YL02. An efficient, reproducible, and stable regeneration system is a prerequisite for the establishment of a genetic transformation system in Eucalyptus. Hence, various studies have developed regeneration systems in Eucalyptus species, such as E. camaldulensis (Mullins et al. 1997), E. urophylla (Huang et al. 2010; Ouyang et al. 2012a, b; Li et al. 2015), E. tereticornis (Aggarwal et al. 2011), E. globulus (Azmi et al. 1997), E. saligna (Silva et al. 2015), and E. grandis × E. urophylla (de Alcantara et al. 2011). Notably, most explants were from seedlings or other sexual reproductive materials, such as cotyledons and hypocotyls, which showed higher regeneration efficiency than asexual materials from plants of selected clones. Unfortunately, adventitious buds induced from seeds or seedlings could produce various phenotypes and variations, which could not be used for further gene function identification and molecular breeding of Eucalyptus. Adventitious shoots originating from clonal material have the same genetic background and retain all the merits of the original elite tree, and the resulting transgenic plants could be applied for gene identification or direct cultivation. Hence, it was necessary to develop an efficient regeneration system for the selected superior clones of Eucalyptus using asexual material. In a previous study, a series of adventitious bud induction experiments were performed on 9 clones of E. urophylla × E. tereticornis by using leaves as explants (Fan et al. 2015). YL02 showed a stable and relatively high regeneration rate, which was suitable for further construction of regeneration and transformation systems. Moreover, a relatively smaller callus was produced in adventitious bud induction medium, which was appropriate for selecting antibiotic transgenic shoots. In addition, it was easier to propagate shoots and induce roots and these could also provide large quantities of explants for developing regeneration and transformation.
Plant growth regulators are of great importance to plant development and regeneration. In the process of adventitious bud induction, cytokinins BAP, ZT and other growth regulators were successfully adopted to construct regeneration systems (Serrano et al. 1996; Mullins et al. 1997; Ho et al. 1998; Diwakar et al. 2010; Huang et al. 2014; Silva et al. 2015). Recently, TDZ was often used as the main plant growth regulator to induce regeneration in Eucalyptus (Deepika et al. 2011; Huang et al. 2014; de França Bettencourt et al. 2020). In the current study, TDZ showed a significant effect on stimulating adventitious shoot initiation and formation. TDZ (0.025 mg·L-1) induced yellow green and compact calluses and generated multiple, healthy, strong shoots, which resulted in the highest percentage of shoot regeneration. In contrast, 0.25 μM TDZ supplemented with 0.1 μM NAA showed the most shoot induction, with a regeneration rate of 43% in the E. grandis × E. urophylla AEC 224 clone (de Oliveira et al. 2017). According to the result, it could be speculated that different optional concentrations of TDZ were related to different species owing to their genetic backgrounds. It was obvious that more TDZ would enlarge the calluses and produce abnormal shoots with vitrification and dwarfing. A similar result was obtained in E. microtheca, which showed that TDZ in small amounts stimulated regeneration and that more additions could decrease regeneration (Shabannejad Mamaghani et al. 2009). This abnormal morphogenesis may be relevant to the nondegradable nature of TDZ, which is a urea-based compound, and there are no specific oxidase enzymes in cells that distinguish TDZ from natural phytohormones (Novikova and Zaytseva 2018).
Furthermore, the type of explant also significantly affected regeneration. The regeneration rate of stems was higher than that of leaves in the present study, which showed a difference from E. gunnii, with only an approximately 10% regeneration rate (Hervé et al. 2001). Interestingly, internodes proximal to the apical meristem showed a higher regeneration rate than those distal to the apical meristem as a result of the higher degree of lignification in the lower stem segments. A similar result was also found in in vitro adventitious bud regeneration of beech, which showed that the morphogenic response varied significantly with the position of the internode (Cuenca et al. 2000).
The selection of optimized concentrations of antibiotics was necessary to construct a stable genetic transformation and avoid the escape of untransformed plants. In a previous study, a vast difference was shown in antibiotic tolerance. Kanamycin (17.5 mg·L-1) significantly inhibited adventitious bud induction in E. grandis (Guo et al. 2012), while 50 mg·L-1 kanamycin in the selection medium was necessary in E. urophylla (de França Bettencourt et al. 2020). However, 90 mg·L-1 kanamycin could completely prevent adventitious bud regeneration in E. urophylla × E. camaldulensis clone DH201-2 (Fan et al. 2009). In the present study, none of the explants regenerated adventitious buds under 130 mg·L-1 kanamycin treatment, while occasional buds emerged under 110 mg·L-1 kanamycin. To avoid killing more transformed buds, 110 mg·L-1 kanamycin was set as a screening pressure. In addition, optimization of hygromycin showed that 15 mg·L-1 and 9 mg·L-1 hygromycin significantly inhibited bud regeneration and root induction, respectively. Hence, hygromycin as a selection abiotic will be tested in the future to determine whether it could improve transformation efficiency.
The achievement of genetic transformation depends on matching the inoculated explants to the suitable Agrobacterium strain (Tzfira and Citovsky 2006). Strain EHA105 showed higher efficiency than other strains in E. tereticornis (Aggarwal et al. 2011), while EHA101 was considered a suitable strain in E. globulus (Moralejo et al. 1998). In contrast, LBA4404 produced higher transformation efficiency in E. grandis × E. urophylla (Wang et al. 2013). In E. urophylla × E. tereticornis clone YL02, the highest transformation efficiency was derived from GV3101- and EHA105-infected explants, which might be due to different sensitivities to Agrobacterium strains in various species of Eucalyptus. Exogenous AS could improve transformation efficiency by enhancing the expression of the vir region of the Ti plasmid and further promoting the transfer of T-DNA to plant genomes (Godwin et al. 1991). In Eucalyptus, it was also found that AS could enhance the efficiency of genetic transformation (de Alcantara et al. 2011; Silva et al. 2011). Similar results showed that 50 μM AS in adventitious bud-inducing medium noticeably improved the frequency of transformation by 8.8% in this study.
In this study, the optimization of the preculture and co-cultivation periods was also important in improving transformation efficiency. Two days of preculture enhanced the expression of the uidA gene compared with explants cultured for 1 d or without preculture in E. grandis × E. urophylla (de Alcantara et al. 2011). In contrast, transformation efficiency showed no significant increases with increasing preculture time. In the co-culture process, the wounds of the explants were fully exposed to Agrobacterium so that the foreign genes had sufficient time to integrate into the genome of the plants. It was reported that 1-day co-cultivation showed better results in the genetic transformation of E. camaldulensis (Ahad et al. 2014). For E. tereticornis, the maximum transient GUS activity occurred when explants were co-cultivated for 2 d (Aggarwal et al. 2011). The longer co-culture period may increase the GUS staining rate slightly, while the prolonged co-culture time could result in overgrowth of bacteria and hardness of sterilization. In our study, the co-culture period had no obvious effect on transformation efficiency, and 48 h was sufficient for the transfer of foreign genes. Based on these results, an Agrobacterium-mediated genetic transformation system was established, and transgenic plants were obtained. To further improve the frequency of transformation for gene function identification and molecular breeding, it was necessary to delicately optimize the transformation protocol in Eucalyptus, such as adjusting selection methods and procedures.
Conclusions
A highly efficient in vitro organogenesis and Agrobacterium-mediated genetic transformation system of E. urophylla × E. tereticornis clone YL02 was developed. The top and middle stem internodes cultured in mWPM medium containing 0.025 mg·L-1 TDZ and 0.10 mg·L-1 IBA showed 85.6% shoot formation. Several factors were optimized for genetic transformation of YL02. Agrobacterium tumefaciens strain GV3101 and the addition of 50 μM AS to adventitious bud-inducing medium significantly increased the frequency of transformation. Three mo later, transgenic plants were obtained with 130 mg·L-1 kanamycin selection, and the transformation frequency was 3.8%.
References
Aggarwal D, Kumar A, Sudhakara Reddy M (2011) Agrobacterium tumefaciens mediated genetic transformation of selected elite clone(s) of Eucalyptus tereticornis. Acta Physiol Plant 33:1603–1611
Ahad A, Maqbool A, Malik KA (2014) Optimization of Agrobacterium tumefaciens mediated transformation in Eucalyptus camaldulensis. Pak J Bot 2:735–740
Andrade G, Shah R, Johansson S, Pinto G, Egertsdotter U (2011) Somatic embryogenesis as a tool for forest tree improvement: a case- study in Eucalyptus globulus. BMC Proceedings 5:128
Azmi APEM, Noin M, Landre P, Prouteau M, Boudet AM, Chriqui D (1997) High frequency plant regeneration from Eucalyptus globulus Labill. hypocotyls: ontogenesis and ploidy level of the regenerants. Plant Cell Tiss Org Cult 51:9–16
Bandyopadhyay S, Cane K, Rasmussen G, Hamill JD (1999) Efficient plant regeneration from seedling explants of two commercially important temperate eucalypt species-Eucalyptus nitens and E. globulus. Plant Sci 140:189–198
Corredoira E, Ballester A, Ibarra M, Vieitez AM (2015) Induction of somatic embryogenesis in explants of shoot cultures established from adult Eucalyptus globulus and E. saligna × E. maidenii trees. Tree Physiol 35:678–690
Cuenca B, Ballester A, Vieitez AM (2000) In vitro adventitious bud regeneration from internode segments of beech. Plant Cell Tiss Org Cult 60:213–220
de Alcantara GB, Filho JCB, Quoirin M (2011) Organogenesis and transient genetic transformation of the hybrid Eucalyptus grandis × Eucalyptus urophylla. Sci Agr 68:246–251
de França Bettencourt GM, Soccol CR, Giovanella TS, Franciscon L, Kestring DR, Gerhardt IR, Degenhardt-Goldbach J (2020) Agrobacterium tumefaciens-mediated transformation of Eucalyptus urophylla clone BRS07-01. J Forestry Res 31:507–519
de la Torre F, Rodríguez R, Jorge G, Villar B, Álvarez-Otero R, Grima-Pettenati J, Gallego PP (2014) Genetic transformation of Eucalyptus globulus using the vascular-specific EgCCR as an alternative to the constitutive CaMV35S promoter. Plant Cell Tiss Org Cult 117:77–84
de Oliveira C, Degenhardt-Goldbach J, de França Bettencourt GM, Amano E, Franciscon L, Quoirin M (2017) Micropropagation of Eucalyptus grandis × E. urophylla AEC 224 clone. J Forestry Res 28:29–39
Deepika R, Veale A, Ma C, Strauss SH, Myburg AA (2011) Optimization of a plant regeneration and genetic transformation protocol for Eucalyptus clonal genotypes. BMC Proceedings 5:P132
Dibax R, Deschamps C, Filho JCB, Vieira LGE, Molinari HBC, De Campos MKF, Quoirin M (2010) Organogenesis and Agrobacterium tumefaciens-mediated transformation of Eucalyptus saligna with P5CS gene. Biol Plant 54:6–12
Diwakar A, Anil K, M SR (2010) Shoot organogenesis in elite clones of Eucalyptus tereticornis. Plant Cell Tiss Org Cult 102:45–52
Fan C, Wang X, Qiu Z, Zeng B, Liu Y, Li X (2015) Effect of TDZ in vitro culture and plant regeneration from leaves of Eucalyptus urophylla × E. tereticornis clones. Chinese J Trop Agr 35:37–40
Fan C, Zeng B, Qiu Z, Liu Y, Li X, Chen L (2009) Establishment of genetic transformation of Eucalyptus urophylla × E. camaldulensis clone DH201. J Zhejiang For Sci Tech 29:15–20
García JR, Anderson N, Le-Feuvre R, Iturra C, Elissetche J, Chapple C, Valenzuela S (2014) Rescue of syringyl lignin and sinapate ester biosynthesis in Arabidopsis thaliana by a coniferaldehyde 5-hydroxylase from Eucalyptus globulus. Plant Cell Rep 33:1263–1274
Godwin I, Todd G, Ford-Lloyd B, Newbury HJ (1991) The effects of acetosyringone and pH on Agrobacterium-mediated transformation vary according to plant species. Plant Cell Rep 9:671–675
Guo L, Zeng B, Liu Y, Li X, Qiu Z (2012) Study on kanamycin and cefotaxime sensitivity of Eucalyptus grandis clone Eg5. J Cent South Univ Forest Technol 32:75–80
Harcourt RL, Kyozuka J, Floyd RB, Bateman KS, Tanaka H, Decroocq V, Llewellyn DJ, Zhu X, Peacock WJ, Dennis ES (2000) Insect- and herbicide-resistant transgenic eucalypts. Mol Breed 6:307–315
Hervé P, Jauneau A, Pâques M, Marien J, Michel Boudet A, Teulières C (2001) A procedure for shoot organogenesis in vitro from leaves and nodes of an elite Eucalyptus gunnii clone: comparative histology. Plant Sci 161:645–653
Ho CK, Chang SH, Tsay JY, Tsai CJ, Chiang VL, Chen ZZ (1998) Agrobacterium tumefaciens-mediated transformation of Eucalyptus camaldulensis and production of transgenic plants. Plant Cell Rep 17:675–680
Huang Z, Ouyang L, Li Z, Zeng F (2014) A urea-type cytokinin, 2-Cl-PBU, stimulates adventitious bud formation of Eucalyptus urophylla by repressing transcription of rboh1 gene. Plant Cell Tiss Org Cult 119:359–368
Huang ZC, Zeng FH, Lu XY (2010) Efficient regeneration of Eucalyptus urophylla from seedling-derived hypocotyls. Biol Plant 54:131–134
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Klocko AL, Ma C, Robertson S, Esfandiari E, Nilsson O, Strauss SH (2016) FT overexpression induces precocious flowering and normal reproductive development in Eucalyptus. Plant Biotechnol J 14:808–819
Lainé E, David A (1994) Regeneration of plants from leaf explants of micropropagated clonal Eucalyptus grandis. Plant Cell Rep 13:473–476
Li L, Ouyang L, Gan S (2015) Towards an efficient regeneration protocol for Eucalyptus urophylla. J Trop For Sci 27:289–297
Manders GNUU, Santos AVPD, Utra Vaz FBD, Davey MR, Power JB (1992) Transient gene expression in electroporated protoplasts of Eucalyptus citriodora Hook. Plant Cell Tiss Org Cult 30:69–75
Matsunaga E, Nanto K, Oishi M, Ebinuma H, Morishita Y, Sakurai N, Suzuki H, Shibata D, Shimada T (2012) Agrobacterium-mediated transformation of Eucalyptus globulus using explants with shoot apex with introduction of bacterial choline oxidase gene to enhance salt tolerance. Plant Cell Rep 31:225–235
Mendonça EG, Stein VC, Balieiro FP, Lima CDF, Santos BR, Paiva LV (2013) Genetic transformation of Eucalyptus camaldulensis by agrobalistic method. Rev Árvore 37:419–429
Moralejo M, F. Rochange AMB, Teulières C (1998) Generation of transgenic Eucalyptus globulus plantlets through Agrobacterium tumefaciens mediated transformation. Aust J Plant Physiol 25:207–212
Mullins KV, Llewellyn DJ, Hartney VJ, Strauss S, Dennis ES (1997) Regeneration and transformation of Eucalyptus camaldulensis. Plant Cell Rep 16:787–791
Muralidharan EM, Mascarenhas AF (1987) In vitro plantlet formation by organogenesis in E. camaldulensis and by somatic embryogenesis by Eucalyptus citriodora. Plant Cell Rep 6:256–259
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Navarro M, Ayax C, Martinez Y, Laur J, El Kayal W, Marque C, Teulières C (2011) Two EguCBF1 genes overexpressed in Eucalyptus display a different impact on stress tolerance and plant development. Plant Biotechnol J 9:50–63
Novikova TI, Zaytseva YG (2018) TDZ-induced morphogenesis pathways in woody plant culture. Springer Singapore, Singapore, pp 61–94
Oberschelp GPJ, Gonçalves AN, Meneghetti EC, Graner ÉM, de Almeida M (2015) Eucalyptus dunnii maiden plant regeneration via shoot organogenesis on a new basal medium based on the mineral composition of young stump shoots. In Vitro Cell Dev Biol - Plant 51:626–636
Oguchi T, Kashimura Y, Mimura M, Yu X, Matsunaga E, Nanto K, Shimada T, Kikuchi A, Watanabe KN (2014) A multi-year assessment of the environmental impact of transgenic Eucalyptus trees harboring a bacterial choline oxidase gene on biomass, precinct vegetation and the microbial community. Transgenic Res 23:767–777
Ouyang L, Wang Z, Li L, Chen B (2020) Physiological parameters and differential expression analysis of N-phenyl-N'-[6-(2chlorobenzothiazol)-yl] urea-induced callus of Eucalyptus urophylla × Eucalyptus grandis. PeerJ 8:e8776
Ouyang L, He W, Huang Z, Zhao L, Peng S, Sha Y, Zeng F, Lu X (2012a) Introduction of the Rs-AFP2 gene into Eucalyptus urophylla for resistance to Phytophthora capsici. J Trop For Sci 24:198–208
Ouyang L, Huang Z, Zhao L, Sha Y, Zeng F, Lu X (2012b) Efficient regeneration of Eucalyptus urophylla × Eucalyptus grandis from stem segment. Braz Arch Biol Technol 55:329–334
Pena L, Seguin A (2001) Recent advances in the genetic transformation of trees. Trends Biotechnol 19:500–506
Prakash MG, Gurumurthi K (2010) Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tiss Org Cult 100:13–20
Rochange F, Serrano L, Marque C, Teulieres C, Boudet AM (1995) DNA delivery into Eucalyptus globulus zygotic embryos through biolistics: optimization of the biological and physical parameters of bombardment for two different particle guns. Plant Cell Rep 14:674–678
Sartoretto LM, Cid LPB, Brasileiro ACM (2002) Biolistic transformation of Eucalyptus grandis × E. urophylla callus. Funct Plant Biol 29:917–924
Serrano L, Rochange F, Semblat JP, Marque C, Teulières C, Boudet A (1996) Genetic transformation of Eucalyptus globulus through biolistics: complementary development of procedures for organogenesis from zygotic embryos and stable transformation of corresponding proliferating tissue. J Exp Bot 47:285–290
Shabannejad Mamaghani M, Assareh MH, Omidi M, Matinizadeh M, Ghamari-Zare A, Shahrzad S, Forootan M (2009) The effect of thidiazuron level on in vitro regeneration type and peroxidase profile in Eucalyptus microtheca F. Muell. Plant Growth Regul 59:199–205
Shwe SS, Leung DWM (2020) Plant regeneration from Eucalyptus bosistoana callus culture. In Vitro Cell Dev Biol - Plant 56:718–725
Silva ALLD, Gollo AL, Brondani GE, Horbach MA, De Oliveira LS, Machado MP, De Lima KKD, Costa JDL (2015) Micropropagation of Eucalyptus saligna sm. from cotyledonary nodes. Pak J Bot 1:311–318
Silva ALLD, Oliveira Y, Costa JDL, Mudry CDS, Scheidt GN, Brondani GE (2011) Preliminary results for genetic transformation of shoot tip of Eucalyptus saligna Sm. via Agrobacterium tumefaciens. J Biotechnol Biodiversity 2:1–6
Teulières C, Grima-Pettenati J, Curie C, Teissie J, Boudet AM (1991) Transient foreign gene expression in polyethylene/glycol treated or electropulsated Eucalyptus gunnii protoplasts. Plant Cell Tiss Org Cult 25:125–132
Tzfira T, Citovsky V (2006) Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Curr Opin Biotechnol 17:147–154
Valério L, Carter D, Rodrigues JC, Tournier V, Gominho J, Marque C, Boudet A, Maunders M, Pereira H, Teulières C (2003) Down regulation of cinnamyl alcohol dehydrogenase, a lignification enzyme, in Eucalyptus camaldulensis. Mol Breeding 12:157–167
Wang P, Jiang F, Cai L, Chen X, Tan Z, Chen B, Wu Y (2013) Construction of genetic transformation system for Eucalyptus grandis × E. urophylla 'GLGU9'. J Forest Eng 27:76–80
Yu X, Kikuchi A, Matsunaga E, Morishita Y, Nanto K, Sakurai N, Suzuki H, Shibata D, Shimada T, Watanabe KN (2013) The choline oxidase gene codA confers salt tolerance to transgenic Eucalyptus globulus in a semi-confined condition. Mol Biotechnol 54:320–330
Acknowledgements
We thank Professor Siming Gan (Research Institute of Tropical Forestry, Chinese Academy of Forestry) for providing E. urophylla × E. tereticornis clone YL02 and we also thank AJE team for English editing.
Funding
This work was supported by the Fundamental Research Funds for the Central Non-profit Research Institution of CAF (Grant No. CAFYBB2020ZB004).
Author information
Authors and Affiliations
Contributions
CJF designed the research. XPW, PL, XDL, and ZFQ conducted the experiments. CJF, XPW, and XDL analyzed the data. The paper was written by CJF and XPW and revised by CJF and BSZ. All the authors read and approved the final manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Luo, P., Qiu, Z. et al. Adventitious bud regeneration and Agrobacterium tumefaciens-mediated genetic transformation of Eucalyptus urophylla × E. tereticornis interspecific hybrid. In Vitro Cell.Dev.Biol.-Plant 58, 416–426 (2022). https://doi.org/10.1007/s11627-021-10240-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-021-10240-x