Abstract
Ischemic stroke and cardiovascular disease can occur from blockage of blood vessels by fibrin clots formed naturally in the body. Therapeutic drugs of anticoagulant or thrombolytic agents have been studied; however, various problems have been reported such as side effects and low efficacy. Thus, development of new candidates that are more effective and safe is necessary. The objective of this study is to evaluate fibrinolytic activity, anti-coagulation, and characterization of serine protease purified from Lumbrineris nipponica, polychaeta, for new thrombolytic agents. In the present study, we isolated and identified a new fibrinolytic serine protease from L. nipponica. The N-terminal sequence of the identified serine protease was EAMMDLADQLEQSLN, which is not homologous with any known serine protease. The size of the purified serine protease was 28 kDa, and the protein purification yield was 12.7%. The optimal enzyme activity was observed at 50°C and pH 2.0. A fibrin plate assay confirmed that indirect fibrinolytic activity of the purified serine protease was higher than that of urokinase-PA, whereas direct fibrinolytic activity, which causes bleeding side effects, was relatively low. The serine protease did not induce any cytotoxicity toward the endothelial cell line. In addition, anticoagulant activity was verified by an in vivo DVT animal model system. These results suggest that serine protease purified from L. nipponica has the potential to be an alternative fibrinolytic agent for the treatment of thrombosis and use in various biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke and cardiovascular disease caused by thrombus formation have significant impacts on mortality (Libby 2006). To overcome these conditions, the thrombus needs to be removed or treated with a thrombolytic agent. Currently, the only thrombolytic agent that has been commercially approved by the United States Food and Drug Administration (US FDA) is tissue-plasminogen activator (t-PA), which is a serine protease found on endothelial cells of blood vessels (Zivin 2009). T-PA reportedly has side effects such as swelling, rashes, hives, short-time window, and reocclusions (Wardlaw et al. 2012). Accordingly, investigation and development of new thrombolytic proteases that can overcome these side effects are required (Nordt and Bode 2003).
Serine protease includes a catalytic triad composed of aspartic acid, histidine, and serine residues that cleaves protein peptide bonds (Brady et al. 1990). In humans, serine protease mainly regulates blood circulation and metabolic processes by influencing thrombolytic events (Carter and Wells 1988). Plasmin is a serine protease that plays a significant role in degrading blood plasma proteins, including fibrin clots, via a process known as fibrinolysis (Wiman and Collen 1978). Owing to its fibrinolytic activity, plasmin should be useful in emergency situations when fibrin clots are not degraded, resulting in thrombosis (Sherry et al. 1959). However, when injury or disease occurs and the conditions cannot be resolved, it is necessary to use effective drugs. Compounds that can dissolve blood clots are known as thrombolytic agents. These drugs are largely divided into two types depending on the thrombosis mechanisms. Direct thrombolytic agents, which include serine proteases such as plasmin, brinase, and trypsin, directly degrade fibrin clots into fibrin degradable products (FDP) (Collen 1999). However, these drugs are toxic and nonspecifically dissolve coagulated clots, resulting in severe damage. Side effects of direct thrombolytic agents can be complemented by indirect fibrinolytic enzymes that activate their downstream enzymes to dissolve fibrin clots such as urokinase, t-PA, streptokinase, and hirudin (Kotb 2015). The indirect fibrinolytic pathway can overcome the disadvantages of directly decomposing proteases by acting on fibrin-specific plasminogen lysis. Nevertheless, indirect thrombolytics still have problems such as low efficacy and side effects such as hemolysis, fever, allergic reactions, topical bleeding, and reocclusions (Bi et al. 2013). Therefore, development of various indirect fibrinolytic agents with high efficacy and fewer side effects as compared to typical thrombolytic agents is important for alternative applications (Victor and Sol 1988).
Previous studies have been conducted to identify serine proteases from many marine organisms. Serine proteases purified from annelids have been shown to have fibrinolytic activity, and this activity has already been demonstrated in various species including Neanthes japonica (Wang et al. 2011), Eisenia andrei (Lee et al. 2007), and Perionyx excavatus (Phan et al. 2011). However, previously identified serine proteases from annelids led to the same side effects caused by conventional thrombolytic agents, such as reocclusion and bleeding.
Lumbrineris nippinica is distributed in mudflats of Korea and Japan which has various protease for digest various food in its habitats. In the present study, we purified and identified a new fibrinolytic serine protease from L. nipponica which is called LN serine protease and evaluated whether the bi-functional fibrinolytic and anti-clotting activity could overcome these side effects. In in vitro assay, the new serine protease strongly expressed indirect fibrinolytic and anticoagulant activities, along with weak direct fibrinolytic activity and non-cytotoxicity to human endothelial cells. Also, in vivo assay showed anticoagulant activities which are promising characteristics required for the treatment of thrombosis and various biomedical applications.
Materials and Methods
Reagents
A Hitrap Benzamidine affinity fast flow column and Hitrap DEAE sepharose ion exchange column were obtained from GE healthcare Life Sciences (Uppsala, Sweden). Azocasein and fibrinogen from human plasma were purchased from Sigma Aldrich (St. Louis, MO,). Thrombin was obtained from Mybiosource (San Diego, CA,). Laemmli's ×5 protein sample buffer was gotten from Elpis-Biotech (Daejeon, Korea) and SeeBlue® Plus2 pre-stained standard was purchased from Thermofisher (Waltham, MA,).
Purification of serine protease
All purification steps were performed at 4°C and followed an ammonium sulfate precipitation purification method (Deng et al. 2010). L.nippionica was harvested from mudflat in Incheon, Korea in winter. Before extracting serine protease, its intestine was cleared out as best as possible through starving for 48 h at 10°C. After incubation, 50 g of washed sample was cut into small pieces and auto-lysed in 100 mL of 20 mM phosphate (Na2HPO4, KH2PO4, and pH 7.0) for 2 h at 4°C. Remaining 4°C for overall precipitation steps, the auto-lysed sample was centrifuged at 8000 g for 30 min, after which the supernatant solution was saturated by the addition of ammonium sulfate to give a final concentration of 20% and it was stayed for 4 h. The suspension was centrifuged at 10,000 g for 20 min, after which the supernatant was adjusted to 55% saturation and incubated for an additional 4 h. The solution including precipitate was then centrifuged at 10,000 g for 15 min. The remaining supernatant was removed and the pellet was dissolved in 5 mL of 20 mM phosphate buffer and stored at −20°C until use.
Fractionation of serine protease
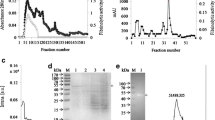
Crude extracts were separated through dialysis membrane which has 14,000 dalton of molecular weight cut-off for 24 h in 20 mM phosphate buffer at 4°C. The sample was then applied to a HiTrap™ Benzamidine affinity fast flow column (GE Healthcare, Uppsala, Sweden, 1 mL) equilibrated with Tris-HCl containing 0.5 M NaCl buffer (pH 7.4) and eluted with 5 mL of 0.5 M glycine buffer (pH 3.0). Following affinity purification, the buffer was exchanged with Tris-HCl buffer in phosphate buffer via dialysis. The sample was then applied to a HiTrap™ DEAE sepharose ion exchange column (GE Healthcare, 1 mL) equilibrated with Tris-HCl. The elution buffer used was NaCl buffer (pH 7.4). At this time, the amount of the eluent was 5 to 10 times that in the column.
Determination of molecular weight
The molecular weight of LN serine protease was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The SDS-PAGE running gel contained 12% acrylamide, 0.1% SDS, and 0.375 M Tris-HCl. (Kim et al. 2015). Seven micrograms of the purified sample was mixed with ×5 protein sample buffer, boiled at 100°C for 5 min, and then electrophoresed with SeeBlue® Plus2 pre-stained standard at 110 V. The electrophoresed gel was stained with Coomassie Brilliant Blue and destained with destaining buffer.
Determination of serine protease amino acid sequence
The purified proteins obtained by SDS-PAGE were transferred to a polyvinylidene difluoride membrane by using electroblotting method. The proteins were electroblotted by TE77X semi dry transfer (Hoefer) in towbin buffer at constant current 110 mA. The sample was submitted to Emass, Seoul, Korea (Moon et al. 2014) to request protein sequencing. The N-terminal sequence of the purified protein was analyzed for 15 amino acid residues, and then subjected to the Edman degradation method. The obtained N-terminal sequence was compared to that of other proteases by BLAST searches of the NCBI database.
Confirmation of protein yield depending on purification steps
The yield of purification steps was determined according to the amount of protein and protein activity. Purification steps were divided into four steps, crude extraction, ammonium sulfate treatment, affinity column chromatography, and ion exchange column chromatography, after which the amounts of protein and protein activity were obtained by bicinchoninic acid (BCA) assay and through the OD value in accordance with the fibrin plate assay, respectively. Specific activity was determined as a total activity/total protein, and yield was determined as the ratio of the protein activity of each step.
Fibrin plate assay
Fibrinolytic activity was confirmed by the fibrin plate method (Astrup and Müllertz 1952). Briefly, a fibrin plate made in our laboratory was formed by the reaction of 1 mL fibrinogen (1.5% human fibrinogen (20 mM Tris-HCl buffer, pH 7.4)) and 30 μL thrombin (100 NIH, 20 mM Tris-HCl buffer, pH 7.4) at 4-well plates for 1 h, after which a small hole was made to test the fibrinolytic activity at room temperature. Next, 10 μg/mL of serine protease and a control sample (u-PA) were added and allowed to react for 2 h at 37°C. Image J (National Institutes of Health) was used to evaluate fibrinolytic activity of samples by measuring the dissolved area of the fibrin plate.
The fibrinolytic activity of the serine protease according to time was determined by spectrophotometric analysis of samples in a 96-well plate at 30 min, 1, 2, and 4 h. The 96-well fibrin plate was formed as described above. For analysis, approximately 1 μg of serine protease was added to each well and the absorbance at 540 nm was measured at each of the aforementioned times. All of the above experiments were proceeded at room temperature.
Azocasein assay
Protease activity was measured by azocasein assay (Iversen and Jørgensen 1995). For this assay, the mixture was composed of 0.5% azocasein, 25 mM Tris-HCl buffer (pH 7.5), and 10 μg/mL of purified enzyme. The azocasein assay was incubated in 1.5 mL microcentrifuge tube at 37°C for 30 min, after which the protease-protease substrate reaction was stopped by the addition of 10% TCA and then centrifuged at 10,000 g for 10 min. Following centrifugation, the pellet was removed and NaOH was added to the supernatant. The supernatant mixture was then measured by ELISA at 450 nm, after which the azocasein assay was used for pH measurement, temperature characterization, and an inhibitor test.
Characterization of enzyme activity dependent on temperature and pH
To determine the optimal temperature for the activity of fibrinolytic enzyme extracted from L. nipponica, the purified sample was evaluated at 4, 10, 24, 30, 37, 50, 60, and 70°C (Ahn et al. 2003). The sample was incubated for 15 min at each temperature before treatment with azocasein, after which the assay was performed as described above. To investigate the optimal pH for the serine protease extracted from L. nipponica, an azocasein assay was performed at pH values of 3.0–10.0. The 25 mM Tris-HCl buffer (pH 7.5) for the azocasein assay was substituted with solution at pH 3 to 6 that was prepared using 25 mM glycine-HCl, while those at pH 7 to 10 were made with 25 mM Tris-HCl. All of the aforementioned experiments were conducted at room temperature and the protease activity was measured by azocasein assay.
In vitro endothelial cell toxicity test
LN serine protease must be tested for cell toxicity before it can be commercially applied for the treatment of actual stroke victims. The cytotoxicity effect of serine protease extracted from L. nipponica on human cerebral micro-vessel endothelial cells (hCMEC/D3) was analyzed by treatment in cell culture media (EBM-2 media containing 5% FBS, 1% penicillin-streptomycin, hydrocortisone (1.4 μM), ascorbic acid (10 μg/mL), ×1 chemically defined lipid concentrate, hydroxyethyl piperazineethanesulfonic acid (HEPES) (10 mM), and basic fibroblast growth factor (bFGF) (1 ng/mL)). The cells were incubated under 5% CO2 at 37°C for 24 h. Samples were tested according to the concentration of fibrinolytic enzyme. This experiment included five controls (5, 10, 20, 50, and 100 μg). Cell viability of hCMEC/D3 was analyzed by MTT assay.
Turbidity assay
A turbidity assay was performed using a modified version of the method described (Park et al. 2013). Briefly, 90 μL of human blood plasma, 10 μL of thrombin (100 NIH), and 25 mM phosphate buffer (pH 7.4) were mixed. The mixture was then incubated for 2 h at 25°C and observed for the formation of clots. Next, 5 μg of LN serine protease was added to the mixture and the samples were incubated for 24 h at 37°C. Finally, the turbidity was measured by ELISA at 405 nm.
In vivo DVT model
A deep vein thrombosis (DVT) animal model was generated using the method described by Zhang et al. (2011). Briefly, after abdominal incision of the rat (SD rat, 8 wk), sub vena cava such as lumber, renal, and iliac were sealed by suturing and clotting inducer (human blood plasma), and fibrinolytic enzyme (10 μg/mL) was injected into the rat tail vein. After 15 s, the vena cava was completely sealed for 1 h, at which time it was cut to determine the number of blood clots. The u-PA was used as a positive control in the DVT model.
Statistical analysis
One-way analysis of variance (ANOVA) was conducted to evaluate the dissolving potential of the fibrin plate, characterization of the optimal temperature and pH, cytotoxicity, and turbidity. All experiments were conducted in triplicate and statistical significance was considered with p values (*< 0.05, **< 0.01, or ***< 0.001). All data are expressed as the average values ± the standard deviation (S.D.).
Results
Purification of serine protease
Using a series of purification procedures, the serine protease was purified and its molecular weight was confirmed by SDS-PAGE (Fig. 1). LN serine protease was shown as a single band that was estimated to be 28 kDa on the SDS polyacrylamide gel. The yield was then measured using the total protein, specific activity, and total activity (Table 1). Determination of the specific activity based on the total activity and protein concentration revealed that it increased with each purification step. The specific activities of ion exchange, the last purification step, were 95.2 units/mg, which was about 1.6-fold higher than the specific activity of the crude extract step. Finally, the purification yield was 12.7%, which was similar to that reported for other reference compounds.
Identification of serine protease from L. nipponica by N-terminal amino sequence analysis
Amino acid sequences of serine protease from L. nipponica were analyzed by the Edman degradation method. N-terminal amino acid sequencing revealed that the LN serine protease is composed of Glu-Ala-Met-Met-Asp-Lys-Ala-Asp-Gln-Leu-Glu-Gln-Ser-Leu-Asn. Table 2 shows the sequence identification results relative to other serine proteases of various species evaluated using the NCBI blast algorithm. High similarity (53.3%) was observed for Oceanobacilus picturae, while the next most similar sequence was 46.6% homologous with Vibrio metschnikovii and Alnus qlutinosa.
The LN serine protease shows both plasminogen-dependent and independent fibrinolytic activities
Fibrinolytic activity assay of the LN serine protease was performed by measurement of clear zones in the fibrin plate (Fig. 2). PBS was used as negative control and plasmin was used as a positive control. As shown in Fig. 2 a, d, there was no fibrionolytic activity in PBS and high fibrinolytic activity in plasmin. In Fig. 2 b, c LN serine protease showed direct fibrinolytic activity which was only 0.3 times of u-PA. In this study, plasminogen-free and plasminogen-rich fibrin plates were used to determine if the serine protease had both low direct fibrinolytic activity and high indirect fibrinolytic activity. The results confirmed this respectively as shown in Fig. 2 e, f: the indirect fibrinolytic activity of LN serine protease over u-PA was confirmed by the plasminogen-rich plate. The indirect fibrinolytic activity of the LN serine protease was 1.3-fold higher than that of the u-PA. The optimal dissolving time was determined to be between 2 and 4 h (Fig. 2 g).
Fibrinolytic activities of LN serine protease and positive control (u-PA) on plasminogen-free (a, b, c, d) and plasminogen-rich (e, f) fibrin plates. (a) PBS; (b, e) LN serine protease; (c, f) u-PA (control); (d) plasmin. The amount of serine protease and u-PA was 10 μg. g The fibrin-dissolving potential of the enzyme was measured at 30 min, 1, 2, and 4 h based on the absorbance at 540 nm. The asterisk (*) indicates significant difference (*** P < 0.001).
Optimal temperature and pH characterization of the LN serine protease
The optimal temperature of LN serine protease was measured by azocasein assay. The temperature range was divided into 4, 10, 24, 30, 37, 50, 60, and 70°C. The optimal temperature was 37°C for the LN serine protease activity, with the activity decreasing below 30°C and above 50°C (Fig. 3 a). The optimal pH of LN serine protease was also measured by azocasein assay. As shown in Fig. 3 b, the serine protease maintained its activity at low to neutral pH’s (pH 3.0–pH 7.0); however, the activity at high pH’s (pH 8.0–pH 10.0) was significantly decreased. Based on these findings, the LN serine protease was identified as an acidic serine protease.
Characterization of the LN serine protease for measurement of optimal temperature (a) and pH (b) in the reaction range. a fibrinolytic enzyme activity according to temperature was measured at pH 7.5. Reactive activity was measured by the azocasein assay (reactive activity = (protease OD value—control OD value)/control OD value). b fibrinolytic enzyme activity according to pH was measured at 37°C. Reactive activity was measured by the azocasein assay.
Cytotoxicity test
Cell viability was measured by MTT assay after subjecting hCMEC/D3 cells to various serine protease concentrations. As shown in Fig. 4, the serine protease exerted no cytotoxicity. The viability of the hCMEC/D3 cells incubated with LN serine protease was unchanged as compared with that of the control cells. These findings indicate that the LN serine protease exerted no toxicity toward cells.
Turbidity assay
Turbidity was measured using a spectrophotometer microplate reader. Human plasma becomes turbid when thrombin reacts with CaCl2. As show in Fig. 5 a, the turbidity of human blood plasma clots was reduced in a concentration-dependent manner when exposed to the LN serine protease. These results demonstrate that this serine protease remained its activity during the purification process.
Measurement of turbidity and thrombus weights. (a) turbidity enhanced due to the dissolution of fibrin clots after adding different concentrations of enzymes. Turbidity was measured by spectrometry at 405 nm. The concentrations of serine protease were used 0, 5, 10, and 20 μg/mL. (b) comparison of thrombus weights 1 h after injection of 10 μg of u-PA or serine protease into the intrarenal vena cava of DVT model SD rats.
Deep vein thrombosis (DVT) model
The DVT model was used to confirm whether serine protease has the ability to act as an anticoagulant by measuring the amount of thrombus in the vena cava. After exposure to the LN serine protease, the vena cava contained fewer blood clots than the control and u-PA (Fig. 5 b). Overall, the experiments showed that serine protease has the ability to act as an anticoagulant.
Discussion
Serine protease was purified and newly identified from L. nipponica. Its N-terminal sequence was verified based on 53.3% homology with serine proteases from Oceanobacillus picturae using the NCBI blast algorithm following Edman degradation analysis (Table 2). When compared with other serine proteases (Oceanobacillus picturae, 44.9 kDa; Vibrio metschnikovii, 58.9 kDa), the molecular weight of the new serine protease from L. nipponica (28 kDa) was unique, confirming its novel nature. In addition, unlike in previous studies (Hahn et al. 1999; Wang et al. 2006), a strategy for purification of the serine protease was more specifically designed using a Benzamidine affinity column, which has a high capacity affinity for both trypsin and trypsin-like serine proteases. Such reduced and specifically developed steps improved purification yield and purity of the enzyme relative to previous studies (Hahn et al. 1999; Wang et al. 2006). This method was also expected to enhance enzymatic activity. As a result, the serine protease specifically identified in the present study can be considered a new isoform of fibrinolytic enzyme from marine species in addition to those that have been reported to date.
In general, serine protease has a difference in direct and indirect activities (Chou et al. 2013; Choi et al. 2014). Serine proteases that have strong direct activity generally have side effects of toxicity and bleeding, highlighting the importance of indirect thrombolysis (Chou et al. 2013). The indirect activities of the LN serine protease were much higher than the direct activity based on comparison with the u-PA. Moreover, the direct activities of the serine protease were lower than those of plasmin. These findings indicate that serine protease has the potential to reduce side effects, toxicity, and bleeding. The LN serine protease has low direct activity and high indirect activity; therefore, the side effects of the serine protease are expected to be lower than those of t-PA and u-PA. As a result, we expect serine protease to have a higher value as a drug because it can react more specifically to the fibrin clots.
The LN serine protease was identified as an acidic serine protease by pH characterization. Serine protease activity was maintained under a low pH environment, which is important because the pH of blood is maintained between 6 and 7 during stroke because of collapsing N-methyl-D-aspartic acid (NMDA) receptors. As the NMDA receptors break down, there is an influx of Ca2+, which results in the release of H+ (Isaev et al. 2008).
A turbidity assay using human plasma was also conducted to investigate whether LN serine protease had fibrinolytic activity (Wang et al. 2006). As shown in Fig. 5 a, the serine protease was effective in human blood plasma in a dose-dependent manner. There are many serine protease inhibitors in human blood plasma, which suppress an important factor to thrombosis. As a result, the serine LN serine protease was more effective in human blood plasma than other enzymes tested against metal chelators as serine protease inhibitors (Park et al. 2013).
To investigate the possibility for use of the serine protease isolated in this study as a thrombolytic agent, the reduction of side effects was focused on. Other studies have identified many problems associated with t-PA, and experiments have been conducted to address them (Wang et al. 1998). The present study verified the reocclusion ability of the serine protease to overcome side effects of t-PA for the treatment of stroke. Moreover, the results of the present study were tentatively confirmed by the DVT in vivo model. In the DVT model, the amounts of clots in the control and u-PA were higher than when serine protease was injected into the vena cava. These findings indicate that the LN serine protease acts as an anticoagulant in the vena cava. Accordingly, the LN serine protease has the potential to overcome the side effects associated with t-PA.
In conclusion, the present study provides the first evidence that LN serine protease has indirect fibrinolytic activities and anti-clotting capabilities, and is noncytotoxic. Moreover, we demonstrated its bi-functional fibrinolytic activities both in vitro and in vivo. Additionally, the DVT model was an effective method to assess the anticoagulant activity. Overall, the results presented herein indicate that LN serine has the potential for use in thrombolytic agents.
References
Ahn MY, Hahn BS, Ryu KS, Kim JW, Kim I, Kim YS (2003) Purification and characterization of a serine protease with fibrinolytic activity from the dung beetles, Catharsius molossus. Thromb Res 112:339–347
Astrup T, Müllertz S (1952) The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys 40:346–351
Bi Q, Han B, Feng Y, Jiang Z, Yang Y, Liu W (2013) Antithrombotic effects of a newly purified fibrinolytic protease from Urechis unicinctus. Thromb Res 132:e135–e144
Brady L, Brzozowski AM, Derewenda ZS, Dodson E, Dodson G, Tolley S, Turkenburg JP, Christiansen L, Huge-jensen B, Norskov L (1990) A serine protesase triad forms the catalytic centre of a triacylglycerol lipase. Nature 34:797–770
Carter P, Wells JA (1988) Dissecting the catalytic triad of a serine protease. Nature 332:564–568
Choi JH, Sapkota K, Kim S, Kim SJ (2014) Starase: a bi-functional fibrinolytic protease from hepatic caeca of Asterina pectinifera displays antithrombotic potential. Biochimie 105:45–57
Chou JH, Sapkota K, Park SE, Kim S, Kim SJ (2013) Thrombolytic, anticoagulant and antiplatelet activities of codiase, a bi-functional fibrinolytic enzyme from Codium fragile. Biochimie 95:1266–1277
Collen D (1999) The plasminogen (fibrinolytic) system. Thromb Haemost 82:259–270
Deng Z, Wang S, Li Q, Ji X, Zhang L, Hong M (2010) Purification and characterization of a novel fibrinolytic enzyme from the polychaete, Neanthes japonica (Iznka). Bioresour Technol 101:1954–1960
Hahn BS, Cho SY, Wu SJ, Chang IM, Baek K, Kim YC, Kim YS (1999) Purification and characterization of a serine protease with fibrinolytic activity from Tenodera sinensis (praying mantis). BBA-Protein Struct M 1430:376–386
Isaev NK, Stelmashook EV, Plotnikov EY, Khryapenkova TG, Lozier ER, Doludin YV, Silachev DN, Zorov DB (2008) Role of acidosis, NMDA receptors, and acid-sensitive ion channel 1a (ASIC1a) in neuronal death induced by ischemia. Biochem Mosc 73:1171–1175
Iversen SL, Jørgensen MH (1995) Azocasein assay for alkaline protease in complex fermentation broth. Biotechnol Tech 9:573–576
Kim DW, Choi JH, Park SE, Kim S, Sapkota K, Kim SJ (2015) Purification and characterization of a fibrinolytic enzyme from Petasites japonicus. Int J Biol Macromol 72:1159–1167
Kotb E (2015) Purification and partial characterization of serine fibrinolytic enzyme from Bacillus megaterium KSK-07 isolated from kishk, a traditional Egyptian fermented food. Appl Biochem Microbiol 51:34–43
Lee CK, Shin JS, Kim BS, Cho IH, Kim YS, Lee EB (2007) Antithrombotic effects by oral administration of novel proteinase fraction from earthworm Eisenia andrei on venous thrombosis model in rats. Arch Pharm Res 30:475–480
Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83:4565–4605
Moon SM, Kim JS, Kim HJ, Choi MS, Park BR, Kim SG, Ahn H, Chun HS, Shin YK, Dk K, Lee SY, Seo YW, Kim YH, Kim CS (2014) Purification and characterization of a novel fibrinolytic α chymotrypsin like serine metalloprotease from the edible mushroom, Lyophyllum shimeji. J Biosci Bioeng 117:544–550
Nordt TK, Bode C (2003) Thrombolysis: newer thrombolytic agents and their role in clinical medicin. Heart 89:1358–1362
Park JW, Park JE, Chou HK, Jung TW, Yoon SM, Lee JS (2013) Purification and characterization of three thermostable alkaline fibrinolytic serine proteases from the polychaete Cirriformia tentaculata. Process Biochem 48:979–987
Phan TTB, Ta TD, Nguyen TX, Van Den Broek LA, Duong GTH (2011) Purification and characterization of novel fibrinolytic proteases as potential antithrombotic agents from earthworm Perionyx excavatus. AMB Express 1:26
Sherry S, Lindemeyer RI, Fletcher AP, Alkjaersig N (1959) Studies on enhanced fibrinolytic activity in man. J Clin Inest 38:810–822
Victor J, Sol S (1988) Thrombolytic therapy: current status. N Engl J Med 318:1512–1520
Wang CT, Ji BP, Li B, Nout R, Li PL, Ji H, Chen LF (2006) Purification and characterization of a fibrinolytic enzyme of Bacillus subtilis DC33, isolated from Chinese traditional Douchi. J Ind Microbiol Biotechnol 33:750–758
Wang S, Deng Z, Li Q, Ge X, Bo Q, Liu J, Cui J, Jiang X, Zhang L, Hong M (2011) A novel alkaline serine protease with fibrinolytic activity from the polychaete, Neanthes japonica. Comp Biochem Physiol B: Biochem Mol Biol 159:18–25
Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA (1998) Tissue plasminogen activator (tPA) increase neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med 4:228–231
Wardlaw JM, Murray V, Berge E, Zoppo G, Sandercock P, Lindley RL, Cohen G (2012) Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 379:2364–2372
Wiman B, Collen D (1978) Molecular mechanism of physiological fibrinolysis. Nature 272:549–550
Zhang Y, Shi H, Wang W, Ke Z, Xu P, Zhong Z, Li X, Wang S (2011) Antithrombotic effect of grape seed proanthocyanidins extract in a rat model of deep vein thrombosis. J Vasc Surg 53:743–753
Zivin JA (2009) Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U. S. Food and Drug Administration (FDA). Ann Neurol 66:6–10
Acknowledgements
This work was supported by INHA U Research Grant.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Yeon, S.J., Chung, G.Y., Hong, J.S. et al. Purification of serine protease from polychaeta, Lumbrineris nipponica, and assessment of its fibrinolytic activity. In Vitro Cell.Dev.Biol.-Animal 53, 494–501 (2017). https://doi.org/10.1007/s11626-017-0137-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-017-0137-2