Abstract
UDP glucose flavonoid 3-O-glycosyltransferase(3GT) is a key enzyme in biosynthesis of stable anthocyanins, which are generally required for flower coloration. Fh3GT1, a cDNA encoding the 3GT from Freesia hybrida, was overexpressed in Petunia hybrida to investigate the influence on flavonoid accumulation. Flowers of transgenic petunia showed color alteration from white to pink and were subjected to further analysis by HPLC-ESI-MS. The results indicated that overexpression of Fh3GT1 resulted in evident increase of cyanidin, peonidin, and kaempferol content in transgenic flowers, whereas the content of quercetin increased slightly. Expressions of endogenous flavonoid biosynthetic pathway genes in transgenic flowers were analyzed by real-time qPCR. The results showed that transcript levels of PhCHS, PhCHI, PhF3H, and Ph3GT were significantly upregulated. Meanwhile, overexpression of Fh3GT1 also led to enhanced expression of AN1 (bHLH) and MYBx (R3MYB) genes, as the mRNA levels of other transcription factors remained constant in control and overexpression lines. Thus, Fh3GT1 overexpression increased the accumulation of anthocyanins and flavonols by modulating the expressions of various flavonoid biosynthesis genes in flowers of transgenic petunia. These results also suggest that Fh3GT1 is a key gene involved in anthocyanin and flavonol biosynthesis and should serve as a useful candidate to modify the components of flavonoid in other plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthocyanins, the largest subclass of plant flavonoids, are ubiquitous in nature, and present in both ornamental and crop plants (Tanaka et al. 2009). They are water-soluble and synthesized in the cytosol, but are sequestered in vacuoles (Tanaka et al. 2008). Although there are only six types of anthocyanin aglycone—pelargonidin, cyanidin, peonidin, delphinidin, petunidin, and malvidin—in the plant kingdom (Bueno et al. 2012), a vast variety of anthocyanins can be synthesized through modification of the basic skeleton by different enzymes such as glycosyltransferases, methyltransferases, and acyltransferases (Nishihara and Nakatsuka 2010). Anthocyanins, a class of colored flavonoid, are largely responsible for diverse pigmentation from shiny orange to pink, red, violet, and blue colors in flowers, fruits, and seeds of some plants (Grotewold 2006), where they serve as visual signals for attracting pollinators and seed dispersers (Castaneda-Ovando et al. 2009). Flavonols, another kind of flavonoid, affect the brightness and brilliance of colors and have important roles in pollen germination (Ferreyra et al. 2012; Zhao and Tao 2015). Furthermore, due to their high antioxidant activity, dietary uptake of anthocyanins and flavonols also play an important role in the prevention of cancer, diabetes, cardiovascular diseases, and many other diseases (Pascual-Teresa 2014; Petroni et al. 2014; Liobikas et al. 2016).
Because of the importance of flavonoids, a number of structural genes involved in the flavonoid biosynthetic pathway have been isolated and well characterized (Cultrone et al. 2010; Kibet 2013). The flavonoid biosynthetic pathway is one of the most extensively studied secondary metabolic pathways in plants (Grotewold 2006; Tanaka et al. 2008). The first enzyme specific for the flavonoid pathway is chalcone synthase (CHS), which catalyzes one molecule of 4-coumaroyl-CoA together with three molecules of malonyl-CoA to form naringenin chalcone. Naringenin chalcone is rapidly isomerized into the colorless naringenin by chalcone isomerase (CHI) (Li et al. 2006; Tanaka et al. 2008). After hydroxylation of the C-ring of naringenin (but not the B-ring) by flavanone 3-hydroxylase (F3H), naringenin is converted to dihydroflavonol (Maleka et al. 2013). Subsequently, both flavonol synthase (FLS) and dihydroflavonol 4-reductase (DFR) utilize dihydroflavonols as substrates to yield flavonol and leucoanthocyanidins, respectively. The colorless leucoanthocyanidins are transformed to corresponding colored anthocyanidins by anthocyanidin synthase (ANS) (Oppermann et al. 2003). Finally, anthocyanidins are glycosylated by the action of 3GT which is the essential enzyme in the flavonoid pathway to produce stable, colorful anthocyanin (Heller and Forkmann 1988).
Glycosylation is often the key reaction in the biosynthesis of flavonoid, and catalyzed by the multi-gene family of glucosyltransferases. The anthocyanidin aglycone is affected by light, temperature, and pH, and is highly reactive (Prior and Wu 2006). Glucosylation of the anthocyanidin aglycone at the 3-hydroxyl group decreases their reactivity and increases their chemical stability, as well as changing the solubility, complexity, and toxicity (Vogt and Jones 2000). Also, the glucosylated metabolites are often required for their transport and storage, because they can be frequently recognized by signal receptors but not their aglycones (Jones and Vogt 2001).
Since the flavonoid biosynthesis has been very well studied, it is possible to alter the flavonoids of flowers and crop plants by overexpression/silencing the genes involved in the flavonoid pathway (Nishihara and Nakatsuka 2010; Chandler and Brugliera 2011). Recently, several reports have discussed the relationship between transcription of 3GT and flavonoid accumulation in different plant species. For example, by expressing 3GT complementary DNA (cDNA) from snapdragon, Schwinn et al. produced transgenic lisianthus with novel 3-O-glucosylated anthocyanins in petals (Schwinn et al. 1997). Similarly, ectopic expression of 3GT gene in somatic embryos of grape caused reddish-purple spots on the grape skin (Kobayashi et al. 2002).
The flowers of Freesia hybrida accumulate high levels of flavonoid. 3GT has been demonstrated to be a key enzyme of flavonoid biosynthesis in Freesia (Wei et al. 2016). In this study, the cDNA encoding flavonoid 3-O-glucosyltransferase from Freesia (Fh3GT1) was overexpressed in petunia to study its influence on flavonoid biosynthesis. Firstly, we performed HPLC to determine the accumulation of anthocyanins and flavonols in transgenic flowers. And then the expression of endogenous flavonoid biosynthetic pathway genes was checked by real-time qPCR. Overexpression of Fh3GT1 increased transcript levels of PhCHS, PhCHI, PhF3H, Ph3GT, AN1 (bHLH), and MYBx (R3MYB) were significantly upregulated. These novel findings will aid future efforts to manage flavonoid biosynthesis in Freesia and in other plants.
Materials and Methods
Plant materials
Petunia hybrida plants (Xinhui Horticulture Co., Chifeng, China) for transformation were grown aseptically on half-strength MS basal medium (Murashige and Skoog 1962) supplemented with 3% (w/v) sucrose. Flowers of transgenic plants at the full-bloom stage and flowers from one Freesia cultivar, Red River®, were frozen in liquid nitrogen and stored at −80°C until required.
Expression vector construction and plant transformation
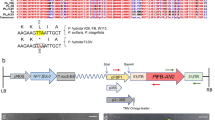
A pair of primers, 5′-CGGTCTAGACAGCAAGCAATGGGATCG-3′ (the XbaI site is underlined) and 5′-CGAGGATCCCAGACTTCAGTCATATTCCGA-3′(the BamHI site is underlined), was designed to amplify the whole coding regions of Fh3GT1 gene, using cDNA from flowers of Red River®, as template. PCR products were digested with XbaI and BamHI, and then inserted into XbaI/BamHI-digested pBI-121 vector containing the constitutive cauliflower mosaic virus 35S promoter (Fig. S1). The binary vectors which were verified to contain entire nucleotide sequences without nucleotide mutations were introduced into Agrobacterium tumefaciens EHA105 competent cells. The A. tumefaciens were grown in YEP medium to A600 of 2.0 at 28°C, followed by centrifugation and resuspension in MS liquid medium with acetosyringone (20 μg l−1) until an A600 of 0.6 was reached. Mature leaves collected from a 2-mo-old petunia cultivar with white flowers were used for transformation. The leaves were sterilized and cut into pieces for immersing into the bacterial suspension. After co-cultivation, the leaves were selected on MS medium with 50 mg l−1 of kanamycin for shoot induction. About 10–12 wk later, the shoots were transferred to the medium for rooting, and then the rooted plantlets were transferred to pot and grown in a greenhouse (Sui et al. 2011). To confirm the expression of Fh3GT1 gene in transformed petunia, total RNA was extracted from flowers of T0 transgenic petunia using the RNAiso Plus (TaKaRa, Dalian, China). Then, the extracted total RNA was treated with RNase-free DNaseI (TaKaRa) and reverse transcribed using M-MLV reverse transcriptase (Promega, Madison, Wisconsin, USA). The 18S rRNA gene was used as an internal control with the following primer sequences: 5′-TACCACATCCAAGGAAGGCA-3′ and 5′-ACCCAAAGTCCAACTACGAG-3′. The PCR cycling parameters were initially denaturized at 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 54°C for 40 s and 72°C for 90 s, and a final extension at 72°C for 5 min. RNA isolated from wild-type plants as described above was used as a negative control, and 10-μl PCR products were analyzed by electrophoresis on 1% (w/v) agarose gels.
Anthocyanin and flavonol analysis
Anthocyanins and flavonols were extracted from 0.25 g blooming flowers of transgenic and control plants with 1 ml of extraction solvent MeOH/HCl (H2O/MeOH/HCl 75/24/1 v/v/v). The extract was incubated at 4°C for 12 h in the dark and then centrifuged at 10,800 g for 10 min at room temperature. Prior to HPLC analyses, the supernatants were filtered with a 0.22-μm filter (Kovinich et al. 2010). Then, 20-μl aliquots were analyzed by a Shimadzu HPLC system coupled with an ACCHROM XUnion 5-μm C18 column (250 × 4.6 mm) and the temperature of the column was 35°C. In HPLC separation, the column was washed at flow rate of 1 mL min−1 by a gradient between 5% formic acid in H2O (eluent A) and methanol (eluent B). The gradient conditions were as follows: 0–10 min, 14–17% B; 10–35 min, 17–23% B; 35–60 min, 23–47% B; 60–67 min, 47–14% B; 67–70 min, 14% B. The chromatogram was monitored at the absorption spectra 520 nm for anthocyanin and 360 nm for flavonol. MS data were collected in the positive mode with mass range m/z 100–1000. The ion spray voltage, capillary temperature, declustering potential, and entrance potential were set at 4500 V, 450°C, +80 V, and 10 V, respectively.
Quantification of anthocyanin and flavonol
Anthocyanin and flavonol were quantitated against authentic cyanidin-3-glucoside chloride and quercetin-3-glucoside (Sigma-Aldrich, St. Louis, MO). Cyanidin-3-glucoside chloride and quercetin-3-glucoside (1 mg ml−1) were dissolved in MeOH/HCl and diluted in the range of: cyanidin-3-glucoside (1–16 μg ml−1) and quercetin-3-glucoside (10–500 μg ml−1) to yield standard curves with R 2 > 0.99 (Zhang et al. 2004). Determinations of each sample were performed three times with three biological replicates. Data shown in Table 2 correspond to means of three independent experiments with three replicates each.
Gene expression analysis of transformants
For real-time qPCR, total RNA was isolated from blooming flowers of non-transgenic control and petunia transformants using the RNAiso Plus (TaKaRa), resuspended in 100 μl of RNase-free water, and treated with RNase-free DNase I (TaKaRa). DNase I was heat inactivated before real-time qPCR. Complementary DNA (cDNA) was synthesized using an oligo-dT primer and M-MLV Reverse Transcriptase (Promega) at 42°C for 60 min. Primers for endogenous structural and regulatory genes of flavonoid biosynthesis in P. hybrida were used as previously (Table S1) (Albert et al. 2011; Koseki et al. 2005). Alpha-tubulin was used as internal control (Koseki et al. 2005). Real-time qPCR was performed on a StepOnePlus system (Applied Biosystems) using SYBR Green Real-Time PCR Master Mix (TOYOBO, Osaka, Japan). Reaction mixtures (10 μl) included the following components: 2× Master Mix, 0.5 μM of each primer, and 1 μl cDNA. Cycling conditions were 95°C for 60 s, followed by 40 cycles of 95°C for 5 s and 60°C for 60 s. To confirm the proper amplification of target fragments, melting curve analysis was carried out at the end of 40 cycles. The reaction specificities were tested by agarose gel electrophoresis and sequencing. Relative expression ratios were calculated with comparative cycle threshold (ΔΔCT) method, with tubulin gene as a reference. In the experiments, three replicates for each RNA sample were included.
Statistical analysis
All data in this work is represented as mean values from at least three independent experiments with three replicates each. Statistical significances of the differences were determined by Student’s t test. Differences between transgenic lines and wild-type were considered significant when P < 0.05 or 0.01 in a two-tailed analysis.
Results
Analysis and deduced structure of Fh3GT1
The full-length cDNA of Fh3GT1 (HM590645) was cloned as described previously (Sui et al. 2011), and its amino acid sequence was aligned with UGT78G1 (flavonoid 3-O-glucosyltransferase from Medicago truncatula, A6XNC6), whose crystal structure has been solved recently (Modolo et al. 2009). As shown in Fig. 1, Fh3GT1 displayed 41.7% overall identity with UGT78G1. Subsequently, Fh3GT1 was submitted to comparative modeling analysis, and structurally conserved regions of the Fh3GT1 model were built from the crystal structure of UGT78G1 using the Pyre2 server (Kelley and Sternberg 2009) (Fig. 2). Fh3GT1 had a typical PSPG motif and highly conserved key residues for recognizing substrate and catalysis of UFGTs: a His residue, His-19 (His-26 in UGT78G1), that is highly conserved among UFGTs acts as a general base and key catalytic residue to activate the hydroxyl group of the glucosyl acceptor substrate. A nearby well-conserved Asp residue, Asp-113 (Asp-124 in UGT78G1), interacting with the His residue to form a hydrogen bond also plays an important role in catalysis. The residues Trp-355, Asp-376, and Gln-377 that were proposed in previous crystallographic studies as major determinants for the sugar specificity (Modolo et al. 2009) are also conserved in Fh3GT1 (Trp-345, Asp-366, and Gln-367). As described above, although the deduced amino acid sequence of Fh3GT1 showed only 41.7% identities with UGT78G1, their secondary and tertiary structures are conserved. These results indicate that the plant UFGT structures are highly similar to each other, albeit with relatively low sequence identities of these enzymes (Wang 2009).
Amino acid sequence alignment of Fh3GT1 against UGT78G1. α-Helices are highlighted in blue and β-strands in pink. The PSPG motif that interacts with the sugar donor, UDP-Glc, is underlined. The conserved residues for recognizing substrate and the catalysis of Fh3GT1 and UGT78G1 are marked with triangle.
Transformation of petunia
To determine the effect of Fh3GT1 on the biosynthesis of anthocyanin and flavonol in petunia, cDNA of Fh3GT1 under the control of CaMV 35S promoter was transformed into petunia cultivar with white flowers. After selection, eight independent transgenic petunia plants (T0) were generated and showed flower color changes, from white to light pink. On the contrary, no anthocyanin hyper-accumulated phenotypes were observed in vegetative tissues. Among them, four lines (clone NO. 1, NO. 4, NO. 5, and NO. 8) showed significant strengthened intensity of flower coloration (Fig. 3 A) and were subjected to further analyses. Firstly, RT-PCR analysis confirmed the expression of Fh3GT1 in transgenic lines of petunia plants (Fig. 3 B). As is well known, the hue of an anthocyanin extract is proportional to flower color. Correspondingly, the color intensities of the solutions extracted from transgenic flowers increasingly enhanced and were consistent with the accumulation of total anthocyanins (Fig. 3 C). In conclusion, the above results indicated that ectopic expression of Fh3GT1 from Freesia could alter the flower color of petunia.
Functional characterization of Fh3GT1 gene following its overexpression in transgenic petunia lines. (A) Differences in color between wild-type (WT) and transgenic petunia flowers. (B) Expression analysis of the Fh3GT1 in WT and transgenic lines. (C) Extracted solutions from flowers. (D) Contents of total anthocyanins. Data correspond to means of three biological replicates.
Anthocyanin and flavonol analysis of transgenic flowers
The amount of anthocyanin and flavonol was measured in flowers from non-transformed (control) and four transgenic lines. Quantitative analyses showed that flowers of transgenic lines expressing Fh3GT1 accumulated higher levels of total anthocyanins, but anthocyanin contents in non-transformed flowers were undetectable (Fig. 3 D). Subsequently, extracts from transgenic flowers were separated by HPLC (Fig. 4) and the corresponding chromatographic peak was further analyzed by HPLC-ESI-MS to determine the individual component of anthocyanidin and flavonol (Table 1). As shown in Table 2, flowers of transgenic petunia presented markedly enhanced accumulation of cyanidin and peonidin derivatives, which were undetectable in flowers of wild-type petunia. Among different transgenic lines, NO.1 accumulated the most abundant anthocyanin, followed by NO.5, NO.4, and NO.8. Moreover, transgenic flowers also produced significantly higher amounts of kaempferol derivatives and slightly higher levels of quercetin derivatives than those of non-transgenic petunia. These results revealed that ectopic expression of the Fh3GT1 gene could affect the biosynthesis and accumulation of anthocyanin and flavonol in petunia flowers.
HPLC analyses of anthocyanins and flavonols in transgenic petunia flowers. (A–J) HPLC chromatograms of the samples from flowers of WT and transgenic lines. (A, F) WT, (B, G) NO. 1, (C, H) NO. 4, (D, I) NO. 5, (E, J) NO. 8. (A–E) Absorbance at 520 nm for analysis of anthocyanins. (F–J) Absorbance at 360 nm for analysis of flavonols. Peak A1 (peonidin-rutinoside), peak A2 (peonidin 3-glucoside), peak A4 (cyanidin 3-rutinoside), peak A5 (cyanidin derivatives), peak A6 (peonidin derivatives), peak F1 (quercetin derivatives), peak F2 (quercetin-glucuronide), peak F3 (kaempferol 3-glucoside), peak F4 (kaempferol 7-glucoside), peak F6 (kaempferol 3,7-diglucoside), peak F7 (kaempferol derivatives).
Expression analysis of endogenous structural and regulatory genes in transgenic flowers
In order to investigate the coordinate interaction of Fh3GT1 gene with other flavonoid biosynthetic genes in transgenic petunia flowers, the structural genes such as PhCHS, PhCHI, PhF3H, PhDFR, PhANS, and Ph3GT were analyzed by real-time qPCR method. Among them, PhCHS, PhCHI, PhF3H, and Ph3GT were consistently upregulated compared with wild-type control. While similar transcript levels of other structural genes (PhDFR and PhANS) were observed in flowers between non-transformed and transgenic flowers (Fig. 5 A). Simultaneously, several regulatory genes including AN1, AN11, JAF13, AN2, and MYBx were also analyzed. The results showed that transcript levels of regulatory factors such as AN11, JAF13, and AN2 were not affected. However, overexpression of Fh3GT1 genes in petunia greatly influenced the expression of AN1 and MYBx, and expression levels of these two genes were 10.3- to 14.6-fold higher in transgenic flowers (Fig. 5 B). In consequence, our results suggested that overexpression of Fh3GT1 could modulate structural gene expression as well as some regulatory genes.
Expression profiles of flavonoid-related biosynthetic genes in flowers of transgenic petunia lines carrying Fh3GT1 genes. All mRNA transcripts expressed in transgenic flowers were quantified relative to those expressed in wild-type petunia flowers. Asterisks indicate statistically significant differences between means of WT and transgenic plants, as judged by Student’s t test (**P < 0.01).
Discussion
Transfer of the glucosyl moiety from uridine diphosphate (UDP)-glucose to the 3-position of anthocyanidins by 3GT has been identified to be the key for anthocyanidin stability and water solubility (Zhao et al. 2012). Thus, 3GT has been regarded as an indispensable enzyme for the biosynthesis of anthocyanins, rather than simply a modifying enzyme (Schijlen et al. 2004). Until now, flavonoid 3-O-glucosyltransferase genes have been isolated and characterized in Brassica oleracea (Saleh et al. 1976), Gentiana triflora (Tanaka et al. 1996), Antirrhinum majus (Schwinn et al. 1997), P. hybrida (Yamazaki et al. 2002), Iris hollandica (Yoshihara et al. 2005), black soybean (Glycine max (L.) Merr.) (Kovinich et al. 2010), Vitis labrusca (Hall et al. 2012), and potato (Solanum tuberosum L.) (Hu et al. 2011).
It has been reported that most glycosyltransferases in the flavonoid biosynthesis pathway have broader substrate specificity and can glucosylate both anthocyanidins and flavonols (Fukuchi-Mizutani et al. 2003). For instance, flavonoid 3-O-glucosyltransferase from G. triflora (Tanaka et al. 1996), Vitis vinifera (Ford et al. 1998), P. hybrida (Yamazaki et al. 2002), and Medicago (Modolo et al. 2009) can glucosylate the 3-hydroxy group of cyanidin, pelargonidin, and flavonols. Overexpression of Fh3GT1 genes resulted in the accumulation of anthocyanins (cyanidin and peonodin derivatives) as well as flavonols (quercetin and kaempferol derivatives) in transgenic flowers. Fh3GT1 may therefore accept not only anthocyanidins but also flavonols as substrates. Indeed, substrate specificity studies indicated that Fh3GT1 can catalyze the transfer of a glucosyl moiety from UDP-glucose to the 3-hydroxyls of several anthocyanidins and flavonols (Wei et al. 2016).
Accumulation of anthocyanidin and flavonol derivatives was significantly enhanced in flowers of transgenic petunia compared with wild-type control. As for anthocyanins, only cyanidin-based pigments were detected. In flavonoid biosynthesis, DFR converts dihydroflavonols into leucoanthocyanidins and competes with flavonol synthase (FLS) for the substrate to produce unit for anthocyanin biosynthesis (Davies et al. 2003). As the DFR enzyme of petunia does not accept dihydrokaempferol as a substrate, the biosynthesis of pelargonidin-type anthocyanins is blocked (Peter et al. 1987). Simultaneously, the increased formation of kaempferol derivatives by FLS might occur. As shown in Fig. 4, a decrease in peak F1 which had been identified as quercetin derivatives was detected. This may be because the biosynthesis of cyanidin and peonidin derivatives was activated, and the conversion of dihydroquercetin to quercetin was decreased. Also, it has been reported that seedlings of fls1 mutants accumulate higher levels of anthocyanins in Arabidopsis (Owens et al. 2008; Stracke et al. 2009), whereas inactivation of DFR showed enhanced quercetin levels (Pelletier et al. 1999; Gou et al. 2011). Results in this study concurred with these findings, indicating that metabolic flux of the flavonoid pathway might be controlled by substrate competition between DFR and FLS. In addition, transgenic petunia flowers overexpressing Fh3GT1 genes showed an obvious change from white to pink, which were accompanied by the changes in cyanidin and peonidin pigment content. This suggests that manipulation of Fh3GT1 may contribute to modulations of flower colors in other plants.
The influence of Fh3GT1 overexpression on expression of endogenous genes of the flavonoid pathway was analyzed, and the results confirmed that four structural genes, PhCHS, PhCHI, PhF3H, and Ph3GT, were upregulated in transgenic flowers. It has been reported that CHS is the key enzyme in an early stage of the anthocyanin biosynthetic pathway; silencing of CHS gene can be easily monitored by the loss of pigmentation in tobacco and petunia flowers (Napoli et al. 1990; Wang et al. 2006). Similarly, suppression of CHI gene in tobacco also resulted in reduced pigmentation and change of flavonoid components in flower (Nishihara et al. 2005). These results strongly confirm their roles in flavonoid content regulation, and upregulation of PhCHS and PhCHI expression in transgenic flowers might be responsible for the accumulation of anthocyanin and flavonol.
F3H enzymatic protein belongs to 2-oxoglutarate-dependent dioxygenase family and is essential for catalyzing the formation of flavonols. Accumulation of flavonols in tobacco flowers expressing MdANR has been demonstrated under the transcriptional regulation (Han et al. 2012). Thus, upregulation of PhF3H in Fh3GT1-overexpressing petunia might activate the biosynthesis of flavonols. Moreover, F3H enzyme also plays a critical role in anthocyanin biosynthesis. For example, F3H mutants of Dahlia, Streptocarpus, Verbena, and Zinni exhibit white flowers which do not contain anthocyanin. Suppression of F3H gene in Carnation leads to complete loss of its original reddish color (Forkmann and Stotz 1984; Zuker et al. 2002). Catalyzing the conversion of anthocyanidin to anthocyanidin 3-glycoside by 3GT is a crucial step in the biosynthesis of anthocyanin. The relation of anthocyanin accumulation and expression of 3GT has been reported in several plants (Wei et al. 2011; Zhao et al. 2012). Upregulation of Ph3GT expression in flowers of transgenic petunia expressing Fh3GT1 was well correlated with higher levels of anthocyanins. Positive feedback regulation of flavonoid biosynthetic genes by pathway intermediates has been previously identified in Arabidopsis thaliana mutant (Pourcel et al. 2013). Therefore, it is likely that alteration of structural gene expression might be due to the positive feedback regulation by accumulation of anthocyanins and flavonols (flavonoid pathway intermediates) resulting from Fh3GT1 overexpression.
Coordinated transcriptional regulation of structural biosynthetic genes is a major mechanism that determines the final accumulation of flavonoid in plant cells (Petroni and Tonelli 2011). Consequently, transcript levels of regulatory genes were also investigated in transgenic flowers. In this study, MYBx repressing anthocyanin synthesis showed higher levels of expression in transgenic flowers expressing Fh3GT1. This result is in agreement with previous studies of strawberry and gentian in which the transcription repressors were upregulated so as to balance the levels of anthocyanin pigments (Aharoni et al. 2001; Nakatsuka et al. 2013). In the current study of petunia, AN1 acts in concert with AN11 and different R2R3-MYB factors to control anthocyanin production in flowers (Albert et al. 2011). Interestingly, flowers of all transgenic petunia lines showed significantly higher levels of expression of AN1 than those of wild-type petunia. Therefore, it seems that overexpression of Fh3GT1 may affect the regulatory role of MYBx and AN1 genes. However, it is not clear whether the Fh3GT1 protein can interact with structural or transcriptional regulation proteins, or that the increased transcription observed was due to feedback from the increased level of intermediates.
Conclusion
In our study, transgenic petunia lines expressing Fh3GT1 genes accumulated more anthocyanins up to 7.12 μg g−1 than wild-type petunia plants, and showed obvious changes in flavonol accumulation compared with controls. These results suggested that overexpression of the exogenous Fh3GT1 gene would redirect the flavonoid common pathway to anthocyanin branch. Recently, there has been an increasing interest in developing functional foods that are rich in anthocyanins because of their antioxidant properties. The successful manipulation of Fh3GT1 from F. hybrida indicated that this gene could be used for modifying coloration in other important floricultural plants and/or improving nutritive values in plant species of interest.
References
Aharoni A, De Vos C, Wein M, Sun Z, Greco R, Kroon A, Mol JN, O'Connell AP (2001) The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J 28:319–332
Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE, Davies KM (2011) Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J 65:771–784
Ando T, Tatsuzawa F, Saito N, Takahashi M, Tsunashima Y, Watanabe H, Kokubun H, Hara R, Seki H, Hashimoto G (2000) Differences in the floral anthocyanin content of red petunias and Petunia exserta. Phytochemistry 54:495–501
Bueno JM, Sáez-Plaza P, Ramos-Escudero F, Jiménez AM, Fett R, Asuero AG (2012) Analysis and antioxidant capacity of anthocyanin pigments. Part II: chemical structure, color, and intake of anthocyanins. Crit Rev Anal Chem 42:126–151
Castaneda-Ovando A, Pacheco-Hernández MDL, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA (2009) Chemical studies of anthocyanins: a review. Food Chem 113:859–871
Chandler SF, Brugliera F (2011) Genetic modification in floriculture. Biotechnol Lett 33:207–214
Cultrone A, Cotroneo PS, Recupero GR (2010) Cloning and molecular characterization of R2R3-MYB and bHLH-MYC transcription factors from Citrus sinensis. Tree Genet Genomes 6:101–112
Davies KM, Schwinn KE, Deroles SC, Manson DG, Lewis DH, Bloor SJ, Bradley JM (2003) Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 131:259–268
Downey MO, Harvey JS, Robinson SP (2003) Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust J Grape Wine 9:15–27
Ferreyra MLF, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3:222
Ford CM, Boss PK, Høj PB (1998) Cloning and characterization of Vitis vinifera UDP-glucose: flavonoid 3-O-glucosyltransferase, a homologue of the enzyme encoded by the maize bronze-1locus that may primarily serve to glucosylate anthocyanidins in vivo. J Biol Chem 273:9224–9233
Forkmann G, Stotz G (1984) Selection and characterisation of flavanone 3-hydroxylase mutants of Dahlia, Streptocarpus, Verbena and Zinnia. Planta 161:261–265
Fukuchi-Mizutani M, Okuhara H, Fukui Y, Nakao M, Katsumoto Y, Yonekura-Sakakibara K, Kusumi T, Hase T, Tanaka Y (2003) Biochemical and molecular characterization of a novel UDP-glucose: anthocyanin 3′-O-glucosyltransferase, a key enzyme for blue anthocyanin biosynthesis, from gentian. Plant Physiol 132:1652–1663
Gou JY, Felippes FF, Liu CJ, Weigel D, Wang JW (2011) Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 23:1512–1522
Griesbach RJ, Asen S (1990) Characterization of the flavonol glycosides in petunia. Plant Sci 70:49–56
Griesbach RJ, Asen S, Leonnarat BA (1991) Petunia hybrida anthocyanins acylated with caffeic acid. Phytochemistry 30:1729–1731
Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57:761–780
Hall D, Yuan XX, Murata J, Luca VD (2012) Molecular cloning and biochemical characterization of the UDP-glucose: flavonoid 3-O-glucosyltransferase from Concord grape (Vitis labrusca). Phytochemistry 74:90–99
Han Y, Vimolmangkang S, Soria-Guerra RE, Korban SS (2012) Introduction of apple ANR genes into tobacco inhibits expression of both CHI and DFR genes in flowers, leading to loss of anthocyanin. J Exp Bot 63:2437–2447
Heller W, Forkmann G (1988) Biosynthesis, Harbome edn. Chapman and Hall, London
Hu C, Gong Y, Jin S, Zhu Q (2011) Molecular analysis of a UDP-glucose: flavonoid 3-O-glucosyltransferase (UFGT) gene from purple potato (Solanum tuberosum). Mol Biol Rep 38:561–567
Jones P, Vogt T (2001) Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta 3(2):164–174
Kelley LA, Sternberg MJ (2009) Protein structure prediction on the web: a case study using the Phyre server. Nat Protoc 4:363–371
Kibet NK (2013) Fruit skin color and the role of anthocyanin. Acta Physiol Plant 35:2879–2890
Kobayashi S, Ishimaru M, Hiraoka K, Honda C (2002) Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 215:924–933
Koseki M, Goto K, Masuta C, Kanazawa A (2005) The star-type color pattern in Petunia hybrida 'red Star' flowers is induced by sequence-specific degradation of chalcone synthase RNA. Plant Cell Physiol 46:1879–1883
Kovinich N, Saleem A, Arnason JT, Miki B (2010) Functional characterization of a UDP-glucose: flavonoid 3-O-glucosyltransferase from the seed coat of black soybean ( Glycine max(L.) Merr.) Phytochemistry 71:1253–1263
Li F, Jin Z, Qu W, Zhao D, Ma F (2006) Cloning of a cDNA encoding the Saussurea medusa chalcone isomerase and its expression in transgenic tobacco. Plant Physiol Bioc 44:455–461
Liobikas J, Skemiene K, Trumbeckaite S, Borutaite V (2016) Anthocyanins in cardioprotection: a path through mitochondria. Pharmacol Res. doi:10.1016/j.phrs.2016.03.036
Maleka M, Albertyn J, Spies J (2013) The floriculture industry and flower pigmentation—a review. Philos Trans Genet 2:55–110
Modolo LV, Li L, Pan H, Blount JW, Dixon RA, Wang X (2009) Crystal structures of glycosyltransferase UGT78G1 reveal the molecular basis for glycosylation and deglycosylation of (iso) flavonoids. J Mol Biol 392:1292–1302
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakatsuka T, Yamada E, Saito M, Fujita K, Nishihara M (2013) Heterologous expression of gentian MYB1R transcription factors suppresses anthocyanin pigmentation in tobacco flowers. Plant Cell Rep 32:1925–1937
Napoli C, Lemieux C, Jorgensen R (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2:279–289
Nishihara M, Nakatsuka T (2010) Genetic engineering of novel flower colors in floricultural plants: recent advances via transgenic approaches. Methods Mol Biol 589:325–347
Nishihara M, Nakatsuka T, Yamamura S (2005) Flavonoid components and flower color change in transgenic tobacco plants by suppression of chalcone isomerase gene. FEBS Lett 579:6074–6078
Oppermann U, Filling C, Hult M, Shafqat N, Wu X, Lindh M, Shafqat J, Nordling E, Kallberg Y, Persson B (2003) Short-chain dehydrogenases/reductases (SDR): the 2002 update. Chem Biol Interact 143:247–253
Owens DK, Alerding AB, Crosby KC, Bandara AB, Westwood JH, Winkel BSJ (2008) Functional analysis of a predicted flavonol synthase gene family in Arabidopsis. Plant Physiol 147:1046–1061
Pascual-Teresa SD (2014) Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins. Arch Biochem Biophys 559:68–74
Pelletier MK, Burbulis IE, Winkel-Shirley B (1999) Disruption of specific flavonoid genes enhances the accumulation of flavonoid enzymes and end-products in Arabidopsis seedlings. Plant Mol Biol 40:45–54
Peter M, Iirs H, Gert F, Heinz S (1987) A new petunia flower colour generated by transformation of a mutant with a maize gene. Nature 677–678
Petroni K, Pilu R, Tonelli C (2014) Anthocyanins in corn: a wealth of genes for human health. Planta 240:901–911
Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci 181:219–229
Pourcel L, Irani NG, Koo AJ, Bohorquez-Restrepo A, Howe GA, Grotewold E (2013) A chemical complementation approach reveals genes and interactions of flavonoids with other pathways. Plant J 74:383–397
Prior RL, Wu X (2006) Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res 40:1014–1028
Saleh NA, Poulton JE, Grisebach H (1976) UDP-glucose: cyanidin 3-O-glucosyltransferase from red cabbage seedlings. Phytochemistry 15:1865–1868
Schijlen EGWM, Vos CHRD, Tunen AJV, Bovy AG (2004) Modification of flavonoid biosynthesis in crop plants. Phytochemistry 65:2631–2648
Schwinn KE, Davies KM, Deroles SC, Markham KR, Miller RM, Bradley JM, Manson DG, Given NK (1997) Expression of an Antirrhinum majus UDP-glucose: flavonoid-3-O-glucosyltransferase transgene alters flavonoid glycosylation and acylation in lisianthus ( Eustoma grandiflorum Grise.) Plant Sci 125:53–61
Stracke R, Vos RCHD, Bartelniewoehner L, Ishihara H, Sagasser M, Martens S, Weisshaar B (2009) Metabolomic and genetic analyses of flavonol synthesis in Arabidopsis thaliana support the in vivo involvement of leucoanthocyanidin dioxygenase. Planta 229:427–445
Sui X, Gao X, Ao M, Wang Q, Yang D, Wang M, Fu Y, Wang L (2011) cDNA cloning and characterization of UDP-glucose: anthocyanidin 3-O-glucosyltransferase in Freesia hybrida. Plant Cell Rep 30:1209–1218
Tanaka Y, Brugliera F, Chandler S (2009) Recent progress of flower colour modification by biotechnology. Int J Mol Sci 10:5350–5369
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749
Tanaka Y, Yonekura K, Fukuchi-Mizutani M, Fukui Y, Fujiwara H, Ashikari T, Kusumi T (1996) Molecular and biochemical characterization of three anthocyanin synthetic enzymes from Gentiana triflora. Plant Cell Physiol 37:711–716
Tatsuzawa F, Saito N, Yokoi M (1996) Anthocyanins in the flowers of Cymbidium. Lindleyana 11:214–219
Vogt T, Jones P (2000) Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci 5:380–388
Wang CK, Chen PY, Wang HM, To KY (2006) Cosuppression of tobacco chalcone synthase using Petunia chalcone synthase construct results in white flowers. Bot Stud 47:71–82
Wang X (2009) Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett 583:3303–3309
Wei Q, Wang QY, Feng ZH, Wang B, Zhang YF, Yang Q (2011) Increased accumulation of anthocyanins in transgenic potato tubers by overexpressing the 3GT gene. Plant Biotechnol Rep 6:69–75
Wei S, Lingj L, Xiangyu M, Yueqing L, Fengzhan G, Xingxue L, Shucai W, Xiang G, Li W (2016) Biochemical and molecular characterization of a flavonoid 3-O-glycosyltransferase responsible for anthocyanins and flavonols biosynthesis in Freesia hybrida. Front Plant Sci. doi:10.3389/fpls.2016.00410
Yamazaki M, Yamagishi E, Gong Z, Fukuchi-Mizutani M, Fukui Y, Tanaka Y, Kusumi T, Yamaguchi M, Saito K (2002) Two flavonoid glucosyltransferases from Petunia hybrida: molecular cloning, biochemical properties and developmentally regulated expression. Plant Mol Biol 48:401–411
Yoshihara N, Imayama T, Fukuchi-Mizutani M, Okuhara H, Tanaka Y, Ino I, Yabuya T (2005) cDNA cloning and characterization of UDP-glucose: anthocyanidin 3-O-glucosyltransferase in Iris hollandica. Plant Sci 169:496–501
Zhang Z, Kou X, Fugal K, McLaughlin J (2004) Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J Agric Food Chem 52:688–691
Zhao D, Tao J (2015) Recent advances on the development and regulation of flower color in ornamental plants. Front Plant Sci 6:261
Zhao ZC, Hu GB, Hu FC, Wang HC, Yang ZY, Lai B (2012) The UDP glucose: flavonoid-3-O-glucosyltransferase (UFGT) gene regulates anthocyanin biosynthesis in litchi (Litchi chinesis Sonn.) during fruit coloration. Mol Biol Rep 39:6409–6415
Zuker A, Tzfira T, Ben-Meir H, Ovadis M, Shklarman E, Itzhaki H, Forkmann G, Martens S, Neta-Sharir I, Weiss D (2002) Modification of flower color and fragrance by antisense suppression of the flavanone 3-hydroxylase gene. Mol Breed 9:33–41
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31170276, 31300271), Jilin Provincial Government of the People’s Republic of China (20130102061JC), the Fundamental Research Fund for the Central Universities (2412015ZH006), and the Initial Fund Key Laboratories of Guizhou Province (grant no. 2011-4005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Sun, W., Meng, X., Liang, L. et al. Overexpression of a Freesia hybrida flavonoid 3-O-glycosyltransferase gene, Fh3GT1, enhances transcription of key anthocyanin genes and accumulation of anthocyanin and flavonol in transgenic petunia (Petunia hybrida). In Vitro Cell.Dev.Biol.-Plant 53, 478–488 (2017). https://doi.org/10.1007/s11627-017-9836-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9836-3