Abstract
The moss Polytrichum juniperinum was widely used by native North Americans and in traditional Chinese medicine to treat several illnesses including burns, wounds, bleeding, fever, kidney stones, and gallstones. This paper reports the efficient establishment of a protonema suspension culture of this moss and evaluation of key factors such as culture medium, trophic condition, initial pH, and inoculum size for increasing biomass production, which has scarcely been studied in this type of biological system. No significant differences were found for the maximum specific growth rate (μmax~0.09 d−1) and the total phenolic content between MS, Knop, and PPNH4 media, although an effect on tissue differentiation was observed. Growth rate in a mixotrophic condition was (μmax 0.27 d−1) three times greater than in autotrophic and heterotrophic conditions, reaching high cell densities (~11 g DW L−1). Moreover, simple sugars were secreted into the medium during the growth phase. P. juniperinum protonema cultures tolerated a wide initial medium pH range (4.5–8). The tissue growth index significantly decreased from ~7.7 to 1.9 with increased inoculum size (45–300 mg DW L−1) under photoautotrophic conditions. Similar responses were obtained under mixotrophic conditions with different sucrose concentrations (15–45 g L−1), but no responses to sucrose concentration and inoculum sizes were seen under heterotrophic growth conditions. Finally, this high cell density culture of P. juniperinum is suitable for further studies aimed at exploring and establishing a production platform for high-value secondary metabolites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The protonemal tissue of mosses represents the early filamentous stage developed from the germinated spore. It consists mainly of two cell types: chloronema, distinguished by a high number of chloroplasts and perpendicular cross walls, and caulonema, which has fewer chloroplasts and oblique cell walls (Reski 1998). Characteristics such as the ability to grow in suspension as a differentiated tissue under photoautotrophic conditions and in a low-cost inorganic medium, the lack of risk of contamination with mammalian pathogens, genetic stability, reduced risk of somaclonal variation, and a high homologous recombination rate observed in the nuclear DNA (Reski 1998) make the suspension-cultured protonemal tissue of mosses suitable for the establishment of large-scale biotechnological processes.
Advances in research on this kind of tissue culture have been slow when compared to advances in suspension culture of vascular plants (Sabovljevic et al. 2009). Despite bryophytes being rich in bioactive compounds (Asakawa 2011), reports on the establishment of moss tissue suspension cultures aimed at producing naturally occurring secondary metabolites are lacking to date. Protonema-derived suspension cultures of Physcomitrella patens have emerged as suitable platforms for the production of recombinant therapeutic proteins (Reski et al. 2015). Furthermore, it has been recently shown that this biological system can be used as a heterologous host of metabolic pathways to produce complex sesquiterpenes with pharmacological applications (Ikram et al. 2015).
Since most of the studies on moss biotechnology have been focused on P. patens, it is important to transfer the techniques used with this organism to others with different characteristics. This would be particularly advantageous for studying moss tolerance to different kinds of abiotic stress as well as its potential for use in phytoremediation and as a production platform for high-value secondary metabolites and recombinant proteins.
In this study, a protonema-derived suspension culture of the cosmopolitan moss Polytrichum juniperinum was established for the first time. Polytrichum species are among the most important plants in Traditional Chinese Medicine (Pant, 1998) and were also used by native North Americans to treat several ailments including burns, wounds, bleeding, fever, pneumonia, kidney stones, gallstones, and leukemia (Flowers 1957; Hart 1981). Furthermore, ethnobotanical uses of P. juniperinum have been supported since it has antibacterial, antifungal, and cytotoxic effects (Krzaczkowski et al. 2009; Savaroglu et al. 2011). In that context, it is important to evaluate and define suitable and uniform baseline growth conditions in order to establish a sustainable production platform for biotechnological products. Therefore, this study evaluated the effects of the basal culture medium, trophic condition, initial medium pH, inoculum size, and carbon source concentration on the biomass production of protonema-derived suspension cultures.
Materials and Methods
Collection and taxonomic identification of specimens
The raw material of the moss P. juniperinum included sporophytes collected in the Antioquia Region, Colombia (2000–2400 m AMSL; 15–20°C). The specimens were identified morphologically, as previously described by Churchill and Linares (1995). In addition, an identification based on molecular markers was carried out according to the methodology proposed by the Barcode of Life initiative (Hebert et al. 2003). Liu et al. (2010) supported the use of rbcL (Kress and Erickson 2007) for DNA barcoding in mosses and proposed the chloroplast intergenic trnH–psbA spacer to be considered as a potential barcode, especially for closely related species, because of its ease of amplification and significant genetic divergence at the interspecific level. Therefore, both molecular markers were amplified and sequenced from growing tissue in vitro. The sequences were deposited in the GenBank database under the accession numbers KT429821 and KT429822, respectively.
Establishment and maintenance of protonemal tissue suspension cultures
Sporophytes of P. juniperinum were disinfected with 0.01% (w/v) sodium dichloroisocyanurate (NaDCC) (Sigma-Aldrich®, St. Louis, Missouri) for 1 h and washed three times with sterile water. The capsule was then broken with a needle to release the spores. Propagation of the protonemal tissue was performed on MS medium solidified with 10 g L−1 Agar-Agar (Merck, Darmstadt, Germany) covered with a cellophane membrane (Flexicolor, Medellin, Colombia), and sterilized at 121°C, 124 kPa for 15 min. The tissue cultures were incubated at 22 ± 2 °C under a 16 h photoperiod with light supplied by cool-white fluorescent tubes (54 μmol m−2 s−1) (Sylvania, Bogota, Colombia). Protonemal cells were propagated by means of mechanical disruption at 19,000 rpm for 15 s (D-160 homogenizer, DS-160/10 dispersing tool) (Almapal, Bogota, Colombia) during each subculture. The suspensions were initiated with a cell density between 30 and 200 mg dry weight (DW) L−1. Protonemal tissue was grown in 100 mL medium in 500-mL shaking flasks in an orbital shaker at 110 rpm, incubated at 25°C and under continuous cool-white fluorescent light at 52 μmol m−2 s−1 (Sylvania). Suspensions were subcultured at 10-d intervals by filtering out the cell cultures and transferring them to a fresh medium. Suspension cultures were disrupted under sterile conditions every 20 d. A sterility test was conducted using Nutrient Agar (CM003: Oxoid, Basingstoke, UK) in each subculture in order to detect contamination, as sometimes it is not visible in the suspension cultures.
Culture growth conditions
Growth kinetics were established for several culture media and trophic conditions. The culture media were Knop (Reski and Abel 1985), PPNH4 supplemented with 500 mg L−1 ammonium tartrate (Ashton et al. 1979), and MS (Murashige and Skoog 1962) (All inorganic salts were obtained from Merck, and MS vitamins were obtained from Sigma-Aldrich®); the trophic conditions were photoautotrophic growth (MS salts + continuous cool-white fluorescent light (Sylvania) at 52 μmol m−2 s−1), mixotrophic growth (MS supplemented with 15 g L−1 sucrose + continuous cool-white fluorescent light (Sylvania) at 52 μmol m−2 s−1), and heterotrophic growth (MS supplemented with 15 g L−1 sucrose + darkness). All media were adjusted to pH 6 with 0.1 N KOH and sterilized at 121°C, 124 kPa for 15 min. In order to determine cell growth characteristics in every culture medium, suspensions were adapted to each one during a 2-mo period and simultaneous growth kinetics were carried out in 100-mL shaking flasks, using the same inoculum size and 20 mL of culture medium.
The effect of initial pH was investigated under photoautotrophic conditions in MS medium ranging between pH 2.7 and 8.8. Ten-day-old tissue suspensions were inoculated into 250-mL shake flasks with 50 mL of culture medium and pre-weighed cotton plugs. The inoculum density was around 100 mg DW L−1. After 12 and 27 d of culture, the growth indexes (GI = (W f − W i )/W i) were determined, where W f is the final biomass DW and W i is the initial biomass DW.
To examine the effect of inoculum size and carbon source concentration on tissue growth, mixotrophic cultures were grown with 0, 15, 25, 35, and 45 g L−1 sucrose in MS medium under continuous cool-white fluorescent light (Sylvania, 52 μmol m−2 s−1); and heterotrophic cultures were grown just with 15 and 45 g L−1 sucrose in MS medium under darkness. All of the treatments were evaluated at three different inoculum densities (45 ± 2.8, 140 ± 10, and 341 ± 32.7 mg DW L−1). GIs were calculated for 15-d suspension cultures in 50 mL medium in 250-mL shaking flasks with pre-weighed cotton plugs. All of the assays were performed in orbital shakers at 110 rpm, with a shaking diameter of 2.5 cm and incubated at 25 °C.
Determination of cell dry weight and total phenolic content
For DW measurement, tissue from 20-mL samples was filtered, washed with distilled water, and transferred to a forced-air oven at 60°C for 24 h.
In order to determine the effect of the culture medium on the production of some types of secondary metabolites, 20-mL samples from the late growth phase (day 28–30) were filtered and lyophilized. The total phenolic content was analyzed from 10 mg of lyophilized tissue by using the Folin–Ciocalteu method (all reagents were obtained from Merck), according to Ainsworth and Gillespie (2007).
Determination of sugars and chlorophylls
Spectrophotometric determination of chlorophylls was performed on cultures grown under different trophic conditions. For each sample, 10 mg oven-dried tissue was milled to a fine powder and chlorophylls were extracted with 2 mL of 95% (v/v) acetone (Merck). The test tubes containing tissue + acetone were immersed in water and placed in an ultrasound bath (Centricol, Medellin, Colombia) for 30 min. The tubes were then removed from the bath and incubated for 24 h in the dark. The absorbance of the extract was measured at 645 and 663 nm, and the chlorophyll content was estimated according to the equations proposed by Arnon (1949).
During mixotrophic growth, 2 mL of culture medium was filtered through 0.2-μm syringe filters (Merck) and the quantitation of sugars (glucose, fructose, and sucrose) (standards from Sigma-Aldrich®) was performed using HPLC (Shimadzu, Kyoto, Japan) equipped with a refractive index detector (RID-10A) and an amino column (Nucleodur® 100–5 NH2-RP; Macherey-Nagel, Düren, Germany) at 30 °C. Acetonitrile–water (80:20 [v/v]) (Merck) was used as the mobile phase at 2 mL min−1 and a sample volume of 20 μL.
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) followed by a Tukey’s multiple comparison test with the XLSTAT software (https://www.xlstat.com/en/) (Addinsoft 2015). However, the effects of culture medium and trophic condition on tissue growth were analyzed by a non-parametric test, Kruskal–Wallis one-way analysis of variance. The analysis was performed using R version 3.3.0 (https://www.r-project.org/) (R Core Team 2014). The data were expressed as means ± standard deviation, and p values below 0.05 were considered as statistically significant. The experiments were repeated independently to guarantee data reproducibility.
Results and Discussion
Effect of culture medium on growth kinetics of protonemal tissue
The protonemal tissue grew under photoautotrophic conditions in the MS, PPNH4, and Knop media, and no significant differences were found for tissue growth according to the Kruskal–Wallis test. In addition, no lag phases were observed when grown in any of the culture media, and the cell DW density reached values around 1 g DW L−1 (Fig. 1a ), a concentration for which light becomes a limiting factor, decreasing the photosynthetic efficiency and biomass productivity (data not shown) (Perner-Nochta et al. 2007). The doubling time was around 7 d in all of the tested culture media (Table 1).
The ammonium concentration was 3.8 times higher in the MS medium than in the PPNH4 medium, and the nitrate/ammonium ratios were 2 and 1.25, respectively. A drastic fall in the pH was observed in MS medium, reaching values around 3 (Fig. 1b ), which could be associated with a preference for ammonium uptake. A similar behavior was reported in the gametophore culture of Sphagnum palustre using Knop medium supplemented with ammonium nitrate (Beike et al. 2015). However, such low pH did not appear to be detrimental to the protonemal tissue growth of P. juniperinum, even though many vascular plant cell suspensions are shown to be sensitive to pH levels below 4 (Orozco-Sanchez et al. 2011). On the other hand, the P. juniperinum culture was mainly growing as chloronema in the MS medium, while caulonema was more abundant in the Knop medium (Fig. 2a , b ). The observed tissue differentiation may have been a consequence of the pH variation (Fig. 1b ).
Some studies have indicated a direct relationship between basal culture medium and secondary metabolites production in plant cell suspension cultures (Kajani et al. 2012). The total phenolic content was analyzed because these compounds are stress indicators in plant cell suspension cultures and are considered the most important group of secondary metabolites along with terpenoids, which are greatly responsible for biological activity in bryophytes such as antibacterial, antifungal, cytotoxic, anti-VIH, insecticide, anti-feeding, and inhibitory activities of several enzymes (Asakawa 2011). However, in this study there were no statistically significant differences between these compounds in the protonemal tissue grown on the three tested culture media under photoautotrophic conditions in the late growth phase (Table 2).
Influence of trophic condition
The protonema of P. juniperinum could be grown in suspension culture under both mixotrophic and heterotrophic conditions, using sucrose as organic carbon source without the need for an adaptation phase. These results contrast with those obtained for P. patens grown on a solid medium (Bricker et al. 2014) and the growth of gametophores in suspension culture of Sphagnum palustre (Beike et al. 2015), both of which required adaptation. The maximum specific growth rate in the mixotrophic cultures was markedly higher than in the heterotrophic and photoautotrophic cultures, decreasing the tissue doubling time by more than 3-fold (Table 3) and achieving a cell density of ~11 g DW L−1 (Fig. 3a ), a density not commonly reported in protonema suspension cultures. These results differ from those of Bricker et al. (2014), in which the heterotrophic growth of P. patens was slower than the photoautotrophic and mixotrophic growth, with no significant differences between the latter two conditions. The results of the present study support the idea that photosynthesis and organic substrate metabolism occur simultaneously and independently in mixotrophic cultures, as has been reported in microalgae, although the mixotrophic growth rate was greater than the sum of the heterotrophic and photoautotrophic growth rates (Table 3), because the stimulatory effect of the organic carbon source depends on the light intensity (Chojnacka and Noworyta 2004; Liang et al. 2009).
Growth kinetics of P. juniperinum tissue suspension cultures growing under different conditions: photoautotrophic growth (MS medium and atmospheric CO2), heterotrophic growth (MS medium supplemented with 15 g L−1 sucrose and darkness), and mixotrophic growth (MS medium supplemented with 15 g L−1 sucrose and continuous white light [52 μmol m−2 s−1]). (a) Biomass production. (b) Specific chlorophyll content. (c) Sugar uptake kinetics under mixotrophic conditions. Error bars represent the standard deviation of three replicates.
On the other hand, the total phenolic content was decreased by half in the mixotrophic cultures (MS + 1.5% sucrose) relative to the photoautotrophic cultures, which suggests that the addition of organic substrates has an effect on the secondary metabolism of P. juniperinum (Table 2). In addition, the specific total chlorophyll content remained higher under photoautotrophic conditions than under the two other growth modes (Fig. 3b ), consistent with previous reports on microalgae (Cheirsilp and Torpee 2012). The specific chlorophyll content increased during the initial days of culture and decreased after day 12, probably due to photolimitation triggered by an increase in cell density. The highest measured chlorophyll content was about 18 mg g−1 DW, a value comparable to that of some microalgae cultures, such as Chlorella sp., Nannochloropsis sp., and Chaetoceros sp., with reported chlorophyll content between 22 and 27 mg g−1 DW (Cheirsilp and Torpee 2012). Furthermore, the chlorophyll content decreased over time in the mixotrophic cultures, indicating that the presence of organic substrates alters the photosynthetic pigment production.
Under mixotrophic growth conditions, the sucrose was completely hydrolyzed after day 15, probably by a cell-wall invertase, resulting in an increase of glucose and fructose (Fig. 3c ). However, the total monosaccharide concentration exceeded the initial sucrose concentration (reaching 20 ± 0.25 g L−1), which suggests carbohydrate production and secretion by the cultured protonema tissue; this behavior has also been reported in microalgae cultures such as Scenedesmus acutus and some species of Chlorella (Maksimova et al. 2004). The drop in monosaccharides after day 20 could be explained by photosynthetic rate decline as a result of a high cell density and photolimitation; however, additional studies are needed to evaluate the factors that affect extracellular carbohydrate accumulation.
Effect of initial medium pH
The protonema cultures of P. juniperinum grew under a broad range of initial pH conditions (4.5–8). The best growth was obtained at pH 4.5–6 and the maximum cell dry weight was ~800 mg DW L−1 after 27 d of culture, while the tissue growth index at pH 8 decreased significantly, (by 34%), resulting in a maximum cell dry weight of 520 mg DW L−1. Alkaline (pH 8.8) and acidic (pH 2.7) initial conditions inhibited growth (Fig. 4), resulting in chlorophyll degradation. Moreover, pH 4.5 prevented protonemal tissue differentiation; a similar effect was reported in P. patens suspensions (Hohe et al. 2002). This response has been associated with the effect of pH on auxin excretion and uptake in culture medium, which is involved in the transition from chloronema to caulonema (Reski 1998). Finally, the versatility of P. juniperinum would allow for adjusting the pH without impairing growth as means of improving secondary metabolite production. Furthermore, it is possible to manipulate this factor to induce metabolite excretion because pH can also affect membrane permeability (Murthy et al. 2014).
Effect of inoculum size and organic carbon source concentration
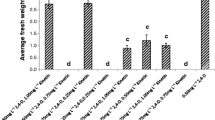
The inoculum size and initial sucrose concentration are considered key factors for plant cell suspension establishment as they affect both growth and secondary metabolism (Murthy et al. 2014). However, these variables have been scarcely studied during the establishment of protonema-derived suspension cultures of mosses. In this study, GI decreased significantly from 7.7 ± 0.16 to 1.9 ± 0.1 with an increase in inoculum size from 45 ± 2.8 to 341 ± 32.7 mg DW L−1 under photoautotrophic conditions. The same response was observed in the mixotrophic culture, but not in the heterotrophic culture, which could be explained by photolimitation in the photoautotrophic and mixotrophic cultures, because the light exposure time decreases for each cell at higher cell density (Fig. 5). This is in accordance with some reports on microalgae such as Haematococcus pluvialis, although at much larger inoculum sizes than those used in this study (<1.5 g DW L−1) (Wang et al. 2013), and also in gametophore cultures of the moss Sphagnum palustre (Beike et al. 2015). In most cases, P. juniperinum GIs were not significantly different at sucrose initial concentrations between 15 and 45 g L−1 sucrose for the same inoculum size (Fig. 5). Previously, 15 g L−1 sucrose was chosen to propagate the tissue suspension cultures under mixotrophic conditions, because sucrose was completely exhausted during growth kinetics (Fig. 3c ).
Effects of inoculum size and organic carbon source concentration on the growth of P. juniperinum tissue suspension cultures. Photoautotrophic growth: light + atmospheric CO2; mixotrophic growth: light +15–45 g L−1 sucrose; heterotrophic growth: 15 and 45 g L−1 sucrose + darkness. Inoculum 1: 45 ± 2.8 mg DW L−1; inoculum 2: 140 ± 10 mg DW L−1; inoculum 3: 341 ± 32.7 mg DW L−1. Means marked with the same letter are not significantly different based on Tukey’s test (significant at p > 0.05). Error bars represent the standard deviation of three replicates.
Under photoautotrophic conditions, chloronema clearly predominated in each of the three tested inoculum sizes (Fig. 6d ). The heterotrophic conditions stimulated protonema differentiation, resulting in a culture consisting mainly of caulonema (Fig. 6e , f ). Under mixotrophic conditions, chloronema predominated in all of the sucrose concentrations at the lowest tested inoculum size (Fig. 6a ), and a predominant trend towards the formation of large aggregates (~5 mm in diameter) was observed at the end of the growth period. The morphology drastically changed with the two other inoculum sizes (inoculum 2 and 3): the chloronema developed brood cells (Fig. 6b , c ), which have been associated with suboptimal growth conditions (Goode et al. 1993). This was particularly evident at increasing concentrations of sucrose, suggesting that there is a combined effect of sucrose supplementation and large inoculum size, probably due to the release of morphoregulatory substances into the medium.
Microscopic view of the protonema cultures and degree of tissue differentiation in different sucrose concentrations and inoculum sizes. (a) Mixotrophic growth, 25 g L−1 sucrose, inoculum 1. (b) Mixotrophic growth, 25 g L−1 sucrose, inoculum 2. (c) Mixotrophic growth, 45 g L−1 sucrose, inoculum 3. (d) Photoautotrophic growth, inoculum 2. (e) Heterotrophic growth, 15 g L−1 sucrose, inoculum 3. (f) Heterotrophic growth, 45 g L−1 sucrose, inoculum 3. Bars = 50 μm.
In conclusion, this study represents a contribution to the knowledge and characterization of protonema-derived suspension cultures of mosses at shake-flask scale. This information is important for establishing a production platform for high-value secondary metabolites. However, to address many engineering aspects, additional studies are needed such as rheological characterization of the tissue suspensions, studies related to the effects of light quality and intensity on biomass production, and systematic studies on resistance to hydrodynamic stress and its impact on the secondary metabolism of the tissue.
References
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2:875–877
Addinsoft (2015). XLSTAT 2015, Data analysis and statistics software for Microsoft Excel
Arnon D (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Asakawa Y (2011) Bryophytes: chemical diversity, synthesis and biotechnology. A review. Flavour Fragr J 26:318–320
Ashton NW, Grimsley NH, Cove DJ (1979) Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants. Planta 144:427–435
Beike A, Spagnuolo V, Lüth V, et al. (2015) Clonal in vitro propagation of peat mosses (Sphagnum L.) as novel green resources for basic and applied research. Plant cell. Tissue Organ Cult 120:1037–1049
Bricker TM, Bell AJ, Tran L, Frankel LK, Theg SM (2014) Photoheterotrophic growth of Physcomitrella patens. Planta 239:605–613
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
Chojnacka K, Noworyta A (2004) Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym Microb Technol 34:461–465
Churchill S, Linares E (1995) Prodromus Bryologiae Novo-Granatensis: introducción a la flora de musgos de Colombia, vol parte 1. Instituto de Ciencias Naturales--Museo de Historia Natural Bogotá, Colombia
Flowers S (1957) Ethnobryology of the Gosuite Indians of Utah. Bryologist 60:11–14
Goode JA, Stead AD, Duckett JG (1993) Redifferentiation of moss protonemata: an experimental and immunofluorescence study of brood cell formation. Can J Bot 71:1510–1519
Hart JA (1981) The ethnobotany of the Northern Cheyenne Indians of Montana. J Ethnopharmacol 4:1–55
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc Biol Sci 270:313–321
Hohe A, Decker E, Gorr G, Schween G, Reski R (2002) Tight control of growth and cell differentiation in photoautotrophically growing moss (Physcomitrella patens) bioreactor cultures. Plant Cell Rep 20:1135–1140
Ikram N, Zhan X, Pan X, King B, Simonsen H (2015) Stable heterologous expression of biologically active terpenoids in green plant cells. Front Plant Sci 6:129
Kajani AA, Moghim S, Mofid MR (2012) Optimization of the basal medium for improving production and secretion of taxanes from suspension cell culture of Taxus baccata L. Daru 20:54
Kress WJ, Erickson DL (2007) A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS One 2:e508
Krzaczkowski L, Wright M, Rebérioux D, Massiot G, Etiévant C, Gairin JE (2009) Pharmacological screening of bryophyte extracts that inhibit growth and induce abnormal phenotypes in human HeLa cancer cells. Fundam Clin Pharmacol 23:473–482
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Liu Y, Yan HF, Cao T, Ge XJ (2010) Evaluation of 10 plant barcodes in Bryophyta (mosses). J Syst Evol 48:36–46
Maksimova IV, Bratkovskaia LB, Plekhanov SE (2004) Extracellular carbohydrates and polysaccharides of the algae Chlorella pyrenoidosa Chick S–39. Izv Akad Nauk Ser Biol:217–224
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy H, Lee E, Paek K (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult 118:1–16
Orozco-Sanchez F, Sepúlveda-Jiménez G, Trejo-Tapia G, Zamilpa A, Rodríguez-Monroy M (2011) Oxygen limitations to grow Azadirachta indica cell culture in shake flasks. Rev Mex Ing Quim 10:343–352
Pant GP (1998) Medicinal uses of bryophytes. In: Chopra RN (ed) Topics in Bryology. Allied Publishers Limited, New Delhi, pp. 112–124
Perner-Nochta I, Lucumi A, Posten C (2007) Photoautotrophic cell and tissue culture in a tubular photobioreactor. Eng Life Sci 7:127–135
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna URL http://www.R-project.org
Reski R (1998) Development, genetics and molecular biology of mosses. Bot Acta 111:1–15
Reski R, Abel WO (1985) Induction of budding on chloronemata and caulonemata of the moss, Physcomitrella patens, using isopentenyladenine. Planta 165:354–358
Reski R, Parsons J, Decker EL (2015) Moss-made pharmaceuticals: from bench to bedside. Plant Biotechnol J 13:1191–1198
Sabovljevic A, Sabovljevic M, Jockovic N (2009) In vitro culture and secondary metabolite isolation in bryophytes. Methods Mol Biol 547:117–128
Savaroglu F, Ilhan S, Filik-Iscen C (2011) An evaluation of the antimicrobial activity of some Turkish mosses. J Med Plants Res 5:3286–3292
Wang J, Han D, Sommerfeld M, Lu C, Hu Q (2013) Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J Appl Phycol 25:253–260
Acknowledgments
This research was supported by the Research Department (DIME) of the Universidad Nacional de Colombia in Medellin (Hermes project 21743). The authors are thankful to Margarita Escobar Acosta from the Universidad de Antioquia for aiding the species taxonomic identification.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Boachun Li
Rights and permissions
About this article
Cite this article
Ruiz-Molina, N., Villalobos-López, M.Á. & Arias-Zabala, M. Protonema suspension cultures of the medicinal moss Polytrichum juniperinum . In Vitro Cell.Dev.Biol.-Plant 52, 419–426 (2016). https://doi.org/10.1007/s11627-016-9783-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-016-9783-4