Abstract
Conservation of Saccharum spp. germplasm as ex situ collections of plants has a high cost, and in natural conditions, the plants remain exposed to pests, pathogens, and natural disasters. Long-term preservation of plant germplasm is important for agricultural biodiversity and food safety, so the aim of this study was to develop a cryogenic procedure for cryopreservation of sugarcane germplasm. The first study compared droplet vitrification and encapsulation-vitrification techniques for cryopreservation of in vitro shoot tips of Saccharum spp. variety Halaii. The best regeneration rate (70.9%) was obtained from 45-min PVS2 vitrification solution-treated shoot tips via the droplet vitrification technique. This technique was tested on two other Saccharum sp. varieties, and the best regeneration rates for varieties NG 57-024 and H 83-6179 were 63.3 and 76.3%, respectively. Shoots derived from cryopreserved shoot tip buds developed well-formed roots, and were easily acclimated to greenhouse conditions. The second study evaluated genetic stability of the cryopreserved varieties using ten inter-simple sequence repeat primers. A total of 211 (Halaii), 198 (H83-6179), and 201 (NG 57-024) reproducible bands, ranging from 125 to 5500 bp, were scored with this technique. One hundred genetic stability was detected from Halaii and H 83-6179 whereas 98.5% genetic stability was detected from varieties of NG 57-024. The PCR reactions showed that there was no crucial variation on genetic stability for all cryopreserved varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term preservation of plant germplasm is important for agricultural biodiversity and food safety. Plant genetic variations provide options to cultivate new and more fruitful crops that are resistant to negative ecological and biological conditions (Rao 2004). Saccharum sp. (sugarcane) germplasm is preserved in some areas of India and the USA as ex situ collections, but it is too expensive to maintain big collections and the collections can be negatively affected by pests and disasters in natural conditions. For this reason, approximately 61% of the clones in the US collections were lost between 1957 and 1977 (Berding and Roach 1987). To prevent these kinds of problem, researchers have developed large numbers of in vitro collections for different plant species (Engelmann 1991).
Long-term conservation by cryopreservation techniques is important for the conservation of the world’s genetic resources and in plant preservation programs (Benson 1999). Biotechnological approaches provide beneficial techniques for plant genetic resources and evaluation (Paunesca 2009). The major advantages of cryopreservation, the most important technique for conservation of biological materials, are ease and practicality of its use for a large range of genotypes (Engelmann 2004; Souza et al. 2016; Kaya et al. 2017).
Synthetic seed technology and vitrification procedures are combined in the encapsulation-vitrification technique, in which explants are encapsulated in alginate beads, then soaked in liquid nitrogen (LN, −196°C) for vitrification (Sakai et al. 2002). This technique has been used successfully for cryopreservation of different plant species such as Ananas comosus (L.) Merr. (pineapple, Gamez-Pastrana et al. 2004), Citrus aurantium amara L. (bitter orange, Al-Ababneh et al. 2002), Daucus carota L. (carrot, Tannoury et al. 1991; Hirai 2001), Ipomoea batatas (L.) Lam. (sweet potato, Hirai and Sakai 2003), Pyrus malus L. (apple, Paul et al. 2000), Manihot esculenta Crantz (cassava, Charoensub et al. 2004; Hirai 2001), and Olea europaea L. (olive, Shibli and Al-Juboory 2000).
The droplet vitrification technique is based on treating shoot tips with small volumes of vitrification solution (3–5 μL) on an aluminum strip, which is then plunged into LN (Panis et al. 2001). This technique has proven effective for many plant species and has produced better regeneration rates than other vitrification methods for Thymus vulgaris L. (thyme, Ozudogru and Kaya 2012), Eucalyptus spp. (Kaya et al. 2013), Musa spp. (banana, Panis et al. 2005), A. comosus (L.) Merr. (pineapple, Souza et al. 2016), and Dioscorea alata L. (yam, Leunufna and Keller 2003).
Genetic stability during cryopreservation will allow the reproduction of conserved plant germplasm that is genetically identical and phenotypically similar to the mother plant (Valles et al. 1993; Ozudogru et al. 2011). Inter-simple sequence repeat (ISSR) markers are frequently used to determine genetic stability and variation of some economically important plant species and to conduct phylogenetic analyses for some systematically difficult and problematic species (Reddy et al. 2002; Vijayan 2005; Kaya 2015).
In this study, the aim was to develop an efficient protocol for cryopreservation of sugarcane germplasm via plant vitrification solution 2 (PVS2)-based procedures. At first, encapsulation-vitrification and droplet vitrification techniques were compared for Saccharum spp. variety Halaii and the best regeneration results were obtained from the droplet vitrification technique. Then, this technique was applied to two other varieties H83-6179 and NG 57-024. The second aim of this study was to confirm the genetic stability of cryopreserved lines of Saccharum spp. using ISSR marker analysis.
Materials and Methods
Plant material
A germplasm collection maintained at the National Center for Genetic Resources Preservation (Fort Collins, CO) as long-term in vitro shoot cultures of three Saccharum officinarum L. varieties consisting of Halaii (Q44830), H 83-6179 (Q42433), and NG 57-024 (Q45251) was used for this study.
Culture conditions
Shoot cultures were subcultured every 4–6 wk in double Magenta® GA-7 vessels (Sigma-Aldrich®, St. Louis, MO) containing 50 mL of MS (Murashige and Skoog 1962) regeneration medium (MS-519, 2.22 g L−1, Sigma-Aldrich®), with 20 g L−1 sucrose, 4.44 μM 6-benzylaminopurine (Sigma-Aldrich®), and 1.25 g L−1 Gelrite™ (P8169, Sigma-Aldrich®). The pH was adjusted with HCl and NaOH to 5.6, with 3 g L−1 charcoal (C325, Sigma-Aldrich®), and medium was autoclaved at 121°C for 20 min. Cultures were maintained in plant growth room at 25 ± 2°C with a 16-h photoperiod under a light intensity of 50 μmol−1 m−2 s−1 provided by cool daylight fluorescent lamps (Osram Sylvania, Wilmington, MA).
Saccharum sp. shoot tips (0.5–1 mm) excised from in vitro plants were cultured as described above for shoot cultures after placing ten shoot tips in each Petri dish (60 × 15 mm) containing regeneration medium (Fig. 1 A, B). The percentage of shoot tips that regenerated, at least, one well-formed shoot with, at least, two nodes was recorded after 4–6 wk for each variety as regeneration control groups.
Encapsulation-vitrification
Excised shoot tips were washed with Ca++-free liquid MS medium followed by immersion into a 3% (w/v) sodium alginate (low viscosity, A2158, Sigma-Aldrich®) solution in MS nutrition medium. Individual shoot tips were then pipetted with a drop of alginate into liquid MS medium containing 100 mM CaCl2 (Dereuddre 1992). The alginate beads, each containing a shoot tip, were kept for 20 min at room temperature (25 ± 2°C) in the 100 mM CaCl2 solution to ensure complete polymerization of the calcium alginate (Fig. 1 C). Alginate beads were then removed from the CaCl2 solution and placed for 24 h on MS semisolid basal medium with 0.625 M sucrose. The encapsulated shoot tips were treated with PVS2 solution (30% [w/v] glycerol, 15% [w/v] ethylene glycol, 15% [w/v] dimethyl sulfoxide, and 0.4 M sucrose in MS regeneration medium) within 1.5-mL cryovials (Nalgene®, Thermo Fisher Scientific®, Paisley, UK) at 0°C for different lengths of time (30, 45, or 60 min.). After PVS2 treatment, ten beads were kept in 1 mL PVS2 solution in a 1.8-mL cryotube and then immersed into LN. The cryotubes were kept there for, at least, 1 d, and each treatment was repeated, at least, three times.
Rewarming was done after a minimum of 24 h in LN by rapidly warming the cryovial for 2 min in a 40°C water bath, followed by removing the beads from the cryovials and transferring them onto MS regeneration medium followed by culture as described above for shoot cultures.
Droplet vitrification
Excised shoot tips of the three varieties were pre-cultured for 24 h on semisolid MS medium containing 0.625 M sucrose. Individual shoot tips were then placed in 4–5-μL drops of PVS2 on sterile aluminum foil strips (5 × 15-mm strips, five PVS2 drops per strip) and kept on ice for 30, 45, or 60 min. Following treatment with PVS2, the aluminum foil strips including the shoot tips in the PVS2 droplets were directly immersed into LN and transferred under LN into 1.5-mL cryovials (Sakai and Engelmann 2007). Rewarming was done after a minimum of 24 h LN exposure by rapidly removing the aluminum foil strips from the cryovials and immediately immersing them into washing solution (plant growth regulator-free liquid MS medium containing 1 M sucrose) at 25 ± 2°C for 15 min prior to transferring the shoot tips onto MS regeneration medium. Control group shoot tips were selected from shoot tips treated with PVS2 for the same time periods as LN-treated material, but not exposed to LN. These shoot tips were immediately placed in washing solution for 15 min following PVS2 exposure. Ten shoot tips were used for each PVS2 treatment time (30, 45, or 60 min), and each treatment was repeated, at least, three times.
Data collection and statistical analyses
The experiments (encapsulation-dehydration, droplet vitrification) were analyzed independently from each other. Growth was recorded 6 wk after each treatment unless noted otherwise and consisted of the percentage of shoot tips that regenerated, at least, one well-formed shoot with, at least, two nodes, and shoot forming capacity (SFC) was calculated (SFC = average number of shoots per regenerating explant × % of regenerating explant / 100, Lambardi et al. 1993). Statistical analyses of the non-parametric data (frequencies) were achieved via a means test for homogeneity of proportions, and major processing differences were detected using the post hoc multiple comparisons test (Marascuilo and McSweeney 1977). Dissimilar data were subjected to ANOVA, followed by the least significant difference (LSD) test at P ≤ 0.05 to compare means.
Inter-simple sequence repeat analyses
Genomic DNA was isolated via a cetyltrimethylammonium bromide (CTAB) method (Doyle and Doyle 1987) from 2 g fresh leaf samples ground with a mortar and pestle collected from sugarcane microshoots before and after cryopreservation. For genetic stability analysis, leaf samples were taken from untreated shoots (in vitro cultures), sucrose pre-cultured shoots, cold hardened shoots, PVS2-treated shoots (chosen from the best cryopreservation result treatment), and cryopreserved shoots (leaf samples of each variety came from the same clone).
ISSR-PCR DNA amplifications were carried out using ten ISRR primers (Table 1, Martins-Lopes et al. 2007; Kaya 2015), and PCR reactions were performed in a 20-μL reaction mixture, containing PCR buffer (1× final concentration, Thermo Fisher Scientific® GeneAmp® 10X PCR Buffer), 2.5 mM MgCl2, 0.4 mM of each dNTP, 0.4 mM ISSR primer, 40 ng genomic DNA, and 1 U Taq DNA polymerase (Thermo Fisher Scientific®, Invitrogen™). Amplification conditions were 3 min at 95°C, followed by 35 cycles of 15 s at 95°C, 30 s at 55°C, and 3 min at 72°C, and a final extension of 10 min at 72°C. After separation on a 1.5% (w/v) agarose gel (Sigma-Aldrich®), the PCR products were stained with a solution of 0.5 mg mL−1 ethidium bromide, and then monitored under UV light. Images were documented using the ChemiDoc™ XRS+ and Image Lab™ Software (Bio-Rad®, Hercules, CA) image analysis system. Bands were scored as present (1) or absent (0), and cluster analysis was used to draw dendrograms, with the Unweighted Pair Group Method with Arithmetic mean (UPGMA) from the similarity data matrices using Jaccard’s coefficient (Rohlf 1998).
Results and Discussion
Cryopreservation of Saccharum spp. variety Halaii (Q44830) via droplet and encapsulation-vitrification techniques
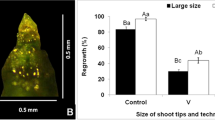
Regeneration rates of variety Halaii shoot tips were high (91.7%) after the sucrose pre-culture period. Gonzalez Arnao et al. (1993) studied the effect of sucrose concentration during the pre-growth treatment prior to cryopreservation on the regeneration of encapsulated apices of six sugarcane varieties, and found the optimal sucrose concentration to be 0.75 M. In the present study, it was observed that shoot tips, treated with PVS2 but not cooled in LN (PVS2 control), maintained their regeneration potential and exhibited regeneration rates of 37.5–75% (Table 2, Figs. 2 A, B and 3 A, B). These shoot tips showed high mean shoot numbers (up to 1.8) and lengths (up to 12.9 mm), indicating that shoot tips which tolerated well the toxic effects of PVS2 and maintained a high regeneration potential were also able to produce multiple adventitious shoots. The regeneration rates of encapsulated shoot tip controls were high (Table 2, Fig. 3 C), and, after the treatment with PVS2, their regeneration rates ranged from 55 to 72.2%.
For the droplet vitrification method, the best regeneration rate (70.9%) was with a 45-min PVS2 treatment after liquid nitrogen exposure. Regeneration rates of cryopreserved shoot tips appeared to be significantly different between droplet and encapsulation-vitrification techniques (Table 2). After cryopreservation, the highest regeneration rate of sugarcane-encapsulated shoots was 27.8%, whereas the highest regeneration rate of shoots cryopreserved via droplet vitrification was 70.9%. However, Barraco et al. (2011) reported better regeneration from in vitro shoot tips of two sugarcane clones cryopreserved via encapsulation-dehydration (53 and 60%) vs. droplet vitrification (27 and 37%). Additionally, Rafique et al. (2015) showed that V cryo-plate is an efficient and practical method for cryopreservation of sugarcane shoot tips in genebanks and they obtained maximum (100%) recovery after cryopreservation.
Although cryoprotective solutions (especially those containing dimethylsulfoxide (DMSO) have protective effects during freezing, they may be toxic to the tissues under conditions of high concentration or temperature, or long exposure times. Kaya et al. (2013) reported superior results using droplet vitrification vs. encapsulation-vitrification for cryopreservation of Eucalyptus spp., reporting up to 84.8% post thaw recovery. Similarly, the droplet vitrification method proved to be the most effective in the present study. Droplet vitrification has been repeatedly reported to have many advantages compared to other cryopreservation techniques. Among these, the most significant is the extremely fast cooling and rewarming rates that can be achieved by using aluminum foil (Towill and Bonnart 2003; Panis et al. 2005; Ozudogru and Kaya 2012).
Cryopreservation of Saccharum spp. varieties H 83-6179 and NG 57-024 via droplet vitrification
The results indicated that this method is effective for the cryopreservation of Saccharum sp. shoot tips (Table 3). Exposure to PVS2 for 45 min yielded the highest regeneration rate for NG 57–024 (63.3%), the treatment found previously to be best for Halaii (Table 2). However, the best regeneration rate for variety H 83-6179 (76.3%) was obtained with a 30-min PVS2 treatment. The regeneration rates of the 0.625-M sucrose pre-treated controls were significantly higher than those of PVS2-treated controls, which demonstrates the toxic effect of PVS2 on Saccharum shoot tips. Additionally, as expected due to the stresses imposed by the cryogenic process, for all varieties there was a significant decrease in regeneration after LN treatment. However, despite this decrease in regeneration from the controls to the LN-treated shoot tips, shoot tip regeneration in all varieties was very respectable varying between 30% (H 83-6179, 45-min PVS2 treatment) and 76.3% (H 83-6179, 30-min PVS2 treatment) after LN exposure. In all lines, after rewarming and transfer to regeneration medium, regenerated shoot tips showed typical shoot development (Fig. 4 A–C).
Evaluation of genetic stability using ISSR primers
Genetic stability is crucial, especially in economically important plant species, and it is essential to set up appropriate micropropagation and cryopreservation protocols that result in genetically identical and genetically stable plants before they are marketed (Saha et al. 2016). ISSR analysis of untreated, sucrose pre-cultured, cold hardened, PVS2-treated, and cryopreserved shoots coming from the same clones was conducted. Complete (100%) genetic stability was detected for Halaii and H 83-6179, and 98.5% genetic stability was detected for NG 57-024 based on ten ISSR primers. A total of 211 (Halaii), 198 (H83-6179), and 201 (NG 57-024) reproducible bands, ranging from 125 to 5500 bp, were scored. However, only three bands (2000, 720, and 625 bp) that were obtained using the ISSR VII and ISSR VIII primers on line NG 57-024 were present in samples of untreated, sucrose pre-cultured, cold hardened treatments and were absent in PVS2-treated shoots and cryopreserved shoots (Fig. 5). These bands were lost from NG 57-024 samples after treatment with PVS2 including DMSO, and these differences could be the result of DMSO toxicity. DMSO toxicity has been reported in several studies which indicated that DMSO increases benzene metabolism and the toxicity of other aromatic hydrocarbons (Kocsis et al. 1968; Ozudogru et al. 2011; Kaya et al. 2013). Although there were a few differences of ISSR band profiles, these profiles indicate that three lines of sugarcane had high rate of genetic stability after cryopreservation.
Products produced from lines of NG 57-024 using ISSR VII and ISSR VIII primers. M 1-kb ladder; untreated shoots (from in vitro cultures) (1); sucrose pre-cultured shoots (2); cold hardening shoots (3); PVS2-treated shoots (4); and cryopreserved shoots (5). All leaf samples of each line came from the same clone. Arrows indicate polymorphic band profiles
Martin et al. (2014) used molecular markers (RAPD and AFLP) to compare two cryopreservation techniques for Mentha piperita L. (mint) and Chrysanthemum morifolium Ramat. (chrysanthemum): encapsulation-dehydration and droplet vitrification for mint, and encapsulation-dehydration and vitrification for chrysanthemum. They observed more genetic instability after encapsulation-dehydration protocol for both species and the instability occurred after treatments where osmotic stress was imposed, and their results showed the importance of testing genetic stability of the recovered material. On the other hand, Choudhary et al. (2013) examined genetic stability using RAPD and ISSR markers for cryopreserved dormant buds of Morus (mulberry) germplasm. Both types of markers showed monomorphic banding patterns among the mother plant and in vitro regenerants before and after cryopreservation, suggesting that cryopreservation, using two-step freezing, does not affect genetic stability of mulberry germplasm. Similarly in the present study, regenerated plants derived from cryopreserved shoots were 100% (Halaii and H 83-6179) and 98.5% (NG 57-024) genetically similar. Distinct DNA polymorphism may not be induced by cryopreservation (Harding 2004). Clonal fidelity is one of the most important aspects of long-term conservation studies of vegetatively propagated species.
The vitrification process using PVS2 before LN treatment provides a satisfactory method by which sugarcane shoot tips may resist cryopreservation damage. The results of this study showed a simple and useful technique that might be used to predict the tolerance of sugarcane shoot tips to LN treatment. All three varieties of Saccharum spp. benefited from droplet vitrification before storage in LN in terms of regeneration after cryopreservation. Shoots derived from cryopreserved shoot tips had well-formed roots, genetically stable shoots, and they acclimated easily to in vivo conditions.
References
Al-Ababneh SS, Karam NS, Shibli RA (2002) Cryopreservation of sour orange (Citrus aurantium L.) shoot tips. In Vitro Cell Dev Biol Plant 38:602–607
Barraco G, Sylvestre I, Engelmann F (2011) Comparing encapsulation-dehydration and droplet-vitrification for cryopreservation of sugarcane (Saccharum spp.) shoot tips. Sci Hortic 130:320–324
Benson EE (1999) Cryopreservation. In: Benson EE (ed) Plant conservation biotechnology. Taylor and Francis, London, pp 83–95
Berding N, Roach BT (1987) Sugarcane improvement through breeding. In: Heinz DJ (ed) Developments in crop science. Elsevier, Amsterdam, pp 143–210
Charoensub R, Hirai D, Sakai A (2004) Cryopreservation of in vitro-grown shoot tips of cassava by encapsulation-vitrification method. CryoLetters 25:51–58
Choudhary R, Chaudhury R, Malik SK, Kumar S, Pal D (2013) Genetic stability of mulberry germplasm after cryopreservation by two-step freezing technique. Afr J Biotechnol 12:5983–5993
Dereuddre J (1992) Cryopreservation of in vitro cultures of plant cells and organs by vitrification and dehydration. In: Dattee Y, Dumas C, Gallais A (eds) Reproductive biology and plant breeding. Springer, Berlin, pp 291–300
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Focus 12:13–15
Engelmann F (1991) In vitro conservation of tropical plant germplasm—a review. Euphytica 57:227–243
Engelmann F (2004) Plant cryopreservation: progress and prospects. In Vitro Cell Dev Biol Plant 40:427–433
Gamez-Pastrana R, Martinez-Ocampo Y, Beristain CI, GonzalezArnao MT (2004) An improved cryopreservation protocol for pineapple apices using encapsulation-vitrification. Cryo Letters 25:405–414
Gonzalez Arnao MT, Engelmann F, Huet C, Urra C (1993) Cryopreservation of encapsulated apices of sugarcane: effect of freezing procedure and histology. CryoLetters 14:303–308
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. CryoLetters 25:3–22
Hirai D (2001) Studies on cryopreservation of vegetatively propagated crops by encapsulation vitrification method. Rep Hokkaido Pref Agric Exp Sta 99:1–58
Hirai D, Sakai A (2003) Simplified cryopreservation of sweet potato [Ipomoea batatas (L.) Lam.] by optimizing conditions for osmoprotection. Plant Cell Rep 21:961–966
Kaya E (2015) ISSR analysis for determination of genetic diversity and relationship in some Turkish olive (Olea europaea L) cultivars. Not Bot Horti Agrobot Cluj Napoca 43:96–99
Kaya E, Alves A, Rodrigues L, Jenderek M, Hernandez-Ellis M, Ozudogru A, Ellis D (2013) Cryopreservation of Eucalyptus genetic resources. CryoLetters 34:608–618
Kaya E, Souza F, Yilmaz-Gokdogan E, Ceylan M, Jenderek M (2017) Cryopreservation of citrus seed via dehydration followed by immersion in liquid nitrogen. Turk J Biol 41:242–248
Kocsis JJ, Harkaway S, Santoyo MC, Snyder R (1968) Dimethyl sulfoxide: interactions with aromatic hydrocarbons. Science 160:427–428
Lambardi M, Sharma KK, Thorpe TA (1993) Optimization of in vitro bud induction and plantlet formation from mature embryos of Aleppo pine (Pinus halepensis Mill.) In Vitro Cell Dev Biol Plant 29:189–199
Leunufna S, Keller ERJ (2003) Investigating a new cryopreservation protocol for yams (Dioscorea spp.) Plant Cell Rep 21:1159–1166
Marascuilo LA, McSweeney M (1977) Non-parametric and distribution free methods for the social sciences. In: Marascuilo LA (ed) McSweeney M. Books/Cole Publication, Belmont, pp 141–147
Martin C, González I, Kremer C, González-Benito ME (2014) Cryopreservation and genetic stability of shoot cultures in vegetatively propagated species: mint and chrysanthemum. Acta Hortic 1039:127–132
Martins-Lopes P, Lima-Brito J, Gomes S, Meirinhos J, Santos L, Guedes-Pinto H (2007) RAPD and ISSR molecular markers in Olea europaea L.: genetic variability and molecular cultivar identification. Genet Res Crop Evol 54:117–128
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ozudogru EA, Kaya E (2012) Cryopreservation of Thymus cariensis and T. vulgaris shoot tips: comparison of three vitrification-based methods. CryoLetters 33:363–375
Ozudogru EA, Kaya E, Kirdok E, Issever-Ozturk S (2011) In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cell Dev Biol Plant 47:309–320
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55
Panis B, Swennen R, Engelmann F (2001) Cryopreservation of plant germplasm. Acta Hort 560:79–86
Paul H, Daigny G, Sangwan-Norreel BS (2000) Cryopreservation of apple (Malus×domestica Borkh.) shoot tips following encapsulation-dehydration or encapsulation-vitrification. Plant Cell Rep 19:768–774
Paunesca A (2009) Biotechnology for endangered plant conservation: a critical overview. Rom Biotech Letters 14:4095–4104
Rao NK (2004) Plant genetic resources: advancing conservation and use through biotechnology. Afr J Biotechnol 3:136–145
Reddy MP, Sarla N, Siddiq EA (2002) Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 128:9–17
Rafique T, Yamamoto S, Fukui K, Mahmood Z, Niino T (2015) Cryopreservation of sugarcane using the V cryo-plate technique. CryoLetters 36:51–59
Rohlf FJ (1998) NTSYS-pc numerical taxonomy and multivariate analysis system version 2. 0. Exeter publications, New York, USA, pp 1–44
Saha S, Adhikari S, Dey T, Ghosh P (2016) RAPD and ISSR based evaluation of genetic stability of micropropagated plantlets of Morus alba L. variety S-1. Meta Gene 7:7–15
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28:151–172
Sakai A, Matsumato T, Hirai D, Charoensub R (2002) Survival of tropical apices cooled to −196°C by vitrification. In: Li PH, Palva ET (eds) Plant cold hardiness, gene regulation and genetic engineering. Kluwer Academic/Plenum Publishers, New York, pp 109–119
Shibli RA, Al-Juboory KH (2000) Cryopreservation of ‘Nabali’ olive (Olea europea L.) somatic embryos by encapsulation-dehydration and encapsulation-vitrification. CryoLetters 21:357–366
Souza FVD, Kaya E, de Jesus VL, de Souza EH, de Oliveira Amorim VB, Skogerboe D, Matsumoto T, Alves AAC, da Silva Ledo CA, Jenderek MM (2016) Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell Tissue Organ Cult 124:351–360
Tannoury M, Ralambosoa J, Kaminsky M, Dereuddre J (1991) Cryopreservation by vitrification of alginate-coated carnation (Dianthus caryophyllus L.) cultured in vitro plantlets. C. R. Acad. Sci. Paris, pp 633–638
Towill LE, Bonnart R (2003) Cracking in a vitrification solution during cooling or warming does not effect growth of cryopreserved mint shoot tips. CryoLetters 24:341–346
Valles MP, Wang ZY, Montavon P, Potrykus I, Spangenberg G (1993) Analysis of genetic stability of plants regenerated trom suspension cultures and protoplasts of meadow rescue (Festuca pratensis Huds.) Plant Cell Rep 12:101–106
Vijayan K (2005) Inter simple sequence repeat (ISSR) polymorphism and its application in mulberry genome analysis. Int J Indust Entomol 10:79–86
Acknowledgements
This work was supported by Mugla Sitki Kocman University, Scientific Research Projects Coordination Unit (Mugla, Turkey, MSKU-BAP 16/021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Barbara Reed
Rights and permissions
About this article
Cite this article
Kaya, E., Souza, F.V.D. Comparison of two PVS2-based procedures for cryopreservation of commercial sugarcane (Saccharum spp.) germplasm and confirmation of genetic stability after cryopreservation using ISSR markers. In Vitro Cell.Dev.Biol.-Plant 53, 410–417 (2017). https://doi.org/10.1007/s11627-017-9837-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9837-2