Abstract
Purpose
The aim of this study was to investigate the expression of insulin-like growth factor (IGF)-1 and programmed cell death 5 (PDCD5) in osteoarthritis chondrocytes, and to explore the potential correlation between them in the apoptosis process of osteoarthritis chondrocytes.
Methods
Patients with knee osteoarthritis were placed into four categories according to radiological staging. The mRNA and protein levels of IGF-1 and PDCD5 in osteoarthritis chondrocytes were respectively detected by quantitative reverse transcriptase polymerase chain reaction (qPCR) and western blotting. In addition, IGF-1 and PDCD5 protein expression in chondrocytes were also measured by immunohistochemistry. Apoptotic cells were measured by TUNEL staining.
Results
Both the mRNA and protein levels of IGF-1 were down-regulated, while the levels of PDCD5 were up-regulated, and the mRNA and protein levels of IGF-1 were negatively correlated with those of PDCD5, respectively. The apoptotic cell was significantly increased in osteoarthritis chondrocytes compared with control. Importantly, the apoptosis rate was positively correlated with PDCD5 protein expression and negatively correlated with IGF-1 protein expression
Conclusions
We concluded that IGF-1 may down-regulate the expression of PDCD5 and thus inhibit the apoptosis of osteoarthritis chondrocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a chronic arthropathy characterized by degenerative lesions of articular cartilage and peri-articular bone proliferation. OA is the most common arthritis and is also one of the major causes of joint pain in elderly people [1]. Several factors have been implicated in the pathogenesis of OA. For example, epidemiological studies show that OA may be caused by the joint effects of genetic and environmental factors [2, 3]. Loeser suggests articular chondrocytes exhibit an age-related decline in proliferative and synthetic capacity while maintaining the ability to produce pro-inflammatory mediators and matrix degrading enzymes, which contribute to the propensity to develop OA [4]. Recent studies have shown the presence of apoptotic as well as anti-apoptotic mechanisms in OA cartilage [5]. The OA cartilage has a higher proportion of apoptotic chondrocytes than normal tissue [6, 7]. Increased chondrocyte apoptosis may be related to up-regulated expression of both Akt and PKCα in human OA cartilage [8]. ADAM15, a disintegrin metalloproteinase, is up-regulated in OA and further reduces downstream caspase 3/7 activity that controls cell fate and thus the integrity of the entire cartilage [9]. However, the apoptotic and anti-apoptotic mechanism of OA remains not well understood and further study is still needed.
Programmed cell death 5 (PDCD5), formerly designated as TF-1 cell apoptosis-related gene-19 (TFAR19), is first identified as a gene up-regulated in TF-1 cells that are undergoing apoptosis [10]. PDCD5 is located in chromosome 19q12-q13.1 and consists of six exons and five introns. PDCD5 has been demonstrated to be widely expressed within the organizations [11] and PDCD5 can promote the apoptosis process induced by a variety of compounds [12]. Increased expression and nuclear translocation of PDCD5 is also observed in rheumatoid arthritis patient-derived fibroblast-like synoviocytes undergoing apoptosis [13].
Insulin-like growth factor 1 (IGF-1) is a 70-amino-acid straight chain, basic peptide with homology to human proinsulin [14]. It can be synthesized from bone, cartilage and a variety of the other tissues to promote mitosis onset [15]. IGF-1, up to 57.14 ng/mL, has the ability to promote the proliferation of chondrocytes in a dose-dependent manner [16]. Disruption of the IGF-1 gene in osteocytes impairs developmental bone growth [17].
In the present study, we aimed to investigate the expression of IGF-1 and PDCD5 in OA chondrocytes, and explore the potential correlation between them in the apoptosis process of OA chondrocytes.
Materials and methods
Materials
Dulbecco’s modified Eagle’s minimal essential medium was purchased from Life Technologies (USA). Newborn calf serum was obtained from GIBCO (Grand Island, NY, USA). Superscript II and total RNA extraction kit (Trizol) were purchased from Invitrogen (USA). Taqmanmaster mix was obtained from ABI (USA). Primers were synthesized by Invitrogen (USA). Anti-PDCD5 antibody was purchased from Abcam. Collagenase I and mouse monoclonal anti–β-actin antibodies were obtained from Sigma (USA). Liquid DAB was purchased from the Soledad Company (China). Electronic balance was obtained from Mettler Company. The high-speed low-temperature centrifuge was purchased from Beckman (USA). The inverted microscope was obtained from Olympus (Japan), and the Heraeus cell incubator was purchased from GB (USA). The 7500 fluorescent quantitative PCR instrument was obtained from ABI (USA).
The OA patients with total knee replacement surgery or synovectomy (n = 39) and the normal fracture patients (n = 15) were collected from March 2011 to January 2012 at the Joint Clinic Research Centre of Affiliated Hospital of Fudan University. All the patients with OA met the American Rheumatism Association revised diagnostic criteria [18].
Radiographic classification for knee osteoarthritis
For the documentation of knee damage based on radiological findings, four-grade classification was defined as follows: grade 1, doubtful narrowing of joint space and possible osteophytic lipping; grade 2, definite osteophytes and possible narrowing of joint space; grade 3, moderate multiple osteophytes, definite narrowing of joints space, some sclerosis and possible deformity of bone contour; grade 4, large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone contour.
Isolation of chondrocytes
Cartilage fragments were cut into small pieces. After washing with PBS twice, cartilage pieces were digested with 5 ml 0.2 % type II collagen for six hours, at 37 ° C, in a 5 % CO2 humidified incubator, then the cartilage suspension was harvested in sterile tubes containing 5 ml DMEM, and centrifuged at 1,500 rpm for five minutes. Finally, the cells were resuspended using DMEM. After straining with 200 mesh stainless steel screen, cells were seeded in T25 flasks at a density of 1 × 105 cells per flask.
Primary culture of isolated chondrocytes
After trypsinized, cells were seeded in T25 flasks at a density of 2–3 × 105/ml (ten flasks, each contains 2 ml cell suspension), then the cells were incubated at 37 ° C in a 5 % CO2 humidified incubator. The cultures were examined for growth 72 hours later, and the culture medium was changed every two days.
Subculture of chondrocytes
The culture cells were examined using an inverted phase-contrast microscope. The culture media was removed and the cells were gently washed with PBS. Then, the cells were incubated with 2 ml 0.25 % trypsin at room temperature for five to ten minutes. Finally, the resuspended cells were seeded as described above, and the medium was refreshed every two days with subculturing.
Real-time quantitative PCR
Total mRNA of OA chondrocytes was extracted using TRIzol according to the manufacturer’s instructions (Life Technologies, Rockville, MD, USA). A total of 1 μg RNA was converted to cDNA and stored at −80 °C. A total of 50 μL of reaction mixture was prepared for each sample, containing 25 μL Realtime PCR MasterMix, 2 μL primer mixture (10 μM each), 5 μL cDNA, and 16 μL RNase free water. The PCR amplification cycle was set as 50 °C for two minutes and 94 °C for two minutes for initial denaturation, and then performed for 40 cycles under the following conditions: 15 seconds at 95 °C, and one minute at 60 °C. Copy numbers were obtained through the extrapolation of Ct values of the test samples against the corresponding standard curve, and the housekeeping gene β-actin was used to normalize the amount of mRNA from each sample. The primer sequences are shown in Table 1.
Western blotting
Cells were washed with cold PBS three times and then lysed with 100 μL lysis buffer (20 mM HEPES, pH 7.4, 1 % NP-40, 2 mM EDTA, 100 mM NaF, 10 mM sodium pyrophosphate, 1 mM PMSF,1 mM TPCK, 2 mM Na3VO4, and aprotinin). About 30 μg total proteins were loaded in each well and separated by 15 % sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene fluoride (PVDF) membrane. After electrophoretic transfer, membranes were incubated with the primary anti-bodies for one hour at 4 °C and the second antibody at 37 °C for one hour, and then the immunocomplexes were set up for ECL detection in dark room. β-actin was used for internal control.
TUNEL staining
The slides were rinsed with 0.1 mol/L HCL for 20 minutes, ethanol rinsing, and then dried naturally. Slides were incubated in a solution containing 3 × SSC, 0.02 % Ficoll and 0.02 % polyvinylpyrrolidone at 65 °C for two hours. Subsequently, the slides were fixed with ethanol/acetic acid (3:1) for 20 minutes. After drying naturally, the slides were autoclaved. The treated slides were transferred into a petri dish and the chondrocytes were inoculated. The following culture conditions and time were the same as above. Apoptotic cells in each section were measured by TUNEL assay kit (Wako) according to the manufacturer’s protocol. TUNEL-positive cells in three different areas were counted under a microscope (Axioskop 2 plus; Zeiss, Wetzlar, Germany). The cells with brown nucleus were recognized as positive. The apoptosis index (AI) was calculated as follows: AI = (positive cells/total cells) × 100 %.
Immunohistochemistry
The slides were rewarmed at 37 °C for 45 minutes after incubating with 50 μl antibodies at 4 °C overnight. After washing with PBS three times (five minutes each time), the slides were incubated with 40–50 μL second anti-body at room temperature for one hour followed by rinsing in PBS three times (five minutes each time). The slides were observed under light microscope after incubating freshly prepared DAB solution for five to ten minutes. After rinsing with tap water for ten minutes, the slides were counterstained with hematoxylin for two minutes. Hydrochloric acid alcohol solution (1 %) was used for differentiation, and then the slides were rinsed with tap water for ten minutes. Finally, specimens were dehydrated and mounted, and examined with the microscope equipped with an image analysis system. The yellow or brownish-yellow staining represents positive staining.
Results
The mRNA expression levels of IGF-1 and PDCD5
Real-time quantitative PCR was used to detect the mRNA expression levels of IGF-1 and PDCD5 in chondrocytes of patients with different radiological staging. The results indicated that the mRNA level of IGF-1 was decreased, while the mRNA level of PDCD5 was gradually increased, accompanied by elevated radiological staging. Importantly, the mRNA level of IGF-1 was negatively correlated with the mRNA level of PDCD5 (R 2 = 0.9, P < 0.05; Fig. 1).
Protein levels of IGF-1 and PDCD5
Western blotting was performed to determine the protein expression levels of IGF-1 and PDCD5 in chondrocytes of patients with different radiological staging. As expected, the levels of IGF-1 were also down-regulated, while the levels of PDCD5 were up-regulated in the chondrocytes isolated from our subjects with different radiological staging (Fig. 2).
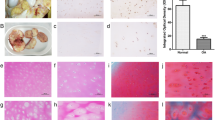
Correlations between radiological staging and relative protein level of IGF-1 and PDCD5. a Protein levels of IGF-1 and PDCD5 were quantified by western blotting. Western blot analysis was performed using the corresponding antibodies to check expression of the proteins, and the β-actin was used as a loading control. The levels of IGF-1 were down-regulated, while the levels of PDCD5 were up-regulated in the chondrocytes isolated from our subjects with different radiological staging. b Correlations between radiological staging and relative level of IGF-1 and PDCD5 were evaluated using the Spearman rank test
Chondrocytes apoptosis
TUNEL positive cells were detected in both the control and osteoarthritis groups. Typical characteristics of apoptosis included the condensed chromatin and the chromatin gathered around the nuclear envelope. The apoptotic cells were significantly higher in the OA groups compared with the control group (Fig. 3).
TUNEL positive cells detected in both control and osteoarthritis groups. Chondrocytes were stained using a TUNEL assay kit. a Control group. b Osteoarthritis groups. Typical characteristics of apoptosis were condensed chromatin and chromatin gathered around the nuclear envelope (magnification, ×400)
The relationship between chondrocyte apoptosis and protein expression of IGF-1 and PDCD5
The cells with brown–yellow granules in the nuclei or cytoplasm were taken as PDCD5 positive cells. The PDCD5-positive cells were increased along the progression of OA grade (Fig. 4; Table 2). IGF-1-positive cells were decreased along the progression of OA grade (Table 2). The apoptosis rate of chondrocytes was negatively correlated with the levels of IGF-1 and was positively correlated with the levels of PDCD5 (Fig. 5).
Expression of PDCD5-immunoreactivity. Examples were examined under a light microscope equipped with an image analysis system. a Grade 1 according to radiological classification. b Grade 2 according to radiological classification. c Grade 3 according to radiological classification. d Grade 4 according to radiological classification. e The CK group. The cells with brown–yellow granules in the nuclei or cytoplasm were taken as PDCD5 positive cells (magnification, ×400)
Discussion
OA, also known as degenerative joint disease, is the most common type of arthritis. OA refers to a form of chronic joint inflammation caused by deterioration of joint cartilage, and poses a serious threat to human health [19, 20]. The pathogenesis of OA has not yet been fully elucidated until now. Previous study has indicated that multiple factors are involved in the pathogenesis of OA, the risk factors include increasing age, gender, race, trauma, obesity, genetics, immunity, inflammation, nutritional deficiencies, and lack of estrogen. Articular cartilage defects can speed up the process of joint degeneration, and eventually lead to arthritis. According to Homandberg et al., the occurrence of arthritis is probably due to the disruption of the collagen network caused by trauma. Cartilage cells are exposed to amounts of inflammatory factors, and the capability of the cartilage to resist external stress is weakened. Besides, cartilage matrix components are isolated from cartilage which can lead to more extensive degenerative changes [21]. The post-traumatic cartilage defect can induce the gradual degeneration of bone, cartilage, and surrounding tissues, which eventually lead to the formation of OA [22].
Apoptosis is considered a vital component of various processes including cell proliferation, proper development and normal cell turnover. The process of apoptosis can be divided into three stages: an initiation phase, a signaling phase and an execution phase. Apoptosis plays critical roles in development, maintenance of homeostasis, and host defense in multicellular organisms, and this process is controlled by multiple genes; therefore, apoptosis is also called programmed cell death (PCD) [23].
Cell death after traumatic cartilage may play an important role in developing OA and the repair of inflammatory joint disease. Two features of cartilage in skeletally mature individuals are important in considering the consequences of chondrocyte death. There are no macrophages in cartilage, meaning that the remnants of dead cells will persist in the cartilage matrix, which may potentially affect matrix structure and the function of viable chondrocytes. There are no vascular or mesenchymal stem cells in articular cartilage, so that precursor cells cannot be replenished. Chondrocytes are surrounded by a considerable extracellular matrix, and some chondrocytes also penetrate into the matrix, indicating that dead cells in the region can be hardly or only slowly regenerated, which can impair the ability of cartilage to maintain and repair extracellular matrix. Regeneration of damaged articular cartilage is very limited because cartilage lacks a blood supply, lymphatic drainage, and innervations. The cartilage defects will not be repaired when reaching a certain level of cell damage. The relationship between reduction of chondrocytes and increase of extensive degenerative changes indicates that cartilage cell death plays an important role in the pathogenesis of OA [24].
IGF-1 can stimulate proliferation of chondrocytes, promote the synthesis of collagen and proteoglycan, and play an important role in maintaining chondrocyte phenotype [25, 26]. IGF-1 may promote the mRNA transcription, protein synthesis and the assembly of organelles, thus promoting cell proliferation [27]. According to Mor et al., IGF-1 is expressed in condylar cartilage, and appears to exhibit pleiotropic effects on osteoblasts [28]. IGF-1 stimulates metatarsal longitudinal growth reflected by the increased percentage of proliferating cells in the epiphyseal and proliferative zones, and IGF-1 is also involved in the differentiation and hypertrophy of chondrocytes [29]. Besides, IGF-1 modulates late G0/G1 progression by a posttranscriptional process that may involve protein modification [30].
PDCD5 is cloned as an increased expression gene whose expression is increased during the apoptotic process of TF-1 cells [31]. In the early stage of apoptosis, PDCD5 translocates from the cytoplasm to the nucleus, which is widespread during the apoptosis of cells, and does not depend on the cell types and apoptosis inducing factors [32]. Excessive proliferation and activation of synovial cells in patients with rheumatoid arthritis is due to an abnormal apoptosis process. Previous studies show that low rate of apoptosis is observed in cells of synovial tissue, the apoptosis rate of sublining stromal fibroblast-like cells is 3 %, and there are almost no apoptotic lining cells [33]. The lack of apoptosis cells can induce synovitis and synovial hyperplasia, leading to increased local pro-inflammatory cytokines and matrix metalloproteinases, eventually resulting in cartilage and bone destruction [34]. These abnormalities are related to abnormal expression of apoptosis-related genes, speculating that PDCD5 may play an important role in cells apoptosis.
In early OA, before collagen structure is destroyed, changes in chondrocytes and cartilage have been observed. The number of apoptotic cells correlates significantly with the severity of osteoarthritis. In addition, proteoglycan synthesis is significantly reduced, and cellular synthetic function is suppressed in chondrocyte apoptosis-existing regional. Therefore, the inhibition of chondrocytes apoptosis may be an effective strategy for osteoarthritis prevention and management [35].
In the present study, the expression of PDCD5 of osteoarthritis chondrocytes at both mRNA and protein levels was detected. The level of PDCD5 was negatively correlated with the rate of chondrocyte apoptosis, indicating PDCD5 may play a role in the regulation of chondrocytes apoptosis. However, molecular mechanisms of the role of PDCD5 in apoptosis of chondrocytes still remains unclear; further research is required to better understand the mutual relations of IGF-1 and PDCD5, and the role they play in apoptosis resistance or defectiveness.
References
Kouri JB, Lavalle C (2006) Do chondrocytes undergo “activation” and “transdifferentiation” during the pathogenesis of osteoarthritis? A review of the ultrastructural and immunohistochemical evidence. Histol Histopathol 21(7):793–802
Spector TD, MacGregor AJ (2004) Risk factors for osteoarthritis: genetics. Osteoarthr Cartil 12:39–44
Valdes AM, Spector TD (2010) The genetic epidemiology of osteoarthritis. Curr Opin Rheumatol 22(2):139
Loeser RF (2009) Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr Cartil 17(8):971–979
Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez M, Lavalle C, Kouri J (2010) Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of osteoarthritis within an experimental model. Apoptosis 15(5):631–638
Blanco FJ, Guitian R, Vázquez-Martul E, de Toro FJ, Galdo F (2004) Osteoarthritis chondrocytes die by apoptosis: a possible pathway for osteoarthritis pathology. Arthritis Rheum 41(2):284–289
Musumeci G, Loreto C, Carnazza ML, Martinez G (2011) Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc 19(2):307–313
Chen Q, Zhang B, Yi T, Xia C (2012) Increased apoptosis in human knee osteoarthritis cartilage related to the expression of protein kinase B and protein kinase Cα in chondrocytes. Folia histochemica et cytobiologica/Polish Academy of Sciences. Polish Histochem Cytochem Soc 50(1):137
Böhm B, Hess S, Krause K, Schirner A, Ewald W, Aigner T, Burkhardt H (2010) ADAM15 exerts an antiapoptotic effect on osteoarthritic chondrocytes via up-regulation of the X-linked inhibitor of apoptosis. Arthritis Rheum 62(5):1372–1382
Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen G, Tang J, Ma D (1999) TFAR19, a novel apoptosis-related gene cloned from human leukemia cell line TF-1, could enhance apoptosis of some tumor cells induced by growth factor withdrawal. Biochem Biophys Res Commun 254(1):203–210
Chen Y, Zhang Y, Sun R, Song Q, Di C, Ma D (2000) Preparation and identification of monoclonal antibodies against human apoptosis-related protein TFAR19. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 22(6):502–504
Li H, Zhang X, Song X, Zhu F, Wang Q, Guo C, Liu C, Shi Y, Ma C, Wang X (2012) PDCD5 promotes cisplatin-induced apoptosis of glioma cells via activating mitochondrial apoptotic pathway. Cancer Biol Ther 13(9):822–830
Wang N, Lu HS, Guan ZP, Sun TZ, Chen YY, Ruan GR, Chen ZK, Jiang J, Bai CJ (2007) Involvement of PDCD5 in the regulation of apoptosis in fibroblast-like synoviocytes of rheumatoid arthritis. Apoptosis 12(8):1433–1441
Landin-Wllhelmsen K, Wllhelmsen L, Lappast G, Rosen T, Lindstedt G, Lundberg PA, Bengtsson BÁ (2008) Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol 41(3):351–357
Mairet-Coello G, Tury A, DiCicco-Bloom E (2009) Insulin-like growth factor-1 promotes G1/S cell cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J Neurosci 29(3):775–788
Li Y, Qin J, Lin B, Zhang W (2010) The effects of insulin-like growth factor-1 and basic fibroblast growth factor on the proliferation of chondrocytes embedded in the collagen gel using an integrated microfluidic device. Tissue Eng C Methods 16(6):1267–1275
Sheng MHC, Zhou XD, Bonewald LF, Baylink DJ, Lau KHW (2013) Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone 52(1):133–144
Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS (2005) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Videan EN, Lammey ML, Lee DR (2011) Diagnosis and treatment of degenerative joint disease in a captive male chimpanzee (Pan troglodytes). J Am Assoc Lab Anim Sci 50(2):263–266
Rubenhagen R, Schuttrumpf JP, Sturmer KM, Frosch KH (2012) Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop 83(1):59–64
Homandberg GA (2001) Cartilage damage by matrix degradation products: fibronectin fragments. Clin Orthop Relat Res 391(Suppl):S100–S107
Halstead J, Bergin D, Keenan AM, Madden J, McGonagle D (2010) Ligament and bone pathologic abnormalities more frequent in neuropathic joint disease in comparison with degenerative arthritis of the foot and ankle: implications for understanding rapidly progressive joint degeneration. Arthritis Rheum 62(8):2353–2358
Khanson KP (1997) Programmed cell death (apoptosis): molecular mechanisms and the role in biology and medicine. Vopr Med Khim 43(5):402–416
Newman AP (1998) Articular cartilage repair. Am J Sports Med 26(2):309–324
Hock JM, Centrella M, Canalis E (1988) Insulin-like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology 122(1):254–260
Fortier LA, Lust G, Mohammed HO, Nixon AJ (1999) Coordinate upregulation of cartilage matrix synthesis in fibrin cultures supplemented with exogenous insulin-like growth factor-I. J Orthop Res 17(4):467–474
Kelley KM, Johnson TR, Ilan J, Moskowitz RW (1999) Glucose regulation of the IGF response system in chondrocytes: induction of an IGF-1-resistant state. Am J Physiol 276(4 Pt 2):R1164–R1171
Maor G, Laron Z, Eshet R, Silbermann M (1993) The early postnatal development of the murine mandibular condyle is regulated by endogenous insulin-like growth factor-I. J Endocrinol 137(1):21–26
Wu S, Fadoju D, Rezvani G, De Luca F (2008) Stimulatory effects of insulin-like growth factor-I on growth plate chondrogenesis are mediated by nuclear factor-kappaB p65. J Biol Chem 283(49):34037–34044
Olashaw NE, Van Wyk JJ, Pledger WJ (1987) Control of late G0/G1 progression and protein modification by SmC/IGF I. Am J Physiol 253(4 Pt 1):C575–C579
Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen G, Tang J, Ma D (1999) TFAR19, a novel apoptosis-related gene cloned from human leukemia cell line TF-1, could enhance apoptosis of some tumor cells induced by growth factor withdrawal. Biochem Biophys Res Commun 254(1):203–210
Chen Y, Sun R, Han W, Zhang Y, Song Q, Di C, Ma D (2001) Nuclear translocation of PDCD5 (TFAR19): an early signal for apoptosis? FEBS Lett 509(2):191–196
Kameda H, Ishigami H, Suzuki M, Abe T, Takeuchi T (2006) Imatinib mesylate inhibits proliferation of rheumatoid synovial fibroblast-like cells and phosphorylation of Gab adapter proteins activated by platelet-derived growth factor. Clin Exp Immunol 144(2):335–341
Pope RM (2002) Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol 2(7):527–535
Chen Q, Liu SQ, Du YM, Peng H, Sun LP (2006) Carboxymethyl-chitosan protects rabbit chondrocytes from interleukin-1beta-induced apoptosis. Eur J Pharmacol 541(1–2):1–8
Acknowledgements
This study was supported by National Natural Science Foundation of China(30700853).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, C., Ma, C., Xie, Z. et al. Down-regulation of programmed cell death 5 by insulin-like growth factor 1 in osteoarthritis chondrocytes. International Orthopaedics (SICOT) 37, 937–943 (2013). https://doi.org/10.1007/s00264-012-1744-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-012-1744-x