Abstract
Ipomoea turbinata Lagasca and Segura (Purple Moonflower) belongs to the largest flowering genus Ipomoea in the Convolvulaceae family. Ipomoea turbinata has not been previously explored for its in vitro potential. This is the first study focused on thidiazuron-induced callus culture for efficient biosynthesis of commercially important phenolic compounds in this plant species. Among the two plant growth regulators tested on leaf, stem, and root explants, 5 mg L−1 thidiazuron (TDZ) induced the highest biomass accumulation (61.4 g L−1 fresh weight, 6.3 g L−1 dry weight) in leaf-derived callus cultures after 5 wk of culture. The highest total phenolic and flavonoid contents recorded were 9.04 mg g−1 and 1.16 mg g−1, respectively, in optimized callus cultures. High-performance liquid chromatography analysis indicated high levels of pharmacologically important anticancer compounds such as chlorogenic acid (13.48 mg g−1), arctigenin (11.67 mg g−1), quercetin (6.19 mg g−1), and kaempferol (5.48 mg g−1), along with other phenolic acids. Furthermore, the antioxidant activity was also evaluated, and leaf-derived callus culture displayed a maximum of 62.6% antioxidant potential. The induction of improved biomass accumulation in callus culture and the production of multipotent bioactive metabolites shows the potential of the multifunctional thidiazuron hormone as an efficient elicitation tool in callus culture of I. turbinata.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Decades of clinical understanding and indigenous facts of traditional medicines are still pivotal sources for current drug discovery. In the ancient culture of medicine, the extracts of medicinal plants established the foundation for the treatment of a wide variety of disorders. The unique nature and phytochemical diversity in extracts of medicinal plants plays an important role in the search for novel drug discovery and development (Awale et al. 2006; Osbourn and Lanzotti 2009; Wink 2012).
Plants produce a variety of secondary metabolites to adapt to environmental fluctuations, to survive in stressful conditions, and to defend themselves against pathogenic invasions. Of these metabolites, phenolics have gained considerable attention because of the multidimensional applications discovered over the past few years including anti-mutagenic, anti-inflammatory, antioxidant and anti-clotting ability, which have potential roles to treat cardiovascular disorders and cancer (Han et al. 2007; Fresco et al. 2010; Loke et al. 2010; Ostertag et al. 2010).
Plant in vitro culture techniques provide valuable insights for the production of phenolics (Hiroyuki et al. 2002). In vitro cultures are more profitable than whole plant extracts in several ways, such as continuous production of valuable metabolites (Blando et al. 2004), large-scale production depending on market needs (Smith and Pépin 1999) and low expenditure and extrapolation of biosynthetic pathways (Curtin et al. 2003; Plata et al. 2003).
Phenolics are naturally synthesized during a plant’s metabolic reactions. These are bioactive compounds that act as antioxidants by scavenging reactive oxygen species (ROS) and free radicals that are produced during metabolic reactions in the body (Gill and Tuteja 2010; Nadeem et al. 2018a). Phenolic acids such as chlorogenic acid, caffeic acid, and ferulic acid have been shown to inhibit the promotion of skin tumors in female CD-1 mice (Huang et al. 1988). Similarly, the antiviral properties of these phenolic compounds are also reported to act against hepatitis B virus (Wang et al. 2009). Quercetin and kaempferol have anti-inflammatory potential (Hämäläinen et al. 2007). Protocatechuic acid has been shown to inhibit hepatotoxicity, due to its antioxidant and anti-inflammatory properties (Liu et al. 2002). In addition to these properties, phenolic compounds also exhibit antibacterial, anti-allergenic and antithrombotic properties (Ahmad et al. 2012).
Ipomoea turbinata Lagasca and Segura, also known as Purple Moonflower, belongs to the family Convolvulaceae. Ipomoea genus is known for its entheogenic potential and presence of psychoactive compounds (Meira et al. 2012). This genus grows across the tropical and subtropical countries. The Ipomoea genus is composed of annual and perennial climbing shrubs and herbaceous plants. According to Gunn (1969), I. turbinata was introduced as an ornamental plant in the USA, but now, it is listed as introduced weed species in 46 of the 50 United States. In China and Sri Lanka, the pedicels, fruits and juvenile seeds of I. turbinata are consumed. Its leaves are consumed for treating stomach aches and trauma in China (Gunn 1969; Chandler et al. 1977). There is no previous report available on callus culture and phenolic profiles of I. turbinata. Therefore, the objective of the current study was to explore and establish an in vitro callus culture system to produce phenolic compounds. This study will possibly open new areas to produce multifunctional health-promoting bioactive ingredients in callus cultures of I. turbinata.
Materials and Methods
Seed germination
Seeds of I. turbinata were collected from their natural habitat in the rural region of Peshawar, Khyber Pakhtunkhwa, Pakistan. Seed inoculation was performed as described by Zahir et al. (2018a) with minor modifications. Briefly, the seeds were surface sterilized with 0.1% (w/v) mercuric chloride and 70% (v/v) ethanol for 60 s each treatment, followed by washing with autoclaved distilled water three times. Sterilized seeds were dried on autoclaved filter paper and inoculated onto MS basal medium (Murashige and Skoog 1962) modified with 30 g L−1 sucrose, 8 g L−1 plant tissue culture micropropagation grade agar (Phytotech Laboratories® Lenexa, KS), and the pH was adjusted to 5.6 using 2 M NaOH. For seed inoculation, locally manufactured jam glass jars tightly closed with plastic caps were used. Each jar contained 40 mL of medium and was autoclaved at 121 °C under 103 kPa for 20 min prior to inoculation. The seed-containing jars were maintained at 25 ± 2°C with a 16-h photoperiod and light intensity of 40 μmol m−2 s−1 supplied by Philips TLD 35 fluorescent lamps (Signify Philips, Eindhoven, Netherlands) in a growth room. All the chemicals used in the current study were purchased from Sigma-Aldrich® (St. Louis, MO).
Establishment of callus cultures
To establish callus cultures, stem, leaf and root explants (approximately 1.0 cm each) were excised from 4-wk-old in vitro germinated seedlings. All of the leaf, stem and root explants were separately inoculated onto MS basal medium containing 30 g L−1 sucrose and augmented with 0.1 to 20 mg L−1 thidiazuron (TDZ) alone or in combination with 1.0 mg L−1 α-naphthaleneacetic acid (NAA), and the pH of all media was adjusted to 5.6 using 2 M NaOH followed by the addition of 8 g L−1 plant tissue culture micropropagation grade agar (Phytotech Laboratories®) in 100-mL Erlenmeyer flasks (Pyrex®, Corning, NY) containing 30-mL medium, tightly closed with sterile cotton plugs and aluminum foil. The medium-containing flasks were then autoclaved at 121°C under 103 kPa for 20 min. Plant growth regulator (PGR)-free medium (MS0) was used as the control. The explanted tissues were cultured in the same culture conditions previously described. The experiment was executed in triplicate and repeated two times. Optimum growing conditions were determined after initial culture incubation for 35 d. Afterwards, growth curves were obtained by recording growth-associated parameters periodically at an interval of 7 d for a total of 49 d.
Biomass determination
For biomass determination, the respective calluses were harvested and washed with distilled water to remove any attached medium. Callus tissue was placed and rubbed gently on filter paper to remove any extra water and weighed to determine the fresh weight (FW) and subsequently oven-dried at 45°C for 48 h for dry weight (DW) determination.
Extract preparation
Extracts of callus cultures were prepared according to the protocol of Zahir et al. (2018b) with minor modifications. Briefly, 100 mg of powdered callus was mixed with 500 μL of methanol in a 1.5-mL Eppendorf tube. These mixtures were vortexed for 1 min each, and then sonicated at 60 kHz and 25°C for 30 min (Elmasonic P, Elma Schmidbauer GmbH, Singen, Germany). This process was repeated twice, and the resultant mixtures were maintained at constant agitation at 80 rpm, on a gyratory shaker at 25 ± 2°C for 24 h. The vortexing and sonication step was repeated. Finally, the mixtures were centrifuged (SpectrafugeTM 24D, Labnet International, Edison, NJ) at 9200×g for 10 min. The resultant supernatants were syringe filtered (0.45 μm, Merck Millipore, Saint Quentin Falavier, France) and stored at 4 °C for further analysis.
Total phenolic content and production
To determine the total phenolic content (TPC), the protocol described by Zahir et al. (2014) was followed using Folin-Ciocalteu (FC) reagent. Absorbance of the mixture was recorded at 630 nm, with the aid of an ELx808 microplate reader (BioTek, Winooski, VT). Total phenolic content was expressed as equivalent of gallic acid (GAE g−1 of DW). Equation 1 was then used to calculate the total phenolic production (TPP). Total phenolic content was expressed in mg gallic acid g−1 DW and TPP in mg gallic acid L−1.
Total flavonoid content and production
To determine the total flavonoid content (TFC), the protocol described by Nadeem et al. (2018b) was followed. Absorbance Microplate Reader (ELx808, BioTek) was used to evaluate the absorbance of the samples at 415 nm. Total flavonoid content (TFC) was expressed as equivalent of quercetin (QE g−1 DW). Equation 2 was then used to calculate the total flavonoid production (TFP). Total flavonoid content was expressed in mg quercetin g−1 of DW and TFP in mg quercetin L−1.
Free radical scavenging assay
To determine the antioxidant potential, the free radical scavenging assay (FRSA) was performed using 1,1-diphenyl-2-picrylhydrazyl (DPPH) as described by Amarowicz et al. (2004). Methanolic extracts of DPPH solution were used as the control. Equation 3 was then used to calculate FRSA. The antioxidant potential was expressed as a percentage.
where AC is the absorbance of the solution when the sample extract was mixed at a specific concentration and AS is the absorbance of the standard (DPPH solution).
Reverse phase high-performance liquid chromatography (RP-HPLC) analysis
To identify the flavonoids and phenolic acids in I. turbinata callus extracts, HPLC analysis was conducted. Following ultrasound-assisted extraction as described previously in the “Extract Preparation”, extracts were evaporated and subjected to enzymatic hydrolysis using 5 U mL−1 of β-glucosidase from almond (Sigma-Aldrich®, Saint Quentin Falavier, France), in 1 mL of pH 4.8 0.1 M citrate–phosphate buffer, for 6 h at 37°C, to release aglycones and simplify the analysis. The samples were then centrifuged (Spectrafuge™ 24D) at 12,000×g for 5 min, and the supernatant was filtered using a 0.45-μm syringe filter (Merck Millipore) prior to HPLC analysis. The separation was performed on a Varian ProStar system (Agilent Technology, Les Ulis, France) comprising a pump Varian Prostar 230, a Degasser MetaChem Degasit, an autosampler Varian Prostar 410 and a Photodiode Array Detector PAD, Varian Prostar 335. The system was driven by Galaxie v1.9.3.2 software (Agilent Technology). A Purospher® RP-18 column (250 × 4 mm i.d.: 5 μm) (Merck Millipore) was used for the separation at 40°C. The mobile phase was composed of 0.2% (v/v) acetic acid-acidified water as solvent A and HPLC-grade methanol as solvent B. The mobile phase composition varied according to a nonlinear gradient at a flow rate of 0.8 mL min−1 as follows: from 0 to 40 min of A–B: 90:10 (v/v) to 30:70 (v/v), from 41 to 50 min of A–B: 30:70 (v/v) to 0:100 (v/v), and A–B: 0:100 (v/v) from 51 to 60 min. A range of authentic standards were used, and compounds were identified by comparing their retention times, and UV spectra to those of authentic standards, and by standard additions.
Identification of metabolites
Quantification was performed at 280 nm using calibration curves of each identified standard, ranging from 0.0125 to 0.5 mg mL−1 with a correlation coefficient of at least 0.999. Quantification was done on the basis of retention times compared to commercial reference standards of chlorogenic acid, caffeic acid, ferulic acid, vanillic acid, protocatechuic acid, p-coumaric acid, p-hydroxybenzoic acid, quercetin, kaempferol and arctigenin (Sigma-Aldrich®). O-coumaric acid was used as an internal standard at 0.05 mg mL−1 final concentration in the extract.
Statistical analysis
Experiments were conducted in a completely randomized design and repeated twice. The statistical comparison was conducted using version 8.5 OriginPro software (OriginLab® Corporation, Northampton, MA). Duncan’s multiple range test was conducted with Windows v7.5.1. SPSS® software platform (IBM, Chicago, IL) was used to determine the significance at P < 0.05. All figures were generated using version 8.5 OriginPro software.
Results and Discussion
Callus morphogenesis and biomass accumulation
Thidiazuron is reported to be a highly efficient growth regulator for callogenesis in various plant species (Prathanturarug et al. 2005). In the current study, TDZ and NAA alone or in combination (almost all concentrations) successfully induced callogenesis. The maximum callus induction frequency (92%) was recorded in leaf-derived explants inoculated onto MS medium supplemented with 5.0 mg L−1 TDZ (Table 1). No significant difference was observed in callogenesis of all the three types of explants, but the concentration and type of PGR affected the morphology of callus. Moreover, the type of explant also played a role in morphological variations in callus culture (Table 1). However, 5.0 mg L−1 TDZ was found to be highly efficient for callus organogenesis (Fig. 1). Generally, from leaf-derived callus, higher FW and DW accumulation occurred in cultures treated with TDZ alone compared to NAA alone or in combination. Furthermore, the color of the resultant callus was green (Table 1). Thidiazuron has a potential role in the production of purine cytokinins to improve cell growth (Arinaitwe et al. 2000). Accordingly, TDZ stimulated biomass accumulation and other related growth parameters in callus cultures of I. turbinata. From all the three types of explants, NAA alone or in combination with higher concentrations of TDZ, displayed lower callogenesis compared to TDZ alone. However, auxins have been reported to be potential candidates in callogenesis in a number of plant species (Sané et al. 2012). No previous report exists on the effect of NAA or combined treatment of NAA and TDZ, in tissue culture responses in I. turbinata. However, Alam et al. (2010) reported the potential role of TDZ in callus induction in Ipomoea batatas (L.) Lam. The callus-inducing ability of TDZ in combination with NAA has been reported by Cheruvathur et al. (2015) in Ipomoea marginata (Desr.) Verdc. The MS medium devoid of any PGR did not favor any callogenesis. Conclusively, leaf explants exhibited a higher callogenic response (92%), than stem-derived (90%) and root-derived (85%) explants along with maximal FW (61.4 g L−1) and DW (6.3 g L−1) accumulation (Table 2). The divergence in the response of explants of the same plant species to the same hormonal treatment is probably due to the particular biochemical and physiological potential of different tissues. Usually, a number of parameters are involved in callus formation such as the type of explant, concentration, nature of PGR, plant genotype and in vitro growth-promoting conditions (Mathur and Shekhawat 2013). Thidiazuron in lower and higher concentrations than the optimum concentration poorly promoted callus formation (Table 1). These results are in agreement with results reported by Ali and Abbasi (2014) and Khan et al. (2016) for the Artemisia absinthium L. and Fagonia indica Burm.f., respectively.
To determine the trends in growth kinetics and biomass accumulation, data were recorded at a 7-d interval for a total of 49 d. The growth curves of callus cultures of I. turbinata grown at 5.0 mg L−1 TDZ showed a 7-d lag phase, 21-d exponential phase starting from day 14 to day 35, followed by a 7-d stationary phase from day 35 to day 42. From day 42 onward, a death phase was observed (Fig. 2).
Phenolic and flavonoid accumulation in callus cultures
Phenolic compounds are synthesized by plants as secondary metabolites to scavenge reactive oxygen species (ROS) and possess other numerous biochemical and pharmacological properties (Kasote et al. 2015; Nadeem et al. 2018c). Maximum TPC (9.04 mg g−1) and TFC (1.16 mg g−1) were noted from leaf-derived callus (Figs. 3 and 4) grown on 5.0 mg L−1 TDZ compared to stem-derived callus (TPC 8.39 mg g−1 and TFC 1.01 mg g−1) and root-derived callus (TPC 8.56 mg g−1 and TFC 1.01 mg g−1; Table 2). A TDZ-based enhanced TPC and TFC in vitro culture system was reported by Pourebad et al. (2015) and Khan et al. (2016). In the current study, the dependent behavior of explants on the TDZ concentration for increased FW and DW accumulation (Fig. 2) and higher TPC and TFC (Figs. 3 and 4) has also established the link with the role of TDZ in callus organogenesis. Thidiazuron at a mild concentration of about 5.0 mg L−1 increased the biosynthesis of TPC and TFC in callus cultures, while it inhibited the accumulation of TPC and TFC at higher concentrations. A possible reason for the accumulation of secondary metabolites in this trend is that TDZ stimulates the production of ethylene at higher concentrations, and ethylene represses the biosynthesis of secondary metabolites (Shibli et al. 1997).
Antioxidant potential of callus cultures
The profusion of ROS and other related free radicals induces oxidative stress in the body and is responsible for damaging the cells directly or indirectly. As a counter measure, a plant synthesizes different kinds of secondary metabolites, mainly phenolics and flavonoids, as antioxidants to scavenge these free radicals (Gill and Tuteja 2010). These metabolites can be employed in the body as an antidote for several disorders (Raskin et al. 2002). Guo et al. (2011) have reported that thidiazuron induces the expression of stress marker compounds such as abscisic acid, proline, and 4-aminubutyrate. When peanut seedlings were cultured on TDZ-fortified media, the proline content was enhanced, which resulted in high oxidative stress. Moreover, TDZ also affected embryogenesis and organogenesis by inducing oxidative stress in plant cells (Younas et al. 2018).
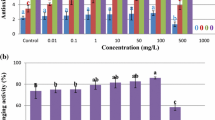
To evaluate the presence of such antioxidants in callus, a DPPH-free radical scavenging assay (FRSA) was performed. In the current study, a high percentage of FRSA was observed in callus cultures (Fig. 5). In the cases of all three types of explants, an increasing trend was observed with increased TDZ concentrations. Maximum FRSA (62.6%) was observed in leaf-derived callus cultures (Table 2).
RP-HPLC-assisted quantification of phenolics
HPLC was used to identify and quantify the main phenolic acids and flavonoids accumulated in I. turbinata callus cultures. Due to unavailability of any reports on HPLC-based phytochemical profiles of I. turbinata, a number of reference compounds previously identified in other related species of genus Ipomea were tested (Páska et al. 2002; Lin et al. 2008; Oluyori et al. 2016; Khan et al. 2018). Chlorogenic acid, arctigenin, quercetin and kaempferol were the major compounds accumulated in I. turbinata. Upon evaluation of growth dynamics, it was noted that chlorogenic acid (13.48 mg g−1), quercetin (6.19 mg g−1) and kaempferol (5.48 mg g−1) were accumulated in the highest amounts at day 28 after inoculation (14 d into exponential phase), while arctigenin was produced in the highest concentration (11.67 mg g−1) at day 35 (end of exponential phase), as shown in Fig. 6a. Additional phenolic acids such as caffeic acid, ferulic acid, vanillic acid, protocatechuic acid, p-coumaric acid and p-hydroxybenzoic acid were also detected in lower concentrations (0.12 to 1.19 mg g−1). The accumulation of phenolic acids such as p-coumaric acid and protocatechuic acid in the early and late stationary phase showed their role to alleviate nutrient depletion-induced stress (Fig. 6b). In accordance with the results on the total phenolic and flavonoid content, the leaf-derived callus cultures grown on MS medium supplemented with 5.0 mg L−1 TDZ accumulated higher amounts of these compounds than all other treatments (Table 2). Interestingly, arctigenin, a pharmacologically important dibenzylbutyralactone lignan, is produced in substantial amounts in callus cultures of I. turbinata. This is of particular interest when considering the biological activities of this compound such as anti-diabetic (Huang et al. 2012), anti-inflammatory (Zhao et al. 2009), antiviral (Hayashi et al. 2010), neuroprotective (Li et al. 2014) and anti-cancer (Han et al. 2016). Furthermore, other accumulated phenolics are not devoid of potent applications and could have pharmaceutically and industrially valued as well. This first report on the induction of improved biomass accumulation and production of multipotent bioactive metabolites in callus cultures of I. turbinata showed the high potential of the multifunctional TDZ synthetic hormone as a stimulation tool for the production and future valuation of bioactive compounds in this species.
(a) Quantification of major phenolics in callus culture of Ipomoea turbinata Lagasca and Segura at different d of growth. Values are means of three replicates ± SD. Means with different letters are statistically different (P < 0.05). (b) Quantification of minor phenolics in callus culture of I. turbinata at different growth phases. Values are means of three replicates ± SD. Means with different letters are statistically different (P < 0.05).
Conclusions
In the current study, a callus culture system for I. turbinata was established for the improved and feasible production of commercially important phenolic acids. Thidiazuron is an influential growth regulator for inducing callus production in I. turbinata, irrespective of the type of explant used. Thidiazuron has positively affected the biomass accumulation and biosynthesis of phenolic compounds. Thidiazuron at a low level can promote the cost efficient and easy production of these bioactive secondary metabolites. The system presented in this study is a step towards the establishment of a cell suspension culture and ultimate scale-up for industrial production of commercially important metabolites.
References
Ahmad N, Fazal H, Ahmad I, Abbasi BH (2012) Free radical scavenging (DPPH) potential in nine Mentha species. Toxicol Ind Health 28:83–89
Alam I, Sharmin SA, Naher K, Alam J, Anisuzzaman M, Alam MF (2010) Effect of Growth Regulators on Meristem Culture and Plantlet Establishment in Sweet Potato ['Ipomoea Batatas'(L.) Lam.]. Plant Omics 3:35–39
Ali M, Abbasi BH (2014) Thidiazuron-induced changes in biomass parameters, total phenolic content, and antioxidant activity in callus cultures of Artemisia absinthium L. Appl Biochem Biotechnol 172:2363–2376
Amarowicz R, Pegg R, Rahimi-Moghaddam P, Barl B, Weil J (2004) Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem 84:551–562
Arinaitwe G, Rubaihayo P, Magambo M (2000) Proliferation rate effects of cytokinins on banana (Musa spp.) cultivars. Sci Hortic 86:13–21
Awale S, Lu J, Kalauni SK, Kurashima Y, Tezuka Y, Kadota S, Esumi H (2006) Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res 66:1751–1757
Blando F, Gerardi C, Nicoletti I (2004) Sour cherry (Prunus cerasus L) anthocyanins as ingredients for functional foods. Biomed Res Int 2004:253–258
Chandler J, Munson R, Vaughan C (1977) Purple moonflower: emergence, growth, reproduction. Weed Sci 25:163–167
Cheruvathur MK, Abraham J, Thomas TD (2015) In vitro micropropagation and flowering in Ipomoea sepiaria Roxb. An important ethanomedicinal plant. Asian Pac J Reprod 4:49–53
Curtin C, Zhang W, Franco C (2003) Manipulating anthocyanin composition in Vitis vinifera suspension cultures by elicitation with jasmonic acid and light irradiation. Biotechnol Lett 25:1131–1135
Fresco P, Borges F, Marques M, Diniz C (2010) The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr Pharma Des 16:114–134
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gunn CR (1969) History and taxonomy of the purple moonflower, Ipomoea turbinata Lagasca y Segura. Proc Assoc Official Seed Analysts North Am 59:116–123
Guo B, Abbasi BH, Zeb A, Xu L, Wei Y (2011) Thidiazuron: a multi-dimensional plant growth regulator. Afr J Biotechnol 10:8984–9000
Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E (2007) Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat Inflamm 2007:45673. https://doi.org/10.1155/2007/45673
Han X, Shen T, Lou H (2007) Dietary polyphenols and their biological significance. Int J Mol Sci 8:950–988
Han Y-H, Kee J-Y, Kim D-S, J-g M, Jeong M-Y, Park S-H, Choi B-M, Park S-J, Kim H-J, Um J-Y (2016) Arctigenin inhibits lung metastasis of colorectal cancer by regulating cell viability and metastatic phenotypes. Molecules 21:1135. https://doi.org/10.3390/molecules21091135
Hayashi K, Narutaki K, Nagaoka Y, Hayashi T, Uesato S (2010) Therapeutic effect of arctiin and arctigenin in immunocompetent and immunocompromised mice infected with influenza A virus. Biol Pharm Bull 33:1199–1205
Hiroyuki H, Kousuke H, Eiji N, Mariko O, Yoshihito K, Setsuro H, Takeshi K (2002) Enhanced anthocyanin production from grape callus in an air-lift type bioreactor using a viscous additive-supplemented medium. J Biosci Bioeng 94:135–139
Huang M-T, Smart RC, Wong C-Q, Conney AH (1988) Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res 48:5941–5946
Huang S-L, Yu R-T, Gong J, Feng Y, Dai Y-L, Hu F, Hu Y-H, Tao Y-D, Leng Y (2012) Arctigenin, a natural compound, activates AMP-activated protein kinase via inhibition of mitochondria complex I and ameliorates metabolic disorders in ob/ob mice. Diabetologia 55:1469–1481
Kasote DM, Katyare SS, Hegde MV, Bae H (2015) Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 11:982–991
Khan T, Abbasi BH, Khan MA, Shinwari ZK (2016) Differential effects of thidiazuron on production of anticancer phenolic compounds in callus cultures of Fagonia indica. Appl Biochem Biotechnol 179:46–58
Khan MZI, Zahra SS, Ahmed M, Fatima H, Mirza B, Haq I-U, Khan SU (2018) Polyphenolic profiling of Ipomoea carnea Jacq. by HPLC-DAD and its implications in oxidative stress and cancer. Nat Prod Res 33:2099–2104
Li D, Liu Q, Jia D, Dou D, Wang X, Kang T (2014) Protective effect of arctigenin against MPP+ and MPTP-induced neurotoxicity. Planta Med 80:48–55
Lin R-J, Chen C-Y, Lo W-L (2008) Cytotoxic activity of Ipomoea cairica. Nat Prod Res 22:747–753
Liu C-L, Wang J-M, Chu C-Y, Cheng M-T, Tseng T-H (2002) In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem Toxicol 40:635–641
Loke WM, Proudfoot JM, Hodgson JM, McKinley AJ, Hime N, Magat M, Stocker R, Croft KD (2010) Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E–knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol 30:749–757
Mathur S, Shekhawat GS (2013) Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: an in vitro approach for production of stevioside. Acta Physiol Plant 35:931–939
Meira M, Silva EP, David JM, David JP (2012) Review of the genus Ipomoea: traditional uses, chemistry and biological activities. Rev Bras 22:682–713
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nadeem M, Abbasi BH, Garros L, Drouet S, Zahir A, Ahmad W, Giglioli-Guivarc’h N, Hano C (2018a) Yeast-extract improved biosynthesis of lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult 135:347–355
Nadeem M, Abbasi BH, Younas M, Ahmad W, Zahir A, Hano C (2018b) LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J Photochem Photobiol B Biol 190:172–178
Nadeem M, Ahmed W, Zahir A, Hano C, Abbasi BH (2018c) Salicylic acid-enhanced biosynthesis of pharmacologically important lignans and neo lignans in cell suspension culture of Linum ussitatsimum L. Eng Life Sci 19:168–174
Oluyori AP, Shaw AK, Preeti R, Reddy S, Atolani O, Olatunji GA, Fabiyi OA (2016) Natural antifungal compounds from the peels of Ipomoea batatas Lam. Nat Prod Res 30:2125–2129
Osbourn AE, Lanzotti V (2009) Plant-derived natural products. Springer, New York, NY, pp 361–384
Ostertag LM, O'kennedy N, Kroon PA, Duthie GG, De Roos B (2010) Impact of dietary polyphenols on human platelet function–a critical review of controlled dietary intervention studies. Mol Nutr Food Res 54:60–81
Páska C, Innocenti G, Ferlin M, Kunvári M, László M (2002) Pinoresinol from Ipomoea cairica cell cultures. Nat Prod Lett 16:359–363
Plata N, Konczak-Islam I, Jayram S, McClelland K, Woolford T, Franks P (2003) Effect of methyl jasmonate and p-coumaric acid on anthocyanin composition in a sweet potato cell suspension culture. Biochem Eng J 14:171–177
Pourebad N, Motafakkerazad R, Kosari-Nasab M, Akhtar NF, Movafeghi A (2015) The influence of TDZ concentrations on in vitro growth and production of secondary metabolites by the shoot and callus culture of Lallemantia iberica. Plant Cell Tissue Organ Cult 122:331–339
Prathanturarug S, Soonthornchareonnon N, Chuakul W, Phaidee Y, Saralamp P (2005) Rapid micropropagation of Curcuma longa using bud explants pre-cultured in thidiazuron-supplemented liquid medium. Plant Cell Tissue Organ Cult 80:347–351
Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N (2002) Plants and human health in the twenty-first century. Trends Biotechnol 20:522–531
Sané D, Aberlenc-Bertossi F, Diatta LID, Guèye B, Daher A, Sagna M, Duval Y, Borgel A (2012) Influence of growth regulators on callogenesis and somatic embryo development in date palm (Phoenix dactylifera L.) Sahelian cultivars. Sci World J:2012. https://doi.org/10.1100/2012/837395
Shibli RA, Smith M, Kushad M (1997) Headspace ethylene accumulation effects on secondary metabolite production in Vaccinium pahalae cell culture. Plant Growth Regul 23:201–205
Smith MAL, Pépin M-F (1999) Stimulation of bioactive flavonoid production in suspension and bioreactor-based cell cultures Plant biotechnology and in vitro biology in the 21st century. Springer, Dordrecht, The Netherlands pp 333-336
Wang G-F, Shi L-P, Ren Y-D, Liu Q-F, Liu H-F, Zhang R-J, Li Z, Zhu F-H, He P-L, Tang W (2009) Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir Res 83:186–190
Wink M (2012) Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules 17:12771–12791
Younas M, Drouet S, Nadeem M, Giglioli-Guivarc'h N, Hano C, Abbasi BH (2018) Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. J Photochem Photobiol B Biol 184:61–70
Zahir A, Abbasi BH, Adil M, Anjum S, Zia M (2014) Synergistic effects of drought stress and photoperiods on phenology and secondary metabolism of Silybum marianum. Appl Biochem Biotechnol 174:693–707
Zahir A, Ahmad W, Nadeem M, Giglioli-Guivarc'h N, Hano C, Abbasi BH (2018a) In vitro cultures of Linum usitatissimum L.: Synergistic effects of mineral nutrients and photoperiod regimes on growth and biosynthesis of lignans and neolignans. J Photochem Photobiol B Biol 187:141–150
Zahir A, Nadeem M, Ahmad W, Giglioli-Guivarc’h N, Hano C, Abbasi BH (2018b) Chemogenic silver nanoparticles enhance lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult 136:589–596
Zhao F, Wang L, Liu K (2009) In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. Ethnopharmacology 122:457–462
Acknowledgments
Dr. Bilal Haider Abbasi acknowledges the research fellowship of Le Studium-Institute for Advanced Studies, Loire Valley, Orléans, France. This research was in part supported by Cosmetosciences, a global training and research program dedicated to the cosmetic industry. The authors appreciate language editing of manuscript by Muhammad Younas.
Funding
Located in the heart of the cosmetic valley, this program led by University of Orléans, France, is funded by the Region Centre-Val de Loire.
Author information
Authors and Affiliations
Contributions
WA performed experiments of establishment of callus culture, optimization with TDZ, and analyses of TPC, TFC, and antioxidant activity. AZ and MN assisted WA with all of these experiments. BHA conceived idea, supervised research, and critically evaluated MS. CH contributed to HPLC analysis and critical revision of manuscript. MZ assisted with experiments and manuscript preparation.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no conflicts of interest.
Additional information
Editor: David Songstad
Rights and permissions
About this article
Cite this article
Ahmad, W., Zahir, A., Nadeem, M. et al. Thidiazuron-induced efficient biosynthesis of phenolic compounds in callus culture of Ipomoea turbinata Lagasca and Segura. In Vitro Cell.Dev.Biol.-Plant 55, 710–719 (2019). https://doi.org/10.1007/s11627-019-10027-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-019-10027-1