Abstract
Background
The triglyceride-glucose index (TyG index), calculated as the logarithmic product of fasting triglyceride and glucose concentrations, is recognized as a simple marker of insulin resistance. However, the association between the TyG index and future decline of renal function remains unclear in the general population.
Objective
To investigate whether the TyG index was associated with future decline of renal function in the general population who had not progressed to chronic kidney disease stage G2.
Design
Retrospective longitudinal observational cohort study.
Participants
Individuals who received a population-based health checkup at JA Ehime Kouseiren Checkup Center from 2010 to 2019 (n = 134,007). Individuals without data of baseline fasting triglyceride or glucose levels, or baseline and follow-up data of estimated glomerular filtration rate (eGFR), or those with baseline eGFR < 60 mL/min/1.73 m2 were excluded.
Main Measures
Future renal function decline, defined as a ≥ 25% decrease in eGFR from baseline.
Key Results
Of 10,758 participants, 8,076 were classified into the low TyG index group (TyG index < 8.76, 1st to 3rd quartiles) and 2,682 into the high TyG index group (TyG index ≥ 8.76, 4th quartile). The mean follow-up period was 37.8 ± 23.6 months. The incidence rates of renal function decline were 0.31 and 0.69 per 100 person-years in the low and high TyG index groups, respectively. In multivariate Cox proportional hazard models, high TyG index was significantly associated with future renal function decline (hazard ratio 2.25, 95% CI 1.40–3.60). This association was consistent across subgroups stratified by age, sex, body mass index, baseline eGFR, and diagnosed hypertension, diabetes, or dyslipidemia.
Conclusion
In the general population, high TyG index was associated with future renal function decline. The TyG index may be useful in identifying individuals at high risk for future renal function decline in the setting of health checkups.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Chronic kidney disease (CKD) is a growing global health concern with increasing prevalence and associate risks, including death and cardiovascular disease.1,2,3 Importantly, CKD is characterized by progressive renal function deterioration.4 Early CKD diagnosis and intervention are crucial to prevent death and cardiovascular disease.5
Insulin resistance is recognized as a risk factor of CKD and a potential target for CKD treatment and prevention.6 Previous cross-sectional studies have demonstrated that insulin resistance and hyperinsulinemia occur early in CKD progression, even in patients with normal estimated glomerular filtration rate (eGFR).7,8 Furthermore, several longitudinal studies have shown that insulin resistance was associated with incident of CKD and kidney function decline.9,10 Thus, insulin resistance may serve as a novel marker for CKD development.
The insulin clamp test is the gold standard for evaluating insulin resistance but is impractical for large studies due to time and cost constraints.11 In 2008, the triglyceride-glucose (TyG) index was proposed as a less invasive marker, calculated using fasting triglycerides (TG) and blood sugar (FBS).12 The TyG index predicts hypertension and cardiovascular disease independently of diabetes.13 Whereas longitudinal studies have reported that a high TyG index predicts the progression of kidney dysfunction under specific conditions such as the elderly population, patients with type 2 diabetes mellitus, or those with preserved eGFR (≥ 75 mL/min/1.73 m2) and no medication,14,15,16,17 the association between the TyG index and future renal function decline has not been fully explored in the general population.
Whereas health checkups target early disease detection in the general population, they may not predict CKD risk solely based on eGFR and proteinuria assessments.18 This study investigates the association between the TyG index and future renal function decline in individuals not yet at CKD stage G2, using annual health checkup data.
METHODS
Study Population
This was a retrospective cohort study using a population-based health checkup data. The dataset was derived from the annual health-screening program conducted by JA Ehime Kouseiren Checkup Center between 2010 and 2019. This study was approved by the local ethics committee at Kyushu University Hospital (22181–00) and Ehime University (Approval no. 1912011). This study complied with the Declaration of Helsinki. An opt-out consent procedure was used in this retrospective and noninterventional study.
Data Collection
All participants included in this study underwent health checkup at least twice between 2010 and 2019. Baseline data was obtained from the data of the first visit during the period from 2010 to 2018. Individuals without baseline eGFR, baseline FBS, or fasting TG were excluded. Individuals with baseline eGFR < 60 mL/min/1.73 m2, suggestive of possible CKD stage G2 or higher, were also excluded from this study. The TyG index was calculated using the formula ln [FBS (mg/dL) × fasting TG (mg/dL) /2].12 Other details of the data collection are shown in Appendix 1.
Outcomes
Future renal function decline was defined as a 25% or greater decrease in eGFR from baseline, using the values at baseline and each subsequent visit. As sensitivity analyses, we also examined the association for (1) a 25% or greater decrease in eGFR from baseline to less than 60 mL/min/1.73 m2, (2) a 30% or greater decrease in eGFR from baseline, and (3) a 20% or greater decrease in eGFR from baseline. The eGFR was calculated using the following formula: eGFR (mL/min/1.73 m2) = 194 × (serum creatinine concentration [mg/dL])−1.094 × Age−0.287 for males, or (eGFR for males) × 0.739 for females, which is revised formula for Japanese.19
Statistical Analysis
Participants were stratified into four groups based on quartiles for their baseline TyG index. Baseline differences between participants in the four groups were compared using Jonckheere-Terpstra test for continuous variables and Cochran-Armitage test for categorical variables.
Univariate and multivariate Cox proportional hazards models were employed to calculate hazard ratios (HRs) for the incidence of future renal function decline. Models 1, 2, and 3 were developed to adjust for potential confounders (details in Appendix 1 and footnotes in Tables 2 and 4). Multivariate Cox regression analysis with Model 3 using the TyG index as a continuous variable was also performed. The associations between the TyG index and the incidence of future renal function decline were examined using restricted cubic splines with the same covariates used in Model 3.
In addition, we performed the analyses stratifying the participants into two groups: those with a low TyG index (1st, 2nd, and 3rd quartiles) and those with a high TyG index (4th quartile). Baseline differences between participants in the two groups were compared using t-test for continuous variables and chi-square test for categorical variables. The cumulative incidence of eGFR decline events in the low and high TyG index groups, adjusted for age and sex, was estimated using a Cox proportional hazards model. Additional analyses were conducted by including fasting TG and FBS in Model 3, respectively. In these two analyses, the TyG index was included only as a categorical variable due to collinearity issues. Subgroup analyses were also performed using Model 3 to determine whether the association between the TyG index and future renal function decline was affected by sex, age, body mass index (BMI), eGFR, or the diagnosis of hypertension, diabetes mellitus, or dyslipidemia.
All P values were two-sided except for trend tests, in which one-sided P values were used. A P value < 0.05 indicated statistical significance. All analyses were conducted with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
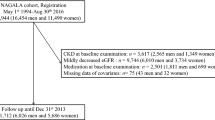
Figure 1 shows a flowchart of participants selection. A total of 134,007 individuals received health checkups at JA Ehime Kouseiren Checkup Center at least once between 2010 and 2018. Exclusions were made for individuals without baseline eGFR data (n = 76,081), those with baseline eGFR < 60 mL/min/1.73 m2 (n = 7,044), those without follow-up eGFR data (n = 21,841), and those without baseline FBS or fasting TG data (n = 18,283). After applying these exclusion criteria, 10,758 people were included in this study. The median TyG index was 8.32, with an interquartile range of 7.94–8.76. The mean follow-up period was 37.8 ± 23.6 months (median 35.5 months, interquartile range: 13.2–59.0 months).
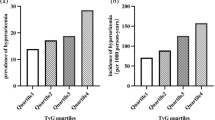
Table 1 shows the baseline characteristics of the participants divided by quartiles of the TyG index. The incidence of future renal function decline was observed in 136 out of 10,758 participants (1.26%). The incidence rates of future renal function decline were 0.35, 0.21, 0.37, and 0.69 per 100 person-years in Q1 (TyG index < 7.94), Q2 (7.94 ≤ TyG index < 8.32), Q3 (8.32 ≤ TyG index < 8.76), and Q4 (8.76 ≤ TyG index), respectively, suggesting that individuals in Q2 had the lowest risk for renal function decline.
Table 2 shows the HRs for the incidence of future renal function decline in comparison with Q2 of the TyG index, representing the lowest risk population, derived from the univariate and multivariate Cox regression analyses. In the multivariate analysis using Model 3, compared with individuals in Q2, those in Q1 had a high risk of renal function decline, and those in Q4 also had a higher risk. However, the risk in Q3 was not significantly different from that in Q2.
We also performed multivariate Cox regression analysis using the TyG index as a continuous variable and showed that an elevated TyG index was significantly associated with the renal function decline (HR 1.69, 95% CI 1.22–2.34, P = 0.002).
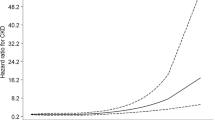
Figure 2 shows the restricted cubic spline regression analysis with the TyG index of 8.76 (upper quartile) as the reference, adjusted for the same variables using Model 3. There was a minimal HR of less than 0.5 observed at a TyG index of approximately 8.0, indicating a lower risk of renal function decline at this level. A slightly increased risk was observed at lower TyG index levels. As the TyG index increased beyond approximately 8.0, the risk of renal function decline continued to increase.
This result of the spline analysis aligns with the findings from the multivariate analyses conducted across the four groups stratified by quartiles of the TyG index, as well as the analysis treating the TyG index as a continuous variable. Detecting high-risk populations by categorizing individuals based on a specific cutoff value is crucial. To further explore this, we performed additional analyses by stratifying participants into two distinct groups: individuals with a low TyG index (Q1–3: TyG index < 8.76) and those with a high TyG index (Q4: TyG index ≥ 8.76).
Table 3 shows the baseline characteristics of the participants in the low and high TyG index groups. Similar to the TyG index quartile analysis, the high TyG group had significantly more known cardiovascular risk factors. The mean follow-up period was 37.8 ± 23.5 months and 37.9 ± 23.9 months in the low and high TyG index groups, respectively, with no significant difference between the groups (P = 0.89).
The incidence rates of future renal function decline were 0.31 and 0.69 per 100 person-years in the low and high TyG groups, respectively. Figure S1 in Appendix 2 shows the cumulative incidence of future renal function decline adjusted by age and sex. The incidence of future renal function decline was significantly higher in the high TyG index group compared to the low TyG index group.
Table 4 shows the unadjusted and adjusted HRs for future renal function decline comparing the high TyG index group with the low TyG index group. Both unadjusted and adjusted analyses revealed a significant association between the high TyG index group and future renal function decline. Sensitivity analyses using alternative renal endpoints showed a similar association between the TyG index and risk of eGFR decline (Table S1 in Appendix 3). Additional analyses, including TG or FBS, respectively, as a continuous variable in addition to the variables in Model 3, showed that the association between a high TyG index and worsening renal function remained significant (including TG: HR 2.06, 95% CI 1.27–3.36, P = 0.004; including FBS: HR 2.19, 95% CI 1.36–3.53, P = 0.001).
The results of subgroup analyses were shown in Fig. 3. The subgroup analysis based on sex, age (≥ 65 vs < 65 years), BMI (≥ 25 vs < 25 kg/m2), baseline eGFR (≥ 90 vs < 90 mL/min/1.73 m2), or the presence of hypertension, diabetes mellitus, or dyslipidemia presented no significant interaction between the TyG index level and these factors for the association with future renal function decline. The positive association between the TyG index and future renal function decline basically remained consistent across these subgroups.
Association between TyG index and the risk for future renal function decline in the subgroups. Hazard ratios were calculated by a multivariate Cox proportional hazards model with Model 3. Abbreviations: BMI, body mass index; DM, Diabetes mellitus; eGFR, estimated glomerular filtration rate; HR, hazard ratio; TyG index, triglyceride-glucose index.
DISCUSSION
This study provides novel evidence that individuals with a high TyG index have a higher incidence of future renal function decline, defined as a ≥ 25% decline in eGFR from baseline, compared to those with a low TyG index in the general population without evidence of CKD using data from annual health checkups. Importantly, this association remained significant even after adjusting for potential confounding factors such as age, hypertension, diabetes mellitus, baseline eGFR, and urine protein. This association remained significant after further adjustment for TG or FBS. Subgroup analyses demonstrated the consistency of this association across different subgroups, including sex, age, BMI, baseline eGFR, and the presence of hypertension, diabetes mellitus, or dyslipidemia. These findings highlight the potential utility of the TyG index as a screening tool for identifying individuals at high risk for future renal function decline in the general population, regardless of their specific backgrounds.
It should be noted that the primary renal outcome of our study, defined as a ≥ 25% decrease in eGFR from baseline, differs from commonly used surrogate outcomes such as end-stage kidney disease. We aimed to assess the clinical utility of the TyG index for early identification of high-risk individuals in the general population not yet at CKD stage G2. Therefore, a less robust surrogate outcome was set as the primary outcome, and individuals with baseline eGFR < 60 mL/min/1.73 m2 were excluded. Our sensitivity analysis also showed that a high TyG index was associated similarly with other renal outcomes, such as eGFR decline of ≥ 20% or ≥ 30%, supporting its utility in detecting individuals at high risk for early-stage CKD. Furthermore, given that a ≥ 30% eGFR decline predicts future end-stage kidney disease,20 the TyG index may potentially prove useful in identifying individuals at high risk for renal dysfunction, including end-stage kidney disease. A previous study demonstrated an association between a high TyG index and the development of end-stage kidney disease during a 22.7 ± 6.9-year follow-up in the general population.21 Further studies are needed to assess how the TyG index's association with future renal decline varies across different CKD stages.
There were significant differences in demographic and clinical characteristics between the high and low TyG index groups, as well as between the four groups stratified by quartiles of the TyG index. Patients in the high TyG index group had more risk factors for worsening renal function, including hypertension, diabetes, and dyslipidemia.22,23,24 Thus, the increased incidence of renal function decline in individuals with a high TyG index may partly stem from these accompanying risk factors. However, after adjusting for these potential confounders, the high TyG index remained associated with renal function decline. Subgroup analyses also demonstrated the consistency of this association across different subgroups: the HRs for renal function decline were greater than 1.0 in all subgroups with no significant interaction between the TyG index and subgroup factors, which is consistent with previous studies.14,25,26 Therefore, the elevated TyG index itself may contribute to the progression of renal dysfunction.
The TyG index is a minimally invasive and cost-effective measure of insulin resistance.12,27 Insulin resistance can disrupt glucose utilization in adipocytes, affecting lipid profiles and fasting glucose levels,28 which are components of the TyG index. While previous studies have reported associations between blood TG levels and worsening kidney function,29 our results showed that a high TyG index correlates with renal function decline even after adjusting for TG or FBS levels. This suggests that the TyG index captures additional information beyond TG or FBS levels alone, serving as a comprehensive indicator of metabolic abnormalities and insulin resistance that contribute to kidney dysfunction progression. Considering the TyG index could help identify individuals at higher risk of renal function decline, even with normal TG or FBS levels.
Insulin resistance impacts kidney function and CKD development diversely.30,31 Hyperinsulinemia, a characteristic feature of insulin resistance, can directly contribute to kidney dysfunction by promoting glomerular hyperfiltration, impairing endothelial function, and increasing vascular permeability.32,33 Insulin resistance-related hyperglycemia prompts metabolic acidosis, systemic inflammation, and oxidative stress, causing renal fibrosis and tubular damage.34,35 Blood sugar fluctuations in insulin-resistant individuals increase oxidative stress and risk of complications, including mortality, independent of hemoglobin A1c levels.36,37 Furthermore, insulin resistance-induced secondary hypertriglyceridemia directly impacts kidney function through lipid accumulation and lipotoxicity.38,39 Moreover, insulin resistance itself exacerbates renal disease progression by worsening renal hemodynamics through mechanisms such as sympathetic activation, sodium retention, and downregulation of the natriuretic peptide system.30,31 These factors collectively contribute to CKD development in individuals with high insulin resistance, as indicated by a high TyG index. Importantly, the TyG index outperforms other simple indicators such as homeostasis model assessment of insulin resistance (HOMA-IR) and TG/high-density lipoprotein cholesterol ratio in assessing insulin resistance.40,41 The strong association between a high TyG index and future renal function decline may reflect its superior detectability for insulin resistance.
Currently, no established TyG index cutoff exists to diagnose insulin resistance or predict diseases such as metabolic syndrome, diabetes mellitus, or CKD. Previous studies on TyG index and metabolic syndrome or diabetes mellitus suggest a cutoff around 8.6–8.9.42,43 In our study, individuals with a TyG index ≥ 8.76 had a higher risk of worsening renal function compared to those with a TyG index < 8.76, aligning with prior research. Furthermore, the HRs in the quartile stratified analysis and the restricted cubic spline curve analysis suggest a J-curve shaped risk by TyG index for renal function decline.
This study has several limitations. First, we could not analyze the association between the TyG index and hard renal endpoints because of low event incidence in our study, likely due to a slower GFR decline in Japanese individuals compared to Caucasians.44 Second, potential influences of treatments for hypertension, diabetes mellitus, and dyslipidemia (e.g., diet, exercise, medication) on the TyG index, insulin resistance, or renal function,45,46,47,48 were not considered due to lack of data. Certain medications can directly impact fasting TG and FBS levels, affecting the TyG index independent of true insulin resistance. Although we couldn’t assess these treatments’ effects, subgroup analysis consistently showed an association regardless of hypertension, diabetes mellitus, or dyslipidemia. Third, reasons for follow-up discontinuation were unavailable. The study would be further strengthened if the reasons for censoring patients, such as mortality and long-term hospitalization, were available. Fourth, many individuals were excluded due to missing TG, FBS, and eGFR data. This is probably attributed to the fact that Japanese law permits non-fasting blood draws, the exceptional omission of blood tests in individuals under 39 years of age, and the omission of serum creatinine from blood tests across all age groups in health checkups. Nonetheless, TG and FBS tests are common in clinical practice and health checkups. This study underscores the potential of routine blood tests in identifying high-risk individuals for renal dysfunction, advocating for fasting blood samples and serum creatinine inclusion in health checkups.
In conclusion, using health checkup data, we found a higher incidence of eGFR decline ≥ 25% was in the high TyG index group compared to the low TyG index group. Evaluation of the TyG index may be useful in identifying individuals at high risk for future renal function decline in the setting of health checkups in the general population.
Data Availability:
The datasets analyzed during the current study are not publicly available due to ethical restrictions but are available from the corresponding author on reasonable request.
References
Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2020;395(10225):709-733. https://doi.org/10.1016/S0140-6736(20)30045-3.
Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet (London, England). 2018;392(10159):2052-2090. https://doi.org/10.1016/S0140-6736(18)31694-5.
Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157-1172. https://doi.org/10.1161/CIRCULATIONAHA.120.050686.
Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294-1304. https://doi.org/10.1001/jama.2019.14745.
Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34-47. https://doi.org/10.1016/j.kint.2020.10.012.
Sarafidis PA. Obesity, insulin resistance and kidney disease risk: insights into the relationship. Curr Opin Nephrol Hypertens. 2008;17(5):450-456. https://doi.org/10.1097/MNH.0b013e328305b994.
Fliser D, Pacini G, Engelleiter R, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53(5):1343-1347. https://doi.org/10.1046/j.1523-1755.1998.00898.x.
Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16(4):1091-1098. https://doi.org/10.1681/ASN.2004090742.
Cheng HT, Huang JW, Chiang CK, Yen CJ, Hung KY, Wu KD. Metabolic syndrome and insulin resistance as risk factors for development of chronic kidney disease and rapid decline in renal function in elderly. J Clin Endocrinol Metab. 2012;97(4):1268-1276. https://doi.org/10.1210/jc.2011-2658.
Huh JH, Yadav D, Kim JS, et al. An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism. 2017;67:54-61. https://doi.org/10.1016/j.metabol.2016.11.003.
Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19(7):527-534. https://doi.org/10.1046/j.1464-5491.2002.00745.x.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299-304. https://doi.org/10.1089/met.2008.0034.
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. https://doi.org/10.1186/s12933-022-01511-x.
Lei L, Liang H, Qu Y, et al. Association between triglyceride-glucose index and worsening renal function in the elderly. Front Nutr. 2022;9:951564. https://doi.org/10.3389/fnut.2022.951564.
Xu X, Tang X, Che H, et al. Triglyceride-glucose product is an independent risk factor for predicting chronic kidney disease in middle-aged and elderly population: a prospective cohort study. Nan Fang Yi Ke Da Xue Xue Bao. 2021;41(11):1600-1608. https://doi.org/10.12122/j.issn.1673-4254.2021.11.02.
Lv L, Zhou Y, Chen X, et al. Relationship between the TyG index and diabetic kidney disease in patients with type-2 diabetes mellitus. Diabetes Metab Syndr Obes. 2021;14:3299-3306. https://doi.org/10.2147/DMSO.S318255.
Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Triglyceride-glucose index is a predictor of incident chronic kidney disease: a population-based longitudinal study. Clin Exp Nephrol. 2019;23(7):948-955. https://doi.org/10.1007/s10157-019-01729-2.
Takeuchi M, Shinkawa K, Yanagita M, Kawakami K. Prevalence, recognition and management of chronic kidney disease in Japan: population-based estimate using a healthcare database with routine health checkup data. Clin Kidney J. 2021;14(10):2197-2202. https://doi.org/10.1093/ckj/sfab016.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982-992. https://doi.org/10.1053/j.ajkd.2008.12.034.
Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821-835. https://doi.org/10.1053/j.ajkd.2014.07.030.
Fritz J, Brozek W, Concin H, et al. The triglyceride-glucose index and obesity-related risk of end-stage kidney disease in Austrian adults. JAMA Netw Open. 2021;4(3):e212612. https://doi.org/10.1001/jamanetworkopen.2021.2612.
Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis Off. 2009;53(3 Suppl 3):S4-16. https://doi.org/10.1053/j.ajkd.2008.07.048.
Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70(10):1694-1705. https://doi.org/10.1038/sj.ki.5001794.
Tuttle KR, Alicic RZ, Duru OK, et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Netw Open. 2019;2(12):e1918169. https://doi.org/10.1001/jamanetworkopen.2019.18169.
Ren X, Jiang M, Han L, Zheng X. Association between triglyceride-glucose index and chronic kidney disease: A cohort study and meta-analysis. Nutr Metab Cardiovasc Dis. 2023;33(6):1121-1128. https://doi.org/10.1016/j.numecd.2023.03.026.
Kunutsor SK, Seidu S, Kurl S, Laukkanen JA. Baseline and usual triglyceride-glucose index and the risk of chronic kidney disease: a prospective cohort study. GeroScience. Published online January 2024. https://doi.org/10.1007/s11357-023-01044-5.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347-3351. https://doi.org/10.1210/jc.2010-0288.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi:https://doi.org/10.1186/s12933-018-0762-4.
Shimizu M, Furusyo N, Mitsumoto F, et al. Subclinical carotid atherosclerosis and triglycerides predict the incidence of chronic kidney disease in the Japanese general population: results from the Kyushu and Okinawa Population Study (KOPS). Atherosclerosis. 2015;238(2):207-212. https://doi.org/10.1016/j.atherosclerosis.2014.12.013.
Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12(12):721-737. https://doi.org/10.1038/nrneph.2016.145.
Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087-F1108. https://doi.org/10.1152/ajprenal.00340.2016.
Trevisan R, Bruttomesso D, Vedovato M, et al. Enhanced responsiveness of blood pressure to sodium intake and to angiotensin II is associated with insulin resistance in IDDM patients with microalbuminuria. Diabetes. 1998;47(8):1347-1353. https://doi.org/10.2337/diab.47.8.1347.
Vedovato M, Lepore G, Coracina A, et al. Effect of sodium intake on blood pressure and albuminuria in type 2 diabetic patients: the role of insulin resistance. Diabetologia. 2004;47(2):300-303. https://doi.org/10.1007/s00125-003-1303-5.
Amorim RG, Guedes G da S, Vasconcelos SM de L, Santos JC de F. Kidney disease in diabetes mellitus: cross-linking between hyperglycemia, redox imbalance and inflammation. Arq Bras Cardiol. 2019;112(5):577-587. https://doi.org/10.5935/abc.20190077.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058-1070. https://doi.org/10.1161/CIRCRESAHA.110.223545.
Škrha J, Šoupal J, Škrha JJ, Prázný M. Glucose variability, HbA1c and microvascular complications. Rev Endocr Metab Disord. 2016;17(1):103-110. https://doi.org/10.1007/s11154-016-9347-2.
Klimontov VV, Saik OV, Korbut AI. Glucose variability: How does it work? Int J Mol Sci. 2021;22(15). https://doi.org/10.3390/ijms22157783.
Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens. 2010;19(4):393-402. https://doi.org/10.1097/MNH.0b013e32833aa4ac.
Castro BBA, Foresto-Neto O, Saraiva-Camara NO, Sanders-Pinheiro H. Renal lipotoxicity: insights from experimental models. Clin Exp Pharmacol Physiol. 2021;48(12):1579-1588. https://doi.org/10.1111/1440-1681.13556.
Huang R, Cheng Z, Jin X, et al. Usefulness of four surrogate indexes of insulin resistance in middle-aged population in Hefei, China. Ann Med. 2022;54(1):622-632. https://doi.org/10.1080/07853890.2022.2039956.
Luo P, Cao Y, Li P, et al. TyG index performs better than HOMA-IR in Chinese type 2 diabetes mellitus with a BMI < 35 kg/m2: a hyperglycemic clamp validated study. Medicina (Kaunas). 2022;58(7). https://doi.org/10.3390/medicina58070876.
Tahapary DL, Pratisthita LB, Fitri NA, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16(8):102581. https://doi.org/10.1016/j.dsx.2022.102581.
Lee DY, Lee ES, Kim JH, et al. Predictive value of triglyceride glucose index for the risk of incident diabetes: a 4-year retrospective longitudinal study. PLoS One. 2016;11(9):e0163465. https://doi.org/10.1371/journal.pone.0163465.
Imai E, Horio M, Yamagata K, et al. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res. 2008;31(3):433-441. https://doi.org/10.1291/hypres.31.433.
Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol. 2005;98(1):3-30. https://doi.org/10.1152/japplphysiol.00852.2004.
Toyama K, Sugiyama S, Oka H, Sumida H, Ogawa H. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol. 2010;56(2):142-146. https://doi.org/10.1016/j.jjcc.2010.06.007.
Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: A Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728-741. https://doi.org/10.1053/j.ajkd.2015.10.011.
Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet (London, England). 2022;400(10365):1788-1801. https://doi.org/10.1016/S0140-6736(22)02074-8.
Acknowledgements:
We gratefully acknowledge the staff of the JA Ehime Kouseiren Checkup Center for providing the data used in this study.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest:
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshida, D., Ikeda, S., Shinohara, K. et al. Triglyceride-Glucose Index Associated with Future Renal Function Decline in the General Population. J GEN INTERN MED (2024). https://doi.org/10.1007/s11606-024-08809-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11606-024-08809-4