Abstract
Two cell lines were established from silver crucian carp and goldfish brain tissue and used as the biological tool for monitoring viral diseases. Characterization including optimal growth kinetics study, karyotyping, and mitochondrial ribosomal RNA (rRNA) genotyping were performed. The primary cultures of these cells were generated by the explant technique using the medium 199 supplemented with 20 % fetal bovine serum and epidermal/fibroblast growth factors. The cells grew over the range of 15 to 30°C, while the optimal temperature for culture was 30°C. The cell lines were maintained in vitro and could be subcultured over 40 times. Following cryopreservation in liquid nitrogen, thawed cells exhibit viability of > 90 % after a 13-mo period of storage. The chromosome count of two cell lines were determined to be 154 and 110, respectively, which agreed well with triploid crucian carp brain cells and diploid goldfish brain cells. Polymerase chain reaction amplification and sequence analysis indicated 100 % and 94% match with known crucian carp mitochondrial DNA sequences. Cytopathic effect was continuously observed in both cell lines over 10 passages after inoculation with tissue homogenates of sick or died goldfish from cyprinid herpesvirus 2 (CyHV-2) outbreaks. These newly established cell lines could be a diagnostic tool for viral diseases in fish species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carassius genus is comprised of crucian carp (Carassius carassius) and silver crucian carp (Carassius auratus). Silver crucian carp includes goldfish (Carassius auratus), Prussian carp (Carassius auratus gibelio), and five Japanese subspecies (Apalikova et al. 2011). Goldfish are widely kept as pets in China and the Prussian carp is widely distributed in northern China and Russia. However, since the same species and subspecies of this genus have too many common names, some confusion tends to be made such as Prussian carp or gibel carp for C. auratus gibelio. In FAO website, only silver crucian carp of Carassius genus is listed in Cultured Aquatic Species Information Programme (FAO 2018). About 30 countries/areas in Asia, Europe, and Africa produce silver crucian carp according to the FAO fishery statistics database, which ranked 6th among all cultured freshwater fish globally. Global production in 2013 reached 2,596,295 tons (FAO 2014). Silver crucian carp, crucian carp including their subspecies and strains are widely cultured in China and have been an important food resource for human consumption, due to the success of artificial reproduction. Goldfish was originated from wild crucian carp through artificial breeding and selection (Komiyama et al. 2009; Yi et al. 2012) and has spread worldwide as freshwater ornamental fish. The fish disease becomes worse nowadays and the viral infections have emerged as a significant obstacle to the silver crucian carp and goldfish aquaculture.
Cyprinid herpesvirus group has three members, including carp pox (CyHV-1), Cyprinid herpesvirus 2 (CyHV-2) and koi herpesvirus (CyHV-3) (Davison et al. 2009; Hanson et al. 2011). Among them, CyHV-2 has been confirmed as a causative agent of the acute hematopoietic necrosis disease outbreak in goldfish (Jung and Miyazaki 1995; Groff et al. 1998; Chang et al. 1999; Stephens et al. 2004; Goodwin et al. 2006; Jeffery et al. 2007; Lovy and Friend 2014; Sahoo et al. 2016) and various crucian carp (Daněk et al. 2012; Wang et al. 2012; Fichi et al. 2013; Xu et al. 2013). Diagnosis and confirmation of the CyHV-2 outbreaks mainly relied on molecular methods and electron microscopy observation of viral particles. It is reported that cell culture for this virus was difficult since cell lines susceptible to the virus are not easily available. It is reported that the CPE was observed on Cyprinus carpio koi fin (CCKF), fathead minnow (FHM), tilapia ovary (TO-2), and goldfish fin (GFF), but the virus was lost after several passages in vitro (Sahoo et al. 2016; Jung and Miyazaki 1995; Ito et al. 2013; Xu et al. 2013; Shibata et al. 2015; Xia et al. 2016). However, CyHV-2 could proliferate steadily on gibel carp brain cell lines (GiCB) after 40 passages (Ma et al. 2016). Therefore, only cell lines originated from the susceptible host species could support the continuous virus multiplication. Two cell lines from brain tissue of silver crucian carp (CrCB) and goldfish (GFB) were established and have been subcultured more than 40 times in our laboratory for over 2 years. The establishment, optimal growth condition, cryopreservation, species identification, and viral susceptibility of these newly established cell lines were described in this paper.
Materials and Methods
Primary cell cultures
Brain tissue was aseptically collected to initiate primary cell cultures from 6 juvenile silver crucian carp (body weight: 45–60 g, body length: 8–10 cm) and 8 juvenile goldfish (body weight: 35–55 g, body length: 6–8 cm). The tissue was treated with 75 % ethanol two times (15 s each), then rinsed with M199 medium (Gibco, Grand Island, NY) three times. Subsequently, tissue samples were minced into 1–2 mm3 fragments with sterile scissors and the fragments were transferred into a 15-mL sterile centrifuge tube (Corning 430791, Shanghai, China). Two times volume of trypsin solution was added and the mixture was incubated at 25°C for 5 min. After centrifuging at 1000 rpm for 2 min, the supernatant was discarded and the pellet was suspended in an appropriate amount of M199 medium with 10 % FBS. The suspension was centrifuged at 2000 rpm for 2 min and the pellet was explanted into 25 cm2 flasks (Corning 430168, Shanghai, China) using a sterile inoculation ring. The tissue fragments were spread into a single layer and covered with a sterile stainless steel mesh with a wire diameter of 0.20 mm and an aperture of 0.308 mm. For the adhesion of the tissue, the flask was placed into an incubator at 25°C for 24 h and then M199 medium containing 20 % FBS, 100 U/ml penicillin, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 μg/mL fibroblast growth factor, and 10 μg/mL epidermal growth factor was added. After that, the medium was replaced with a fresh one every 4 d. The cultured CrCB and GFB cells were passed every 7 to 10 d. Finally, the medium after passage 10 was changed with growth medium containing 10 % FBS, while no antibiotics and growth factors were added.
Growth studies
Growth characteristics of the cell lines were assessed at selected temperatures: 15, 20, 25, and 30°C over 12 d. The trypsinized cells from passage 26 were seeded in a new 25 cm2 culture flasks (Corning 430168, Shanghai, China) at a density of 2.0 × 105 cells per flask and incubated at selected temperatures. Then, 2 flasks at each temperature were trypsinized every other day, and the number of the cells were counted by a hemocytometer.
Cell plating efficiency
Both CrCB and GFB cell lines at passage 26 were used to determine plating efficiency. Triplicate 25 cm2 flask were seeded with 200, 500, and 1000 cells in growth medium and the cells were incubated at 25°C. On day 12, the medium was discarded and the cells were fixed with 5 mL of crystal violet-formalin stain-fixative for 15 min, rinsed with distilled water, and air-dried. Individual cell colonies were counted under the microscope, and plating efficiency was calculated on the basis of the following equation: (A1/A2) × 100 %, where A1 and A2 represent the number of cell colonies and cell-seeded, respectively.
Cryopreservation of cells
The ability and stability of CrCB and GFB cell lines to be preserved in liquid nitrogen (N2) were assessed in freezing medium. When growing logarithmically the two cells lines were harvested and resuspended at cell densities of 5 × 106 to 6 × 106 cells/mL. Cell suspensions were carefully centrifuged at 1000 rpm for 10 min and the cell pellet was resuspended with freezing medium consisting of 10 % dimethyl sulfoxide DMSO (Sigma D2650), Shanghai, China, 20 % FBS and 70 % M199. Aliquots(1.5 mL) were dispensed into 2-mL sterile plastic vials (Corning 430659, Shanghai, China) and sealed. The temperature of the vials was slowly decreased to − 80°C using cryogenic freezing containers (Nalgene Mr. Frosty) for 24 h before being transferred to liquid nitrogen. After storage of 390 d, the vials containing the cells were thawed at 22°C and then reseeded in 25 cm2 cell culture flasks in which viable cells were counted by trypan blue exclusion.
Ribosomal RNA (rRNA)
DNA extractions from 5.0 × 107 cells for CrCB and GFB cells at passage 23 and 24, respectively, were completed by MagMAX™-96 Viral RNA Isolation Kit (Ambion: AM 1836). Two oligonucleotide primers were designed to amplify the 16S and 18S mitochondrial DNA of Carassius auratus. The 16S fragment (123 bp) was amplified using the following primers: forward (16S-F) 5’-GCGACCACGGAGGAAAAA-3’ and reverse (16S-R) 5’-CGTTGATCGGCTTGTATTAG-3’. The 18S fragment (234 bp) was amplified using the following primers: (18S-F) 5’-GCGAGACGAGCCACCACCTATC-3’ and reverse (18S-R) 5’-CCCCCGGCCGTCCCTCTTA-3’ (Rougée et al. 2007). The PCR reaction mixture (25 μL) contained 2.5 μL PCR buffer (10×), 2.0 μL dNTPs (2.5 mM), 2.0 μL MgCl2(25 mM), 0.5 μL forward primer (50 μmol/L), 0.5 μL reverse primer (50 μmol/L), 0.5 μL Taq-DNA polymerase (5 U/μL), 10 μL DNA template (0.05 μg), and 7.5 μL double-distilled water. The cycling conditions involved an initial denaturing phase at 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, with a final extension phase at 72°C for 5 min. The PCR fragments were sequenced by Nanjing Springen Biotechnology Corporation and compared to known sequences in NCBI.
Viral susceptibility
The brain, kidney, spleen, and intestine of the sick and dead goldfish from Cyprinid herpesvirus 2 outbreak were pooled and detected by PCR primers (Jeffery et al. 2007). The PCR positive tissue samples were homogenized and inoculated into the CrCB and GFB cell monolayer after being filtered through a 0.45-μm filter. When the cytopathic effect (CPE) was observed on 80 % of the cell monolayer the cell suspension was collected and tested by PCR for CyHV-2.
Karyotyping of cells
The chromosome was counted on passage 21 for CrCB and passage 19 for GFB. The subcultured cell lines were seeded in duplicate 25-cm2 tissue culture flasks in the growth medium. The medium was replaced with 5 mL of fresh medium containing a 0.2-mL colcemid solution (0.8 μg/mL) after a 22 h of incubation. The cultures were incubated at 25°C for 24 h before being harvested and prepared for fixation. Microscopy of more than 100 random metaphase spreads fixed to microscope slides were used for each chromosomal count.
Results
Cell morphology
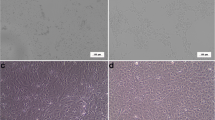
The morphology of CrCB and GFB cells remained consistently fibroblastic from their initial inception to the current passage (Fig. 1).
Growth characteristics
Sizable colonies were established in 25 cm2 tissue culture flasks after 2–3 d of culture for both CrCB and GFB, and cell confluence reached 80–90 % in 5–6 d for CrCB and 6–8 d for GFB. Both CrCB and GFB grew well at temperatures from 25°C to 30°C, with 30°C supporting superior growth (Fig. 2).
Plating efficiency
The CrCB and GFB were also used to determine the plating efficiency in three different groups with 200, 500, or 1000 cells per flask. Moderately, low plating efficiency was observed, with CrCB cells (8, 11.6, and 17.4 %, respectively) and with GFB cells (8.6, 12.4, and 17.8 %, respectively), while no significant differences between replicates. These data also revealed cell plating at a higher seeded density was more efficient than that at lower density, which had difficulties replicating and achieved a confluent monolayer within the 7 d after initial seeding.
Cryopreservation
The viability of CrCB and GFB cells stored in liquid nitrogen was evaluated and the capability of both cells lines to survive following more than 1 yr of storage at − 196°C was confirmed. Over 94% of CrCB and 96% of GFB cell populations from each vial retained the ability to attach and grow at 25°C after the storage period. In addition, both of two cell lines recovered well and no obvious alterations in morphology or growth pattern were observed.
Chromosomal analysis
Chromosomal counts from over 100 random metaphase spreads were used for CrCB and GFB (Fig. 3). Chromosome numbers ranged from 112 to 163 in CrCB and from 90 to 120 in GFB, with a distinct peak at 154 triploid chromosomes and 110 diploid chromosomes respectively (Fig. 4). This finding is consistent with previous karyotyping done in silver crucian carp (Hong et al. 2005) and the goldfish population (Ohno et al. 1967).
Sequence analysis of 16S and 18S rRNA
In order to verify the origin of the cell lines heteroduplex analysis of mitochondrial 16S and 18S rRNA was performed. 16S and 18S mitochondrial DNA were amplified for both cell lines and the expected PCR products of 123 and 234 bp were revealed respectively. After a comparative analysis, the identified sequences were demonstrated a 100% match for 16S and a 94% match for 18S to known Carassius auratus mitochondrial DNA sequence (GenBank Accession Nos. KU896992.1 and KT002365.1, respectively). Our data demonstrate that CrCB and GFB cell lines are indeed derived from silver crucian carp and goldfish (Fig. 5).
(A) PCR amplification of 123 and 234 bp sequences of the silver crucian carp and goldfish genome using oligonucleotide primers designed from the conserved portions of the 16S and 18S mitochondrial DNA. The extracted DNA from CrCB and GFB cells was amplified and then subjected to 1.5 % gel electrophoresis. M (marker): DL 2000; lane 1: CrCB; lane 2: GFB; lane 3: negative control. (B) Nucleotide sequences of the 123 and 234 bp fragments amplified by PCR using the respective primers. Underlined portions represent positions of the PCR primers.
Virus susceptibility
The tissue homogenates of sick or died goldfish from CyHV-2 outbreak were inoculated on CrCB and GFB cell monolayer. CPE was initially observed from 3 d and developed extensively on 7 d post-inoculation. Infected CrCB and GFB suspensions were PCR positive for CyHV-2 (Fig. 6).
Discussion
CyHV-2 has been reported worldwide, including the USA, UK, Australia, New Zealand, China, Italy, Switzerland, Germany, Hungary, Czech Republic, and France following its first report in juvenile goldfish in Japan in 1992 (Radosavljević et al. 2017). Massive mortality outbreaks in an aquaculture pond of silver crucian carp in China have caused quite huge economic loss. Experimental CyHV-2 infections of indigenous Cyprininae species in Japan showed that cumulative mortality was 100, 20, and 10 % in intraperitoneally injected Edonishiki, ginbuna, and nagabuna, respectively (Ito and Maeno 2014). Hybrid goldfish (male goldfish Carassius auratus × female common carp Cyprinus carpio) were completely resistant to experimental CyHV-2 infection while the parental species of goldfish were highly susceptible to the virus (Hedrick et al. 2006) which suggests that the occurrence of CyHV-2 infection is species-specific.
Cell isolation is considered to be the golden standard for virus identification and represents the most accurate method for diagnosis. Several cell line derived from goldfish tissue, such as skin epithelial and fibroblastic cell (GFSk-S1), scale epithelial cell (GAKS), muscle epithelial cell (GFM), and swim bladder fibroblastic cell (GFSB) from goldfish were established but none of them were tested susceptible to CyHV-2 (Lakra et al. 2011). FHM cell line was reported to produce high virus load but the virus was lost after 4 passages (Jung and Miyazaki 1995). It is hypothetically possible that cell lines derived from CyHV-2 target tissues are the best options for the virus replication. In most CyHV-2 outbreaks, there was severe, widespread necrosis of the hematopoietic tissues including the trunk kidney and spleen which were also demonstrated to carry high viral load (Goodwin et al. 2006; Jeffery et al. 2007; Ito et al. 2013; Ding et al. 2014; Lovy and Friend 2014; Giovannini et al. 2016). However, in this study, the cell lines of spleen, kidney, and fin from silver crucian carp and goldfish failed to proliferate CyHV-2. Since common carp brain cell line (CCB) is susceptible to CyHV-3, we decided to establish brain cell lines to isolate CyHV-2.
The brain tissue fragments are easy to float when the medium was added to the flask. Therefore, the flasks with the tissue fragments were placed into the incubator overnight before adding medium to facilitate the attachment and the sterile steel meshes were also used to cover and fix the fragments. It took about 30 d for the CrCB cells to grow to 80 % confluence, and the cell growth gradually accelerated after the first passage. From the 10th passage, it took 24–48 h for the cells to grow into the confluent monolayer.
The growth factors such as epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) were demonstrated to be essential to stimulate the growth of embryo cell lines derived from sea perch, red sea bream, and Japanese flounder (Chen et al. 2003a, 2003b, 2004). In this study, EGF and bFGF were used as potent mitogenic factors in CrCB and GFB cells proliferation for the first 10 passages. After that, the growth medium without the two factors was proved to maintain the cell lines effectively.
The euploid karyotype is an important parameter for characterizing cell lines. Chromosomal typing revealed a triploid chromosome for CrCB and a diploid chromosome for GFB respectively, which was consistent with the normal chromosome number of silver crucian carp and goldfish.
Conclusions
The susceptibility of CrCB and GFB cells lines to CyHV-2 infection is the basis for isolation and characterizing of the virus. The present study demonstrated that CPE occurred in CrCB and GFB from 3 d after infection with CyHV-2, indicating that viral diagnosis might be made with the two cell lines within 3 d. And the CrCB and GFB could be useful tools for isolating and identifying infectious viruses from silver crucian carp and goldfish. It is worth further investigation whether the two cell lines are permissive to CyHV-1, CyHV-3, and other cyprinid-susceptible viruses such as Spring viraemia virus of carp (SVCV). The CrCB cell line has been presented to laboratories in China as request and demonstrated to be a reliable and stable cell line for virus isolation in silver crucian carp and goldfish CyHV-2 outbreaks.
References
Apalikova OV, Podlesnykh AV, Kukhlevsky AD, Guohua S, Brykov VA (2011) Phylogenetic relationships of silver crusian carp Carassius auratus gibelio, C. auratus cuvieri, crucian carp Carassius carassius, and common carp Cyprinus carpio as inferred from mitochondrial DNA variation. Russ J Genet 47(3):322–331
Chang PH, Lee SH, Chiang HC, Jong MH (1999) Epizootic of herpes-like virus infection in goldfish, Carassius auratus, in Taiwan. Fish Pathol 34(4):209–210
Chen SL, Ren GC, Sha ZX, Shi CY (2004) Establishment of a continuous embryonic cell line from Japanese flounder Pararlichthys olivaceus for virus isolation. Dis Aquat Org 60:241–246
Chen SL, Sha ZX, Ye HQ (2003a) Establishment of a pluripotent embryonic cell line from sea perch blastula embryo. Aquaculture 218:141–151
Chen SL, Ye HQ, Sha ZX, Hong Y (2003b) Derivation of a pluripotent embryonic cell line from red sea bream blastulas. J Fish Biol 63:795–805
Daněk T, Kalous L, Vesel T, Krásová E, Reschová S, Rylková K, Kulich P, Petrtýl M, Pokorová D, Knytl M (2012) Massive mortality of Prussian carp Carassius gibelio in the upper Elbe basin associated with herpesviral hematopoietic necrosis (CyHV-2). Dis Aquat Org 102(2):87–95
Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, Pellett PE, Roizman B, Studdert MJ, Thiry E (2009) The order Herpesvirales. Arch Virol 154(1):171–177
Ding Z, Xia S, Zhao Z, Xia A, Shen M, Tang J, Xue H, Geng X, Yuan S (2014) Histopathological characterization and fluorescence in situ hybridization of Cyprinid herpesvirus 2 in cultured Prussian carp, Carassius auratus gibelio in China. J Virol Methods 206:76–83
FAO (2014) Fishery and Aquaculture Statistics. Global aquaculture production. 1950-2014(Fishstat). In: FAO FiSheries and Aquaculture Department [online]. Rome. Updated 2018. www.fao.org/fishery/statistics/software/fishstatj/en
FAO (2018) Cultured Aquatic Species Information Programme. Carassius carassius. Text by Weimin M. In: FAO Fisheries and Aquaculture Department. Rome
Fichi G, Cardeti G, Cocumelli C, Vendramin N, Toffan A, Eleni C, Siemoni N, Fischetti R, Susini F (2013) Detection of Cyprinid herpesvirus 2 in association with an Aeromonas sobria infection of Carassius carassius (L.), in Italy. J Fish Dis 36(10):823–830
Freshney RI ed (1994). Culture of animal cells, A Manual of Basic Techniques New York: pp 486
Giovannini S, Bergmann SM, Keeling C, Lany C, Schütze H, Schmidt-Posthaus H (2016) Herpesviral hematopoietic necrosis in goldfish in Switzerland: early lesions in clinically normal goldfish (Carassius auratus). Vet Pathol 53(4):847–852
Goodwin AE, Khoo L, LaPatra SE, Bonar A, Key DW, Garner M, V. Lee, M & Hanson, Larry. (2006) Goldfish hematopoietic necrosis herpesvirus (cyprinid herpesvirus 2) in the USA: molecular confirmation of isolates from diseased fish. J Aquat Anim Health 18(1): 11–18
Groff JM, LaPatra SE, Munn RJ, Zinkl JG (1998) A viral epizootic in cultured populations of juvenile goldfish due to a putative herpesvirus etiology. J Vet Diagn Investig 10(4):375–378
Hanson L, Dishon A, Kotler M (2011) Herpesviruses that infect fish. Viruses 3(11):216–219
Hedrick RP, Waltzek TB, McDowell TS (2006) Susceptibility of koi carp, common carp, goldfish, and goldfish x common carp hybrids to cyprinid herpesvirus-2 and herpesvirus-3. J Aquat Anim Health 18(1):26–34
Hong YJ, Yu ZJ, Zhou L, Gui JF (2005) A population of red-transparent, triploid Carassius auratus. J Fish Biol 67(4):1139–1143
Ito T, Kurita J, Ozaki A, Sano M, Fukuda H, Ototake M (2013) Growth of cyprinid herpesvirus 2 (CyHV-2) in cell culture and experimental infection of goldfish Carassius auratus. Dis Aquat Org 105(3):192–202
Ito T, Maeno Y (2014) Susceptibility of Japanese Cyprininae fish species to cyprinid herpesvirus 2 (CyHV-2). Vet Microbiol 169(3–4):128–134
Jeffery KR, Bateman K, Bayley A, Feist SW, Hulland J, Longshaw C, Stone D, Woolford G, Way K (2007) Isolation of a cyprinid herpesvirus 2 from goldfish, Carassius auratus (L.), in the UK. J Fish Dis 30(11):649–656
Jung SJ, Miyazaki T (1995) Herpesviral haematopoietic necrosis of goldfish, Carassius auratus (L.). J. Fish Dis 18(3):211–220
Komiyama T, Kobayashi H, Tateno Y, Inoko H, Gojobori T, Ikeo K (2009) An evolutionary origin and selection process of goldfish. Gene 430(1–2): 0–11
Lakra WS, Swaminathan TR, Joy KP (2011) Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37(1):1–20
Lovy J, Friend SE (2014) Cyprinid herpesvirus-2 causing mass mortality in goldfish: applying electron microscopy to histological samples for diagnostic virology. Dis Aquat Org 108(1):1–9
Ma J, Zhou Y, Fan Y, Liu W, Jiang N, Zeng L (2016) The physical-chemical and biological characteristics of Cyprinid herpesvirus 2 and its ultrastructural morphogenesis in vitro. J Fish China 40(3):475–483
Ohno S, Muramoto J, Christian L, Atkin NB (1967) Diploid-tetraploid relationship among old-world members of the fish family Cyprinidae. Chromosom 23:1–9
Radosavljević V, Maksimović ZJ, Veljović L, Ljubojević D, Ćirković M, Marković Z, Vesna M (2017) Cyprinid herpesvirus diseases. Arch Vet Med 10(1):51–60
Rougée L, Ostrander RH, Richmond YL (2007) Establishment, characterization, and viral susceptibility of two cell lines derived from goldfish Carassius auratus muscle and swim bladder. Dis Aquat Org 77:127–135
Sahoo PK, Swaminathan TR, Abraham TJ, Kumar R, Pattanayak S, Mohapatra A, Rath SS, Patra A, Adikesavalu H, Sood N, Pradhan PK, Das BK, Jayasankar P, Jena JK (2016) Detection of goldfish haematopoietic necrosis herpes virus (Cyprinid herpesvirus-2) with multi-drug resistant Aeromonas hydrophila infection in goldfish: first evidence of any viral disease outbreak in ornamental freshwater aquaculture farms in India. Acta Trop 161:8–17
Shibata T, Nanjo A, Saito M, Yoshii K, Ito T, Nakanishi T, Sakamoto T, Sano M (2015) In vitro characteristics of cyprinid herpesvirus 2: effect of kidney extract supplementation on growth. Dis Aquat Org 115(3):223–232
Stephens FJ, Raidal SR, Jones B (2004) Haematopoietic necrosis in a goldfish (Carassius auratus) associated with an agent morphologically similar to herpesvirus. Aust Vet J 82(3):167–169
Wang L, He J, Liang L, Zheng X, Jia P, Shi XJ, Lan W, Xie J, Liu Hongxi XP (2012) Mass mortality caused by Cyprinid Herpes virus 2(CyHV-2)in Prussian carp (Carassius gibelio) in China. Bull Eur Assoc Fish Pathol 32(5):164–173
Xia S, Wang H, Podok P, Xu D, Jiang Y, Lü L, Sano M (2016) The expression analysis of immune genes and microscopic morphology of CCF cells in response to Cyprinid herpesvirus 2 infection. J Fish China 40(12):1915–1922
Xu J, Zeng L, Zhang H, Zhou Y, Ma J, Fan Y (2013) Cyprinid herpes virus 2 infection emerged in cultured gibel carp, Carassius auratus gibelio in China. Vet Microbiol 166(12):138–144
Yi Z, Biqian L, Jie T (2012) Identifying six strains of Carassius auratus by PCR-RFLP on mtDNA ND3/ND4L/ND4 gene. Biotechnol Bull 11:144–149
Funding
This work was financially supported by the science and technology project of Jiangsu Entry-Exit Inspection and Quarantine Bureau of P.R. China (No. 2017KJ01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Xu, Y., Zhou, Y., Wang, F. et al. Development of two brain cell lines from goldfish and silver crucian carp and viral susceptibility to Cyprinid herpesivirus-2. In Vitro Cell.Dev.Biol.-Animal 55, 749–755 (2019). https://doi.org/10.1007/s11626-019-00402-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00402-y