Abstract
Decellularized tissues are an attractive scaffolds for 3D tissue engineering. Decellularized animal tissues have certain limitations such as the availability of tissue, high costs and ethical concerns related to the use of animal sources. Plant-based tissue decellularized scaffolds could be a better option to overcome the problem. The leaves of different plants offer a unique opportunity for the development of tissue-specific scaffolds, depending on the reticulate or parallel veination. Herein, we decellularized spinach leaves and employed these for the propagation and osteogenic differentiation of dental pulp stem cells (DPSCs). DPSCs were characterized by using mesenchymal stem cell surface markers CD90, CD105 and CD73 and CD34, CD45 and HLA-DR using flow cytometry. Spinach leaves were decellularized using ethanol, NaOH and HCL. Cytotoxicity of spinach leaf scaffolds were analysed by MTT assay. Decellularized spinach leaves supported dental pulp stem cell adhesion, proliferation and osteogenic differentiation. Our data demonstrate that the decellularized spinach cellulose scaffolds can stimulate the growth, proliferation and osteogenic differentiation of DPSCs. In this study, we showed the versatile nature of decellularized plant leaves as a biological scaffold and their potential for bone regeneration in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue engineering has become a part and parcel of biomedical research. In tissue engineering, biomaterials need to be bio-compatible to support regeneration of tissues. Various biomaterials (natural and synthetic) have been proposed and are still under investigation to achieve the appropriate morphological, physical, mechanical and biological properties suitable for the regeneration of targeted tissues (Prete et al. 2023).
Stem cell–based tissue engineering begins from cells to the injured tissue or blood vessel. In regenerative medicine, translational research, such as tissue engineering and molecular biology, is concerned with the process of replacing, or repairing cells, tissues or organs in order to resume organ function. However, it is hard to track the transmitted cells and keep them in a specific location. Scaffolds are the central components that are used to carry the cells, drugs and genes into the body. Various types of scaffolds are prepared as typical 3D porous matrix, nanofibrous matrices or porous microspheres, which provide suitable substrates for cell attachment, cell proliferation, differentiated function and cell migration (Eltom et al. 2019). Scaffold matrices have a specific advantage in regenerative medicine. Nature-developed plant-based scaffolds are a new technology of applied science for stem cell transplantation (Bružauskaitė et al. 2016). The varieties of natural polysaccharides and protein have been explored for bone regeneration (Ardeshirylajimi and Hosseinkhani 2013).
Scaffold designing that allows for the structural and functional repair of the bone remains a major task. Our aim is to develop decellularized plant-based scaffolds to support bone regeneration. Bone is made up of a hard and dense type of connective tissue with excellent mechanical properties (Weatherholt et al. 2013). It supports the human body, and stores and releases minerals. It contains osteoblasts, osteoclasts, osteocytes and bone lining cells embedded in the extracellular matrix (ECM). Osteoblasts produced mineralize for new bone matrix, and repair and regeneration of bone. Dental pulp stem cell is an ideal candidate for bone regeneration. Dental pulp stem cells (DPSCs) are possible to isolate from the extracted human permanent third molar pulp. These cells have characteristics as MSCs and fibroblast-like morphology. DPSCs have high proliferation rates, are clonogenic and possess all properties of stem cells (Patil et al. 2018a). DPSCs are multipotent and can differentiate into neural, adipocyte, odontoblast, etc. (Nuti et al. 2016). Dental pulp stem cells have the potential to differentiate in functional osteocyte. DPSCs secrete growth factor (Shekatkar et al. 2022), cytokinin (Bari et al. 2019) and scaffold which serves as a temporary platform that provides structural support, facilitates bone repair and guides bone growth in bone defects.
Plant leaves are structures developed by nature, which can be applied to tissue engineering. Spinach leaves have been the ideal example of plant-borne scaffolds. Various techniques for the development of plant-based scaffolds, like apple-derived cellulose scaffolds, spinach, bamboo sparges, carrot, celery, cucumber, potato, asparagus, green onions, leek and broccoli, have been employed by researchers (Bilirgen et al. 2021). Scaffolds are prepared to influence the physical, chemical and biological environments of a cell population (Howard et al. 2008). The requirement for unique scaffold structure and reproducible manufacture of these approaches results in improved scaffolds that are employed for cell development.
In tissue regeneration, a scaffold should be mechanically stable as well as biodegradable. Its size should be appropriate and it should have a rough surface and porosity which is required for providing a suitable microenvironment for sufficient cell–cell interaction, cell migration, proliferation and differentiation. Pore size plays a major role in cell adhesion, cell-to-cell interaction and other transmigration across the membrane based on the purpose of tissue regeneration (Bružauskaitė et al. 2016; Lee et al. 2022). The scaffold biomaterials should be non-toxic to humans, resistant to quick degradation and with the corresponding pore size or porosity (Krishani et al. 2023). A lot of properties that are needed for biomaterial design are expressed in the structure as well as the function of plants (Fontana 2019). It is also shown that decellularized plant tissue can be used as an adaptable scaffold for culturing human cells by simple bio-functionalization technique; it is possible to qualify the adhesion of human cells on various sets of plant tissue (Fontana 2019).
The hydrophilicity and prime water transfer qualities of plant tissue allow cell expansion. The microstructure of the plant frameworks, cell alignment and shape registration are unique physical characteristics, and the ability to manufacture biomaterials with a range of attainable physical and biological properties is left as a challenge and is an active area of decellularization (Fontana 2019).
Decellularized animal tissue such as human amniotic membrane has been used for a long time (Abazari et al. 2020; Lakkireddy et al. 2022). Plant tissue decellularized scaffold may reduce availableness problems, elevated costs and ethical concerns associated with animal sources. Tissue engineering needs a precise design of engineered biomaterial which is able to assist in the regeneration of misplaced or lost tissues. Fabricated as well as naturally derived biomaterials have been suggested and are still under inspection to accomplish the correct or appropriate mechanical as well as morphological, physical and biological properties to fulfill particular demands for the regeneration of target human tissues (Contessi Negrini et al. 2020).
The generation of vegetal scaffolds by dissociation of plant-based biomaterials has seen an increase in recent years. They are cost-effective and sustainable since the vegetal tissues are obtained from plant leaves, stems, fruits and vegetables. Previous studies have shown that decellularized spinach leaves scaffold are vascularized, which supports mammalian cells (Fontana 2019). Spinach leaves are cost-effective and free from animal-derived components. Most of the biomaterial scaffolds used in tissue engineering are of animal origin such as chitosan and collagen. The leaf surface is covered with a cuticle layer which makes a surface smooth known as the epicuticular wax. Most of the literature suggests use of acids (HCL and nitric acid) to remove the leaf epicuticular wax (Holloway and Baker 1968). This study evaluated viability and differentiation potential of DPSCs. Based on the evidence provided by the European Parliament’s joint motion for innovation to minimize and eliminate animal use and promote plant-based material, a majority of studies focus on utilizing plant tissues to generate scaffolds for tissue engineering (Harris et al. 2021). Our aim to investigate the spinach leaf scaffold supports the osteogenic differentiation; it can be used in regenerative medicine for bone regeneration and repair.
Materials and methods
Selection of leaf samples
Spinach leaves were selected for the study. Fresh spinach leaves were collected from a local supermarket washed with PBS and stored at 4℃ for a maximum of 2 days before use.
Decellularization of the leaf
The leaves were cut longitudinally or transversely into 1–2-mm-thick slices. The leaves were boiled and then the leaf samples were treated with three chemicals, i.e. ethanol, sodium hydroxide and hydrochloric acid. Scaffold A was treated with ethanol, sodium hydroxide and 5% hydrochloric acid. Scaffold B was treated with ethanol, sodium hydroxide and 1% hydrochloric acid and kept under UV light sterilization for 30 min (Dai et al. 2016). Cell adherence on surface is observed by using a phase contrast microscope. For further confirmation, cells were stained with fluorescent antibodies and confirmed by confocal microscopy.
Cell isolation from dental pulp tissue
Stem cell isolation method was studied which was approved by an Institutional Stem Cell Committee for stem cell research. Mesenchymal stem cells were isolated from dental pulp which are also known as dental pulp mesenchymal stem cells. Tissue was obtained from Dr. D.Y. Patil Dental College and Hospital Pune. Tooth were extracted with prior consent from a healthy donor during dental procedure. The extracted tooth was cut vertically to remove the pulp by using air rotor. Dental pulp was cut into 1–2 mm size placed in culture dish in the presence of fetal bovine serum (FBS) and incubated for 24 h in CO2 incubator. After 24 h, the completed media were added in culture dish for cell outgrowth. Cells were cultured by explant culture method (Patil et al. 2018b). DPSCs were characterized by using stem cell surface markers CD90, CD105, CD73 CD34, CD45 and HLA-DR using flow cytometry.
Cell seeding on plant-based leaf scaffold
To maintain pH, leaf scaffold was washed with media and PBS. After maintaining pH, DPSCs (1 × 105 cells/well) were seeded on the leaf scaffold for attachment and bone regeneration in 24-well plates. Then the cells were incubated in 5% CO2 incubator at 37℃ and we let the cells proliferate on the leaf scaffold.
Confocal microscopy and scanning electron microscopy to confirm cell growth
Verification of cell growth on the scaffold was done by using antibody-specific staining DAPI and CD90PE for stem cells and the cells were visualized under confocal microscopy, and further cell growth confirmation was done by scanning electron microscopy (SEM).
Differentiation
The DPSCs were cultured (1 × 106 cell/well) on the spinach leaf scaffold. These were subjected to osteogenic differentiation by inducing the DPSCs with osteogenic induction media containing 1 mM dexamethasone, 1 mM ascorbic acid and 0.1 mM β-glycerolphosphate use as positive control. Cells were cultured on plant-based scaffold and induction was done using osteogenic induction media. Cells were incubated for 18 to 21 d, and induction media were replaced twice a wk. Differentiated cell mineralization was stained using alizarin red. Cell mineralization was dissolved in 0.1% acetic acid for quantitative analysis and measure was taken at 450 nm on Elisa reader.
All experiments were performed in triplicate.
Gene expression
Total RNA was isolated using an RNA isolation kit according to the manufacturer’s protocol. The RNA level and quality were checked using the Qubit Nanodrop technology (Thermofisher, Waltham, MA). A total of 500 ng of RNA was used for reverse transcription using the Superscript III reverse transcription kit (TAKARA Kusatsu, Japan). The quantitative PCR analysis was performed using a QuantStudio 5 real-time PCR system (Thermo Fisher Scientific) and TaqMan gene expression qPCR Master Mix (Thermo Fisher Scientific) following the manufacturers’ instructions. The primer sequences used were osteonectin. GAPDH were used as housekeeping gene (Table 1).
Result
Isolation of dental pulp stem cells
Mesenchymal stem cells were successfully isolated by using the explant culture method. The outgrowth of cells were observed after 1 week of explant culture (Fig. 1A, B). MSCs show fibroblastic structure (Fig. 1C).
DPSCs show mesenchymal stem cell properties. DPSCs were positive for CD90, CD105 and CD73 and negative for CD34, CD45 and HLA-DR surface marker (Fig. 2). DPSCs also show trilineage differentiation properties such as osteogenic, chondrogenic and adipogenic differentiation. In osteogenic differentiation (calcium deposition), stain by alizarin red, chondrogenic differentiation (glycosaminoglycan) stain by alcian blue and adipogenic differentiation (lipid droplet) stain by oil red O stain were used (Fig. 3).
Cell attachment on plant-based scaffold
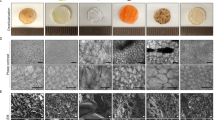
Selection of leaf was done and spinach leaf was selected due to its structure. As spinach leaves have a hierarchical structure with veins interspersed with pores, their structure provides a scaffold that stimulates cell growth and organization. The scaffolds were chlorophyll free by using 99% of ethanol and NaOH (Fig. 4B, C). This chlorophyll-free plant-based scaffold is decellularized by using sodium hydroxide to make it transparent. The cuticle of the leaf was removed by using HCl and they appear completely white. Porous leaf scaffold was obtained from the previous treatment. Scaffold which was treated with 10% HCl was fragile (Fig. 4D). Scaffold treated with 1% HCL showed good physiological properties compared with 5% and 10% HCL (Figs. 4 and 5).
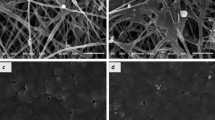
Cells were attached on the leaf surface and penetrated the leaf structure, mimicking their natural function. Cell growth was observed on the surface of the scaffold. DPSC cell growth was confirmed using confocal microscopy and scanning electron microscopy. The MSC cell colonies were observed on the scaffold (Figs. 6 and 7).
Osteogenic differentiation
In scaffold A, calcium deposition is in higher number than that in scaffold B and positive control. The differentiated cells were stained by alizarin red dye to confirm calcium deposition. Calcium deposition was observed under a phase contrast microscope on a scaffold (Fig. 8A). Further confirmation was done by measuring optical density at 450 nm by using 0.1% acetic acid (Fig. 8B).
Gene expression
Osteogenic gene expression was examined. Osteonectin (OSN) gene expression was shown in both control and plant-based base scaffold. At day 14, plant scaffold had significantly higher OSN than the control. In plant-based scaffold, osteonectin expressions increased by 2.5-fold compared with the control group, which is a statistically significant result (Fig. 9).
Osteogenic gene expressions measured by RT‐qPCR. The mRNA levels of osteonectin (OSN) were measured from total RNA extracted from the control and plant-based scaffold after osteogenic induction. Data are expressed as mean ± SD; n = 3. GAPDH gene was used as a reference. The relative expression (fold increases) in plant scaffold compared to control.
Discussion
Nowadays, availability of tissue donors and problems faced with tissue transplantation have become a necessity to grow cells with scaffolds, either natural or synthetic (Sharma et al. 2019). Scaffold is one of the most important three pillars of tissue engineering, inclusive of cell and growth factors which enable to form 3D structures (Hollister 2006).
Biological scaffolds are a valid alternative to traditional therapies and may improve outcomes or offer solution which may not be possible with synthetic scaffolds. Advanced research for different indications using various scaffolds has led to a thorough understanding of the mechanisms involved in in vivo remodeling (Howard et al. 2008; Bružauskaitė et al. 2016; Lacombe et al. 2020).
Recently, plant-derived decellularized scaffolds are of a huge interest replacing animal sources for tissue regeneration (Harris et al. 2021). There is a built-in architecture in the plant leaves in the form of venation. These naturally designed decellularized scaffolds mimic the extracellular matrix of mammalian tissues due to suitable cytocompatibility (Contessi Negrini et al. 2020). Plant-derived scaffolds have been used for neural differentiation in regenerative medicine (Couvrette et al. 2023). Decellularized spinach leaf scaffold could be more successful in differentiating stem cell into the bone formation or bone repair. They allow the exchange of nutrition and oxygen and vascular ingrowth through the scaffold. It is providing a three-dimensional substrate by feeding and delivering oxygen to the cells. Stem cells can be guided to arrange in a specific direction depending upon the structure of the plant material used.
Plant-based scaffolds have a lot of practical benefits such as easy availability, mass production, cost-effectiveness and ready to use for tissue engineering application. Plant leaves are made up of cellulose fiber—a non-toxic (Ilangovan et al. 2020). Large groups of cellulose molecules come together to form microfibrils. These properties make them suitable candidates for scaffolds in tissue engineering (Hickey et al. 2018). They compensate traditional scaffolds by providing a larger surface area, vascular networks, water transfer and retention properties. Our decellularized spinach leaf scaffolds were found to be non-toxic and enhance the DPSC proliferation (Fig. 6C). Earlier studies have demonstrated that plant-based scaffold of Ficus religiosa leaf skeleton architecture exhibited biocompatibility in mammalian cell adhesion, proliferation and functionality (Periasamy et al. 2020; Lacombe et al. 2020).
We used a sequential chemical treatment which is a gold standard approach for decellularizing plant tissue. Aqueous detergent (e.g. sodium dodecyl sulphate (SDS)) is traditionally followed by a surfactant-bleach solution (Adamski et al. 2018). In this study, we used sequential chemical treatment with NaOH, HCL and ethanol for the decellularization of spinach leaves. NaOH destructs the cell wall and removes chlorophyll while retaining the tissue structure. HCL helps in the removal of the cuticle (wax layer) and ethanol enables the sterilization of the leaf (Howell et al. 2022). In regenerative therapy, pore size is very crucial for a better growth of cells. Cells respond strongly to mechanical rigidity and flexibility, and 3D nano topology, as well as extracellular stimuli (Calin and Paun 2022). Our data shows that 5% HCL–treated spinach leaf scaffolds support attachment and proliferation DPSCs compared with 1% HCL. Further, spinach leaf scaffolds must be sterilized before introduction to cell culture environments. Plant-based scaffold does not show toxicity in DPSCs. UV radiation and ethanol methods have been previously employed (Dai et al. 2016). We used spinach leaf scaffold for assessment of the osteogenic differentiation potential of human DPSCs. These decellularized scaffolds enhanced the osteogenic differentiation in terms of calcium deposition and bone-related gene expression such as osteonectin (OSN) (Dhandayuthapani et al. 2011).
In the bone, osteonectin (OSN) is a glycoprotein secreted by osteoblasts that binds to calcium while the bone is being formed. This initiates the mineralization process and encourages the production of mineral crystals. OSN is a noncollagenous extracellular matrix protein and has been suggested to bind selectively to both hydroxyapatite and collagen, and to link the bone mineral and collagen phases, which is likely to lead to active mineralization in normal bone tissue.
The results of the scanning electron microscopy analysis showed that the spinach scaffold in NaOH with 5% HCl provides ideal surface for trapping cells. The decellularized spinach leaf scaffold exhibits better cell attachment, growth, proliferation and differentiation. The plant scaffold could thus be considered an alternative for synthetic scaffolds in tissue engineering (Fontana 2019).
In this investigation, we proposed unconventional alternative tissue engineering plant-based scaffolds for bone regeneration. The spinach leaf scaffolds are promising alternative biomaterials for bone tissue engineering and improving their regenerative abilities for repairing damaged bone. The unique features of the plant scaffolds can offer inner vasculature, optimal fluid transport, etc. to make it an alternative natural model. However, they have few limitations such as high hydrophobicity, absorption of small hydrophobic molecules and non-biodegradability. This finding provides the potential of decellularized leaf scaffold and DPSCs as a therapeutic regenerative medicine for treating bone defect, which could be of great academic and clinical significance. More research needs to be carried out to explore this field as an ideal source for tissue engineering.
Statistical analysis
The ANOVA test was followed by Scheffe’s multiple comparison test to compare in vitro analysis. All results are presented as mean ± SEM or mean ± SD. The mean difference is significant at the 0.05 level.
Conclusion
Our data demonstrate that the decellularized spinach cellulose scaffolds stimulate the growth, proliferation and osteoblastic differentiation of DPSCs in addition to providing a 3D culture environment. Our study represents a case of symbiotic relationship between plant cellulose scaffold and human stem cells.
Data availability
Data is available from the authors upon request.
Code availability
Not applicable.
References
Abazari MF, Soleimanifar F, Enderami SE et al (2020) Decellularized amniotic membrane Scaffolds improve differentiation of iPSCs to functional hepatocyte-like cells. J Cell Biochem 121:1169–1181. https://doi.org/10.1002/jcb.29351
Adamski M, Fontana G, Gershlak JR et al (2018) Two methods for decellularization of plant tissues for tissue engineering applications. J vis Exp 2018:1–7. https://doi.org/10.3791/57586
Ardeshirylajimi A, Hosseinkhani S (2013) Nanofiber-based polyethersulfone scaffold and efficient differentiation of human induced pluripotent stem cells into osteoblastic lineage. Mol Biol Rep 40:4287–4294. https://doi.org/10.1007/s11033-013-2515-5
Bari E, Ferrarotti I, Torre ML et al (2019) Mesenchymal stem/stromal cell secretome for lung regeneration: the long way through “pharmaceuticalization” for the best formulation. J Control Release 309:11–24. https://doi.org/10.1016/j.jconrel.2019.07.022
Bilirgen AC, Toker M, Odabas S et al (2021) Plant-based scaffolds in tissue engineering. ACS Biomater Sci Eng 7:926–938. https://doi.org/10.1021/acsbiomaterials.0c01527
Bružauskaitė I, Bironaitė D, Bagdonas E, Bernotienė E (2016) Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects. Cytotechnology 68:355–369
Calin BS, Paun IA (2022) A review on stimuli-actuated 3D micro/nanostructures for tissue engineering and the potential of laser-direct writing via two-photon polymerization for structure fabrication. Int J Mol Sci 23:14270. https://doi.org/10.3390/ijms232214270
Contessi Negrini N, Toffoletto N, Farè S, Altomare L (2020) Plant tissues as 3D natural scaffolds for adipose, bone and tendon tissue regeneration. Front Bioeng Biotechnol 8:1–15. https://doi.org/10.3389/fbioe.2020.00723
Couvrette LJ, Walker KLA, Bui TV, Pelling AE (2023) Plant cellulose as a substrate for 3D neural stem cell culture. Bioengineering 10. https://doi.org/10.3390/bioengineering10111309
Dai Z, Ronholm J, Tian Y et al (2016) Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J Tissue Eng 7:1–13. https://doi.org/10.1177/2041731416648810
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS (2011) Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci 2011:19. https://doi.org/10.1155/2011/290602
Eltom A, Zhong G, Muhammad A (2019) Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv Mater Sci Eng 2019:19. https://doi.org/10.1155/2019/3429527
Fontana G (2019) Biofunctionalized plants as diverse biomaterials for human cell culture. Physiol Behav 46:248–256. https://doi.org/10.1002/adhm.201601225
Harris AF, Lacombe J, Zenhausern F (2021) The emerging role of decellularized plant-based scaffolds as a new biomaterial. Int J Mol Sci 22:12347
Hickey RJ, Modulevsky DJ, Cuerrier CM, Pelling AE (2018) Customizing the shape and microenvironment biochemistry of biocompatible macroscopic plant-derived cellulose scaffolds. ACS Biomater Sci Eng 4:3726–3736. https://doi.org/10.1021/acsbiomaterials.8b00178
Hollister SJ (2006) Porous scaffold design for tissue engineering (vol 4, pg 518, 2005). Nat Mater 5:590
Holloway PJ, Baker EA (1968) Isolation of plant cuticles with zinc chloride-hydrochloric acid solution. Plant Physiol 43:1878–1879. https://doi.org/10.1104/pp.43.11.1878
Howard D, Buttery LD, Shakesheff KM, Roberts SJ (2008) Tissue engineering: strategies, stem cells and scaffolds. J Anat 213:66–72
Howell MM, Gossmann R, Gee CT (2022) A modified, step-by-step procedure for the gentle bleaching of delicate fossil leaf cuticles. Foss Impr 78:445–450. https://doi.org/10.37520/fi.2022.019
Ilangovan M, Guna V, Prajwal B et al (2020) Extraction and characterisation of natural cellulose fibers from Kigelia africana. Carbohydr Polym 236:115996. https://doi.org/10.1016/j.carbpol.2020.115996
Krishani M, Shin WY, Suhaimi H, Sambudi NS (2023) Development of scaffolds from bio-based natural materials for tissue regeneration applications: a review. Gels 9:100. https://doi.org/10.3390/gels9020100
Lacombe J, Harris AF, Zenhausern R et al (2020) Plant-based scaffolds modify cellular response to drug and radiation exposure compared to standard cell culture models. Front Bioeng Biotechnol 8:932. https://doi.org/10.3389/fbioe.2020.00932
Lakkireddy C, Vishwakarma SK, Raju N et al (2022) Fabrication of decellularized amnion and chorion scaffolds to develop bioengineered cell-laden constructs. Cell Mol Bioeng 15:137–150. https://doi.org/10.1007/s12195-021-00707-7
Lee SS, Du X, Kim I, Ferguson SJ (2022) Scaffolds for bone-tissue engineering. Matter 5:2722–2759. https://doi.org/10.1016/j.matt.2022.06.003
Nuti N, Corallo C, Chan BMF et al (2016) Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep 12:511–523. https://doi.org/10.1007/s12015-016-9661-9
Patil V et al (2018a) Long term explant culture for harvesting homogeneous population of human dental pulp stem cells. Cell Biol Int 42:1602–1610. https://doi.org/10.1002/cbin.11065
Patil VR, Kharat AH, Kulkarni DG et al (2018b) Long term explant culture for harvesting homogeneous population of human dental pulp stem cells. Cell Biol Int 42:1602–1610. https://doi.org/10.1002/cbin.11065
Periasamy VS, Athinarayanan J, Alshatwi AA (2020) Bio-inspired plant leaf skeleton based three dimensional scaffold for three dimensional cell culture. Sustain Chem Pharm 18:100321. https://doi.org/10.1016/j.scp.2020.100321
Prete S, Dattilo M, Patitucci F et al (2023) Natural and synthetic polymeric biomaterials for application in wound management. J Funct Biomater 14:455. https://doi.org/10.3390/jfb14090455
Sharma P, Kumar P, Sharma R et al (2019) Tissue engineering; current status & futuristic scope. J Med Life 2019:225–229. https://doi.org/10.25122/jml-2019-0032
Shekatkar MR, Kheur SM, Kharat AH, Deshpande SS (2022) Assessment of angiogenic potential of mesenchymal stem cells derived conditioned media from various oral sources. J Clin Transl Res 8:323–338. https://doi.org/10.18053/jctres.08.202204.007
Weatherholt AM, Fuchs RK, W SJ (2013) Specialized connective tissue: bone, the structural framework of the upper extremity Alyssa. J Hand Ther 40:363–408
Acknowledgements
The authors thank Dr. D. Y. Patil Dental College & Hospital and Dr. D. Y. Patil Vidyapeeth for the infrastructure.
Author information
Authors and Affiliations
Contributions
Kautubh Raundal and Dr. Avinash Kharat planned and performed experiments and wrote the first draft of the manuscript. Pranjali Potdar and Swapanli Sakhare, gene expression study. Dr. Ramesh Bhonde supervised the work. Dr. Avinash Sanap and Dr. Supriya Kheur edited the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study is approved by the Institutional Committee for Stem cell Research (IC-SCR) no. IC-SCR/001/2022 Dr. D. Y. Patil dental college and Hospital, Pimri Pune.
Consent for publication
All authors agreed to the publication in the submitted form.
Competing interests
The authors declare no competing interests.
Declaration of AI
All authors did not use AI tools to analyse and draw insights from data as part of the research process.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raundal, K., Kharat, A., Sanap, A. et al. Decellularized leaf-based biomaterial supports osteogenic differentiation of dental pulp mesenchymal stem cells. In Vitro Cell.Dev.Biol.-Animal (2024). https://doi.org/10.1007/s11626-024-00937-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11626-024-00937-9