Abstract
Recent studies have demonstrated that insulin-like growth factor-1 (IGF-I) modulates bone mesenchymal stem cell chondrogenic differentiation independent of transforming growth factor beta (TGF-β) signaling in vitro. However, it is unclear whether IGF-I can solely modulate human adipose-derived mesenchymal cell (hAMC) chondrogenic differentiation, or whether it has additive effects with TGF-β1 to induce chondrogenic differentiation in vitro and development of mature cartilage in vivo. We investigated the effect of IGF-I on the induction of hAMC chondrogenic differentiation in the presence or absence of transforming growth factor beta 1 (TGF-β1) in vitro, and chondrogenesis of the induced hAMC in vivo. The results showed that IGF-I alone induced collagen type II, aggrecan, and Sox9 mRNA expression and collagen type II and aggrecan proteins expressions in hAMCs. Notably, there was greater mRNA expression of collagen type II, aggrecan and Sox9, and greater protein expression of collagen type II and aggrecan following TGF-β1 + IGF-I treatment, compared to either TGF-β1 or IGF-I-treated hAMCs. These results were confirmed in cartilage tissues derived from induced hAMCs. These findings indicate that IGF-I alone has the ability to induce chondrogenic differentiation and has additive effects with TGF-β1 to induce chondrogenic differentiation in vitro and in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue engineering is an attractive approach to cartilage repair (Hendriks et al. 2007; Correia et al. 2014). In spite of many advantages, tissue engineering for cartilage repair still faces several challenges, such as finding appropriate cell source and suitable method to induce chondrogenic differentiation. Human adipose-derived mesenchymal cells (hAMCs) are undifferentiated pluripotent cells capable of differentiating into osteoblasts, chondrocytes, epithelial cells, adipocytes, neurons, or myocytes (Zuk et al. 2002; Brzoska et al. 2005; Kingham et al. 2007; Sowa et al. 2013; Gao et al. 2014). Moreover, hAMCs are an attractive cell source of cartilage tissue engineering for their abundance, accessibility, and strong chondrogenic ability (De Ugarte et al. 2003). Compared with bone marrow-derived mesenchymal stem cells (BMSCs), hAMCs have less chondrogenic potential, which can be overcome by bone morphogenetic protein 6 or great doses of growth factor (Hennig et al. 2007; Kim and Im. 2009).
The TGF-β superfamily plays a central role in the chondroinductive differentiation of mesenchymal stem cells (MSCs) derived from bone marrow, adipose, or other mesenchymal tissue (Worster et al. 2001; Zuk et al. 2002; Fukumoto et al. 2003; Kim and Im. 2009). TGF-β1-3 mRNA is expressed throughout the process of chondrogenesis (Pelton et al. 1991; Derynck and Zhang. 2003). TGF-β1 or TGF-β3 is known to primarily induce chondrogenesis (Goude et al. 2014). IGF-I is regarded as one of the most critical growth factors in cartilage development and homeostasis (Nixon et al. 1998; Bonnevie et al. 2014; Madry et al. 2013). IGF-I has been reported to increase in arthritic cartilage to recruit local chondrocytes for cartilage repair (Middleton and Tyler. 1992; Olney et al. 1996; Madry et al. 2013); therefore, decreases in IGF-I may lead to severe osteoarthritis (Denko et al. 1990; Schouten et al. 1993). Several recent studies demonstrated that IGF-I enhances chondrocyte metabolism while maintaining the differentiated phenotype and the chondrogenic ability of the differentiated bone mesenchymal cells (Worster et al. 2001) and periosteal cells (Fukumoto et al. 2003) in a three-dimensional matrix or micromass culture condition in vitro (Nixon et al. 1998). Recent studies have shown that IGF-I can modulate bone mesenchymal stem cell chondrogenic differentiation, independent from transforming growth factor beta (TGF-β) signaling in vitro (Longobardi et al. 2006; Li et al. 2012). However, whether IGF-I can solely modulate hAMC chondrogenic differentiation, and the possibility of additive effects with TGF-β1 to induce hAMC chondrogenic differentiation in vitro and chondrogenesis in vivo, remains unknown. Insulin is often used as an important component in the chondrogenic inducing media at 100 times above physiological levels. Insulin has an affinity that is approximately two orders of magnitude lower for the IGFIR (insulin-like growth factor-I receptor) compared with IGF-I, so high levels of insulin may depress the biological activity of IGF-I (Longobardi et al. 2006). In this study, we evaluated the effects of IGF-I, alone or in combination with TGF-β1, on hAMC chondrogenic differentiation in vitro and chondrogenesis in vivo in the absence of insulin.
Materials and Methods
hAMCs isolation and culture. Subcutaneous fat was obtained from six 18–25-yr-old male subjects by hip surgery. This study was approved by the ethics committee of Huai’an Hospital of Xuzhou Medical College, Huai’an, China, and informed consents were obtained from all the subjects. hAMCs were isolated as described previously (Bogdanova-Jantniece et al. 2014). Briefly, the adipose was extensively washed with phosphate-buffered saline (PBS). Macroscopic blood vessels were removed and the adipose was sheared with a pair of scissors. The sheared adipose was digested at 37°C with an equal volume of 0.075% collagenase I (Sigma, St. Louis, MO) for 2 h, filtered through a 100-μm mesh to remove undigested tissues and centrifuged at 1,200×g for 10 min. The pellet was resuspended in 160 mM NH4Cl at room temperature for 10 min to lyse red blood cells and centrifuged again at room temperature for 10 min. The pelleted cells were resuspended in Dulbecco’s Modified Eagle Medium (DMEM) with low glucose (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic–antimycotic solution (Invitrogen), and incubated in a tissue culture flask at 37°C in a humidified atmosphere containing 5% CO2. The media was replaced every 2 d. The cells were passaged at 80% confluency.
Induction of hAMCs with growth factors. Stem cells generate high-density condensations during chondrocyte differentiation, suggesting that a 3D culture model is ideal to induce stem cells chondrogenesis. However, condensation is difficult to observe in 3D culture, and previous studies have shown that induction of hAMCs in 2D culture is sufficient to obtain cartilaginous tissue formation in vivo (Merceron et al. 2011). Therefore, in our study, we performed chondrogenic differentiation in monolayer to observe stem cells condensation in vitro. hAMCs (5 × 106) at passage 6 were passaged onto T-25 flasks and cultured under the same condition as above. To observe the condensation during chondrogenic induction, the hAMCs were cultured in monolayer. The old media were removed and treated with chondrogenic media or mock treated with fresh media for 30 min. Then the media was replaced with chondrogenic media containing DMEM high glucose and supplemented with 1% FBS, 1% antibiotic–antimycotic solution (Invitrogen), 0.1 mM ascorbic acid-2-phosphate, 10−7 dexamethasone, 6.25 μg/ml transferrin, 6.25 ng/ml selenous acid (Sigma), 10 ng/ml recombinant human TGF-β1 (Pepretech, London, UK), 100 ng/ml recombinant human IGF-I (Pepretech), and TGF-β1 + IGF-I or without any growth factor. The chondrogenic media was changed at a 2-d interval for 14 d.
Histology and immunohistology of the induced hAMCs and cartilage tissues. The hAMCs were harvested 14 d after the induction, and 50 μL of cells suspended in DMEM with low glucose supplemented with 10% FBS and 1% antibiotic–antimycotic solution (1 × 105 cells/ml) were transferred to slides and incubated for 30 min under the same culturing condition to allow the cells adhere to slides. Then the hAMCs on the slides were either used for toluidine blue metachromatic or immunohistochemical staining of collagen type II. For toluidine blue metachromatic staining, the hAMCs were fixed with 10% paraformaldehyde solution at room temperature for 10 min, stained with toluidine blue (Amresco, Solon, OH) for 10 min and washed with double distilled water three times to detect proteoglycans of the induced hAMCs. For immunohistochemical staining of collagen type II, the hAMCs were blocked with normal goat serum at 37°C for 30 min and incubated with polyclonal goat anti-human collagen type II (1:100 in 1% Tween-20/PBS) (Amresco) for 60 min followed by washing with PBS (Amresco) three times. Then the hAMCs were incubated with mouse anti-goat antibody (1:100 in 0.01 M PBS) (Amresco) at 37°C for 60 min in a humidified chamber followed by the same wash as above, and incubated with streptavidin–perosidase at 37°C for 30 min. Diaminobenzidine (DAB) method was used for color development.
Reverse transcription polymerase chain reaction (RT-PCR) of collagen type II, aggrecan, and Sox 9. The hAMCs were harvested 14 d after the induction, and total RNAs from the hAMCs were obtained by TRIzol extraction according to the manufacture’s protocol (Invitrogen). β-actin, collagen type II, aggrecan, and Sox9 were amplified using a PCR kit according to the manufacturer’s instructions (TaKaRa, Otsu, Shiga, Japan). The primer sequences (Lin et al. 2005) are listed in Table 1. The abundance of mRNA was determined by densitometry and the data are expressed as relative values to the amounts of β-actin mRNA.
Western blot analysis of collagen II and aggrecan. The hAMCs were washed with PBS twice and lysed with 500 μL of cell lysis buffer (50 mMTris-cl, 150 mMNaCl, 0.02% sodium azide, 0.1% SDS, 100 mg/l PMSF, 1 mg/l Aprotinin, 1% Triton X-100, and 0.5% Na-deoxycholate). After centrifugation at 13,000×g at 4°C for 30 min, each supernatant was collected and the protein concentrations were determined by a BCA Protein Assay Reagent Kit (Pierce Corporation, Rockford, IL). Equal amounts of protein extracts were run on 7% sodium dodecyl sulfate (SDS)–polyacrylamide gels and electrotransferred to a nitrocellulose membrane. The membranes were blocked with 3% skimmed milk and incubated with mouse monoclonal antibodies (1:100 in PBS) (Amresco) against collagen type II, aggrecan, and β-action, followed by washing three times with TTBS (0.5 ml Tween 20 in 1 L tris buffered saline). Then the membranes were incubated with peroxidase -labeled secondary goat anti-mouse antibody, followed by the same wash as above, and visualized by an ECL detection kit (Pierce) according to the manufacturer’s instructions.
In vivo study of chondrogenesis of the induced hAMCs. Poly lactic-co-glycolic acid (PLGA) (latide: glycolide = 75:25; molwt: 66,000–107,000; porosity ≥ 93%; pore diameter varies from 100 to 200 μm.) was used as scaffolds. PLGA scaffolds were exposed to UV light, soaked in 75% alcohol for 24 h, thoroughly washed with PBS, and stored at 40°C. 5 × 105 hAMCs were induced with IGF-I, TGF-β1, or both for 14 d, trypsinized with 0.25% trypsin-EDTA, and the induced hAMCs were adjusted to 1 × 107cells/ml, then 0.05 ml hAMCs suspension were seeded on PLGA scaffolds. Then the PLGA scaffolds were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 2 h to allow hAMCs to adhere to the PLGA scaffolds. The cells were cultured with chondrogenic media (containing IGF-I, TGF-β1, or both) for 2 d.
Male athymic nude mice were anesthetized with amylobarbitone sodium (30 mg/kg). A 2-cm incision was made on the dorsum of the mice and the hAMCs-attached PLGA scaffolds were subcutaneously transplanted onto the dorsum of the mice (n = 10 per group). Twelve weeks later, the cartilage tissues were harvested and the maxium diameter of the cartilage tissues were measured with an electronic balance. The tissues were fixed in 10% formalin in PBS at room temperature overnight, dehydrated by treatment with a series of alcohol, embedded in paraffin, and cut into sections 5 μm thick. The sections were stained with hematoxylin–eosin or the collagen type II antibody, following the same procedure as above. The animal experiments were performed in compliance with the guidelines of Huai’an Hospital of Xuzhou Medical College, Huai’an, China.
Statistical analysis. All results were expressed as means ± standard deviation. Statistical significance was evaluated by t test using the SPSS 11.0 statistics software and a statistical significance was set at P < 0.05.
Results
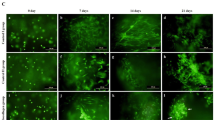
Morphology of hAMCs induced with TGF-β1, IGF-I, or TGF-β1 + IGF-I. To investigate the effect of IGF-I on the chondrogenic differentiation of hAMCs, hAMCs were induced with TGF-β1, IGF-I, TGF-β1 + IGF-I, or growth factor mock treated. All of the hAMCs treated with either or both of the growth factors formed cell clusters and detached 3 d after the treatments, but no cell clusters were found in the mock-treated hAMCs (Fig. 1). The clusters in the tissue culture flask induced with TGF-β1 + IGF-I formed many macroscopic cells masses and could be seen with naked eye (Fig. 1c ), while those induced with either of the two growth factors could only be seen under a light microscope (Fig. 1a and b ). These results indicated that IGF-1 can induce hAMCs condensation alone and enhance hAMCs condense in vitro.
Histology and immunochemistry of collagen type II of induced hAMCs. Toluidine blue metachromatic staining and collagen type II immunohistochemical staining were positive in the IGF-I, TGF β1, and IGF-I + TGF β1 groups, but negative in the growth factor mock-treated group (Fig. 2). hAMCs induced with IGF-I, TGF-β1, or TGF-β1 + IGF-I were round or polygonal morphous (Fig. 2a, b , c, e, f, g), while the growth factor hAMC mocked-treated group maintained their flat appearance (Fig. 2d, h ). These results indicated that hAMCs induced with IGF-1 or TGF-β1 express collagen type IIand proteoglycans.
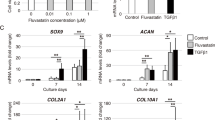
Collagen II, aggrecan and Sox9 mRNA, and collagen II, aggrecan protein expressions of induced hAMCs. The collagen type II, aggrecan, and Sox9 mRNA expressions were detected in all the growth factor-treated groups, but not in the growth factor mock-treated group (Fig. 3a ). The optical density of the mRNA of collagen type II, aggrecan, or Sox9 was the greatest in the TGF-β1 + IGF-I group (P < 0.05), while no statistically significant difference was found between TGF-β1 and IGF-I groups (P > 0.05, Fig. 3b ).
IGF-I induced and enhanced hAMCs chondrogenic-specific genes collagen type II (COL II), aggrecan, and Sox9 mRNA expression after induction with IGF-I, TGF-β1, IGF-I + TGF-β1 or mock treated without any growth factor for 14 d. (a) RT-PCR and, (b) analysis of mRNA expressions. a, P < 0.01 vs growth factor mock-treated group; b, P < 0.01vs TGF-β1-treated group.
The results of Western blot revealed that the optical density of collagen type II or aggrecan was the greatest in the TGF-β1 + IGF-I group (P < 0.05), but no significant difference was found between TGF-β1 and IGF-I-treated groups with respect to collagen type II or aggrecan levels (P > 0.05, Fig. 4). These results indicated that collagen type II, aggrecan, and Sox9 mRNA, and collagen type II and aggrecan proteins were expressed in hAMCs induced with IGF-I alone, and greater levels of collagen type II, aggrecan, and Sox9 mRNA, and collagen type II and aggrecan proteins were expressed in hAMCs induced with IGF-I and TGF-β1.
IGF-I induced and enhanced hAMCs to express chondrogenic-specific proteins COL II and aggrecan after induction with IGF-I, TGF-β1, IGF-I + TGF-β1 or mock treated without any growth factor for 14 d. (a) Western blot and (b) analysis of protein expressions. a, P < 0.01 vs growth factor mock-treated group; b, P < 0.01vs TGF-β1-treated group.
In vivo study of cartilage tissues formed from induced hAMCs. Twelve weeks following implantation, new tissue was formed in the IGF-I, TGF-β1, or IGF-I + TGF-β1-treated hAMCs, while no new tissue was formed in the growth factor mock-treated group (Fig. 5). The maxium diameter of tissue derived from IGF-I + TGF-β1-treated hAMCs was significantly greater compared to either IGF-I or TGF-β1-treated hAMCs (P < 0.05). Hematoxylin and eosin staining revealed that cartilage lacunas were formed in the tissue from IGF-I + TGF-β1, IGF-I, or TGF-β1-treated hAMCs, while no cartilage lacuna was associated with growth factor mock-treated hAMCs (Fig. 6). Collagen type II immunohistochemical staining and toluidine blue metachromatic staining confirmed that collagen type IIand proteoglycans were expressed in all the growth factor treated but not growth factor mock-treated hAMCs. These results indicated hAMCs induced with IGF-1 can form new cartilage and IGF-1 can enhance cartilage formation in vivo.
Hematoxylin and eosin (HE) and toluidine blue metachromatic staining and immunohistochemical (IHC) staining of collagen type IIof the cartilage tissues. Cartilage lacunas were formed in the TGF-β1 + IGFgroup (e, f), TGF-β1 (c, d), or IGF-I-treated group, while no cartilage lacuna was found in growth factor mock-treated group (a, b). Extracellular matrix (ECM) of TGF-β1 + IGF-I (f), TGF-1 (e), or IGF-I treated group was positive in collagen type II immunohistochemical staining and toluidine blue metachromatic staining.
Discussion
To investigate whether IGF-I promotes hAMCs chondrogenic differentiation alone, or may have additive effects on chondrogenic differentiation by TGF-β1, we treated hAMCs with TGF-β1, IGF-I, or TGF-β1 + IGF-I. We found that IGF-I solely induced hAMC chondrogenic differentiation and had an additive effect with TGF-β1 on chondrogenic differentiationin vitro, and mature cartilage formation from hAMCs treated with TGF-β1 in vivo.
IGF-I induced hAMC chondrogenic differentiation in vitro and chondrogenesis in vivo. Histological analysis of hAMCs and the immunohistochemistry of collagen type II in hAMCs demonstrated that IGF-I induced hAMCs to express proteoglycan and collagen type II. Correspondingly, the results of RT-PCR and Western blot showed that collagen type II, aggrecan, and Sox9 mRNA, and collagen type II and aggrecan proteins were expressed in hAMCs induced with IGF-I, respectively. These findings indicate that IGF-I alone induces hAMC chondrocytic differentiation in vitro. Furthermore, the in vivo study demonstrated that cartilage lacuna or collagen type II expression was found in the presence of PLGA compounds in the induced hAMCs with IGF-I. These findings indicate that the hAMCs induced with IGF-I have the ability to induce chondrogenic differentiation in vitro and form mature hyaline cartilage from inoculated hAMC tissue. Taken together, we found that IGF induces chondrogenic differentiation in vitro and in tissue independent of TGF-β1. In fact, prior reports have suggested a robust effect of IGF-I on cartilage repair and regeneration (Schouten et al. 1993; Milne et al. 1998; Nixon et al. 1998; Madry et al. 2013), as well as the ability to enhance extracellular matrix synthesis by cartilage chondrocytes (Nixon et al. 1998; Fortier et al. 2002) and inhibit chondrocyte apoptosis (Schouten et al. 1993; Worster et al. 2001; Fortier et al. 2002; Madry et al. 2002; Yi et al. 2013). IGF-I also regulates chondrogenesis of mesenchymal cells and anabolism of cartilage matrix molecules (Martel-Pelletier et al. 1998; Deng et al. 2013). However, several studies showed that IGF-I has no effect on chondroinduction of mesenchymal stem cells alone (Baddoo et al. 2003; Indrawattana et al. 2004), which is different from the findings in the current study. In accordance with the present study, other studies have demonstrated that IGF-I induces chondrogenic differentiation of mesenchymal cells isolated from limb buds of Hamburger-Hamilton stage 23/24 chicken embryos (Oh and Chun. 2003) and modulates bone mesenchymal stem cells chondrogenesis independent of TGF-β signals (Longobardi et al. 2006). While inducing media is very important in chondrogenic induction, insulin is a widely used component of chondrgenic inducing media. High levels of insulin may depress biological activity of IGF-I (Longobardi et al. 2006). In the present study, insulin-absent chondrogenic media were used to avoid the influence of insulin. In absence of this potential cofounder, our results indicated IGF-1 could induce hAMCs chondrogenic differentiation.
IGF-I had additive effect on TGF-β1 induced hAMC chondrogenic differentiation in vitro and chondrogenesis in vivo. We found that hAMCs treated with TGF-β1 + IGF-I formed significantly more remarkable macroscopic cells masses than hAMCs treated with either TGF-β1 or IGF-I, indicating that IGF-I promoted hAMC condensation, which is an essential step of chondrogenic differentiation. This is consistent with the fact that more collagen type II, aggrecan, and Sox9 mRNA, and greater collagen type II and aggrecan protein expression was found in TGF-β1 + IGF-I compared to either TGF-β1 or IGF-I-treated hAMCs. These findings indicate that IGF-I has an additive effect on TGF-β1-induced chondrogenic differentiation. The maximum diameter of cartilage formed from TGF-β1 + IGF-I-treated hAMCs was significantly greater compared to either IGF-I of TGF-β1-treated hAMCs. The IGF-I additive effects on TGF-β1-induced hAMC chondrogenesis was only in tissue level without direct effect once in vivo because the growth factor treatment was not present once the hAMCs tissue was implanted in vivo.
Indeed, several studies have demonstrated that IGF-I can enhance chondrocytes and MSCs chondrogenesis of TGF-β1 via enhancing metabolically active and depressing cytotoxic activity of TGF-β1 (Worster et al. 2001; Blunk et al. 2002; Fukumoto et al. 2003; Chiou et al. 2006; Sakimura et al. 2006). Cartilage formation is initiated by mesenchymal cells differentiation into chondrocytes, and chondrogenesis is triggered by aggregation of mesenchymal cells that develop into cartilage nodules that can be enhanced by higher inoculum cell density (Takagi et al. 2007). IGF-I regulates the chondrogenesis of mesenchymal cells and maintains differentiated articular chondrocytes phenotype (Oh and Chun. 2003).
In conclusion, the present study demonstrated that IGF-I induced chondrogenic differentiation of hAMCs in the absence of TGF-β1 and has an additive effect on TGF-β1-induced hAMC chondrogenic differentiation in vitro and chondrogenesis in vivo. Our findings may provide an attractive approach to effective tissue engineering for cartilage repair.
References
Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG (2003) Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 89:1235–1249
Blunk T, Sieminski AL, Gooch KJ, Courter DL, Hollander AP, Nahir AM, Langer R, Vunjak-Novakovic G, Freed LE (2002) Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng 8:73–84
Bogdanova-Jantniece A, Berzins U, Kozlovska T (2014) Growth properties and pluriopotency marker expression of spontaneously formed three-dimensional aggregates of human adipose-derived stem cells. Int J Stem Cells 7:143–152
Bonnevie ED, Puetzer JL, Bonassar LJ (2014) Enhanced boundary lubrication properties of engineered menisci by lubricin localization with insulin-like growth factor I treatment. J Biomech 47(9):2183–2188
Brzoska M, Geiger H, Gauer S, Baer P (2005) Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun 330:142–150
Chiou M, Xu Y, Longaker MT (2006) Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem Biophys Res Commun 343:644–652
Correia SI, Pereira H, Silva-Correia J, Van Dijk CN, Espregueira-Mendes J, Oliveira JM, Reis RL (2014) Current concepts: tissue engineering and regenerative medicine applications in the ankle joint. J R Soc Interface 11:20130784
De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH (2003) Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174:101–109
Deng Y, Cao H, Cu F, Xu D, Lei Y, Tan Y, Magdalou J, Wang H, Chen L (2013) Nicotine-induced retardation of chondrogenesis through down-regulation of IGF-1 signaling pathway to inhibit matrix synthesis of growth plate chondrocytes in fetal rats. Toxicol Appl Pharmacol 269:25–33
Denko CW, Boja B, Moskowitz RW (1990) Growth promoting peptides in osteoarthritis: insulin, insulin-like growth factor-1, growth hormone. J Rheumatol 17:1217–1221
Derynck R, Zhang YE (2003) Smad-dependent and smad-independent pathways in TGF-beta family signalling. Nature 425:577–584
Fortier LA, Mohammed HO, Lust G, Nixon AJ (2002) Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br Vol 84:276–288
Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, O’Driscoll SW (2003) Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthr Cartil 11:55–64
Gao S, Zhao P, Lin C, Sun Y, Wang Y, Zhou Z, Yang D, Wang X, Xu H, Zhou F, Cao L, Zhou W, Ning K, Chen X, Xu J (2014) Differentiation of human-adipose derived stem cells into neuron-like cells which are compatible with photocurable three-dimensional scaffolds. Tissue Eng A 20:1271–1284
Goude MC, McDevitt TC, Temenoff JS (2014) Chondroitin sulfate microparticles modulate transforming growth factor-β1-indcuced chondrogenesis of human mesenchymal stem cell spheroids. Cells Tissues Organs 199:117–130
Hendriks J, Riesle J, van Blitterswijk CA (2007) Co-culture in cartilage tissue engineering. J Tissue Eng Regen Med 1:170–178
Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W (2007) Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol 211:682–691
Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A (2004) Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun 320:914–919
Kim HJ, Im GI (2009) Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res 27:612–619
Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G (2007) Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 207:267–274
Li J, Zhao Q, Wang E, Zhang C, Wang G, Yuan Q (2012) Dynamic compression of rabbit adipose-derived stem cells transfected with insulin-like growth factor 1 in chitosan/gelatin scaffolds induces chondrogenesis and matrix biosynthesis. J Cell Physiol 227:2003–2012
Lin Y, Luo E, Chen X, Liu L, Qiao J, Yan Z, Li Z, Tang W, Zheng X, Tian W (2005) Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med 9:929–939
Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A (2006) Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res 21:626–636
Madry H, Padera R, Seidel J, Langer R, Freed LE, Trippel SB, Vunjak-Novakovic G (2002) Gene transfer of a human insulin-like growth factor I cDNA enhances tissue engineering of cartilage. Hum Gene Ther 13:1621–1630
Madry H, Kaul G, Zurakowski D, Vunjak-Novakovic G, Cucchiarini M (2013) Cartilage constructs engineered from chondrocytes overexpressing IGF-I improve the repair of osteochondral defects in a rabbit model. Eur Cell Mater 25:229–247
Martel-Pelletier J, Di BJA, Lajeunesse D, Pelletier JP (1998) IGF/IGFBP axis in cartilage and bone in osteoarthritis pathogenesis. Inflamm Res 47:90–100
Merceron C, Portron S, Masson M, Lesoeur J, Fellah BH, Gauthier O, Geffroy O, Weiss P, Guicheux J, Vinatier C (2011) The effect of two- and three-dimensional cell culture on the chondrogenic potential of human adipose-derived mesenchymal stem cells after subcutaneous transplantation with an injectable hydrogel. Cell Transplant 20:1575–1588
Middleton JF, Tyler JA (1992) Upregulation of insulin-like growth factor I gene expression in the lesions of osteoarthritic human articular cartilage. Ann Rheum Dis 51:440–447
Milne M, Quail JM, Baran DT (1998) Dexamethasone stimulates osteogenic differentiation in vertebral and femoral bone marrow cell cultures: comparison of IGF-I gene expression. J Cell Biochem 71:382–391
Nixon AJ, Lillich JT, Burton-Wurster N, Lust G, Mohammed HO (1998) Differentiated cellular function in fetal chondrocytes cultured with insulin-like growth factor-I and transforming growth factor-beta. J Orthop Res 16:531–541
Oh CD, Chun JS (2003) Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J Biol Chem 278:36563–36571
Olney RC, Tsuchiya K, Wilson DM, Mohtai M, Maloney WJ, Schurman DJ, Smith RL (1996) Chondrocytes from osteoarthritic cartilage have increased expression of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 (IGFBP-3) and -5, but not IGF-II or IGFBP-4. J Clin Endocrinol Metab 81:1096–1103
Pelton RW, Saxena B, Jones M, Moses HL, Gold LI (1991) Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol 115:1091–1105
Sakimura K, Matsumoto T, Miyamoto C, Osaki M, Shindo H (2006) Effects of insulin-like growth factor I on transforming growth factor beta1 induced chondrogenesis of synovium-derived mesenchymal stem cells cultured in a polyglycolic acid scaffold. Cells Tissues Organs 183:55–61
Schouten JS, Van den Ouweland FA, Valkenburg HA, Lamberts SW (1993) Insulin-like growth factor-1: a prognostic factor of knee osteoarthritis. Br J Rheumatol 32:274–280
Sowa Y, Imura T, Numajiri T, Takeda K, Mabuchi Y, Matsuzaki Y, Nishino K (2013) Adipose stromal cells contain phenotypically distinct adipogenic progenitors derived from neural crest. PLoS One 8:e84206
Takagi M, Umetsu Y, Fujiwara M, Wakitani S (2007) High inoculation cell density could accelerate the differentiation of human bone marrow mesenchymal stem cells to chondrocyte cells. J Biosci Bioeng 103:98–100
Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ (2001) Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res 19:738–749
Yi C, Ma C, Xie Z, Zhang G, Song W, Zhou X, Cao Y (2013) Down-regulation of programmed cell death 5 by insulin-like growth factor 1 in osteoarthritis chondrocytes. Int Orthop 37:937–943
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Acknowledgments
This research was supported by grants from the scientific research fund of the Bureau of Public Health of Jiangsu province (no. H201254) and the Huai’an Technology Support Program (social development) funded projects (no. HAS2013046).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Quan Zhou and Baojun Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, Q., Li, B., Zhao, J. et al. IGF-I induces adipose derived mesenchymal cell chondrogenic differentiation in vitro and enhances chondrogenesis in vivo. In Vitro Cell.Dev.Biol.-Animal 52, 356–364 (2016). https://doi.org/10.1007/s11626-015-9969-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9969-9