Abstract
Background
Liver transplantation (LT) has been considered a potential curative treatment for patients with very early intrahepatic cholangiocarcinoma (ICC) and cirrhosis, yet the survival benefit of LT has not been well defined. This study aimed to compare the long-term survival outcomes of patients who underwent LT with that of individuals who received resection and non-curative intent treatment (non-CIT).
Methods
Patients who underwent LT, hepatectomy, and non-CIT between 2004 and 2018 were included in the National Cancer Database. Survival benefits of LT over resection and non-CIT were analyzed relative to overall survival (OS).
Results
Among 863 patients, 54 (6.3%) underwent LT, while 342 (39.6%) underwent surgical resection, and 467 (54.1%) received non-CIT, respectively. While the rates of non-CIT increased over time, the percentages of LT remained consistent during the study period. LT patients had similar 5-year OS to individuals who underwent resection (referent, resection: LT, HR 0.95, 95%CI 0.84–1.58, p=0.84). In contrast, 5-year OS was better among patients who underwent LT versus individuals who had non-CIT after controlling other variables using propensity score overlapping weighting (5-year OS, LT 57.1% vs. LR 25.8%, p<0.001).
Conclusions
The outcomes of very early ICC patients who underwent LT were similar to individuals who underwent hepatectomy, but better than patients treated with non-CIT. LT should be may be a consideration as a treatment option for patients with early stage ICC who are unsuitable candidates for resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer, representing approximately 10–20% of all primary hepatic malignancies.1 The incidence of ICC continues to rise with increasing rates reported worldwide across both Eastern and Western countries.2 The increased incidence is in part due to the improved recognition of characteristic radiologic features and wider implementation of liver cancer surveillance among high-risk populations, which have led to increased detection of early-stage ICC.3,4,5 For patients with early-stage disease, liver resection is the primary curative-intent treatment option; in fact, around 70% of patients with resectable ICC undergo a liver resection with an R0 margin.6 Nonetheless, a subset of ICC patients is considered “unresectable” due to impaired liver function and underlying cirrhosis.5 While liver resection remains the standard of care, it may be challenging in the context of cirrhotic patients as maintaining a sufficient amount of remnant liver volume becomes necessary to avoid post-operative liver failure.7, 8 Given these obstacles, there has been increasing attention towards liver transplantation (LT) as a viable treatment option for ICC.9, 10
LT has been considered a contraindication for ICC due to initial poor survival outcomes after transplantation.11, 12 Sapisochin et al. reported that patients with “very early” ICC (single tumor and ≤ 2cm) and cirrhosis had 5-year overall survival (OS) of 65% and should be considered possible candidates for LT.9 Other pooled analyses of ICC patients undergoing LT noted a 5-year OS among patients with very early ICC of 71%, which was comparable to the currently accepted threshold of 75% 5-year OS for hepatocellular carcinoma.13, 14 Several other studies have suggested that long-term survival outcomes after LT may be comparable to that of resection among patients with ICC, especially individuals with early-stage ICC.15,16,17
Notwithstanding these data, few studies have investigated survival difference among patients undergoing LT versus hepatectomy for ICC. In particular, there is little information on the survival benefit among ICC patients who undergo LT versus other treatments such as resection and non-curative intent treatment (non-CIT). Therefore, the objective of the current study was to investigate the clinical characteristics and survival differences of patients with very early ICC patients receiving different treatment modalities. In particular, we sought to compare the long-term survival outcomes of patients who underwent LT with patients who underwent hepatic resection. Moreover, we assessed the survival benefit of LT versus non-curative intent treatment using propensity score overlapped weighting.
Methods
Data Source and Patient Selection
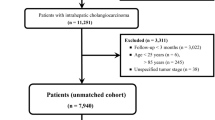
Patients with very early ICC undergoing LT, surgical resection, or non-curative intent treatment (i.e., chemotherapy, radiotherapy, palliative care, others) between 2004 and 2018 were identified from the National Cancer Data Base (NCDB). The NCDB is a comprehensive clinical oncology database that encompasses over 34 million records of individual cancer patients. Data from more than 1500 Commission on Cancer (CoC) accredited facilities throughout the USA are included. The NCDB effectively captures information on over 70% of all newly diagnosed cancer cases across the country at an institutional level. The NCDB 2019 Participant Use File (PUF) was utilized to identify very early ICC patients using the relevant International Classification of Diseases for Oncology, third edition (ICD-O-3) histology code 8160. Patients with single tumors of ≤ 2cm in size were included.9 Patients who had multiple tumors, metastatic diseases, missing information on tumor size and tumor stage based on the American Joint Committee on Cancer 6th or 7th edition,18 and lack of follow-up data were excluded. The Ohio State University Institutional Review Board approved this study.
Variables and Outcomes
Variables of interest included age, gender, race, the Charlson-Deyo comorbidity score (i.e., 0 vs. 1 vs. 2 or more), year of diagnosis (i.e., 2004–2009 vs. 2010–2018), median income, insurance status, attainment of higher education, hospital location (i.e., non-metropolitan vs. metropolitan), hospital type (i.e., non-academic vs. academic), and fibrosis (i.e., Ishak score 1–4 vs. 5–6). Patients were categorized into three groups: LT, liver resection, and non-CIT, which was defined as chemotherapy, radiotherapy, palliative care, or other treatments. Time periods between 2010 and 2018 were referred to as the era of modern chemotherapeutics.15, 19 The primary outcome was overall survival (OS), defined as the time interval between the date of surgery or intervention to the date of death from any cause or last follow-up.
Statistical Analysis
Descriptive statistics were reported as medians (interquartile ranges (IQRs)) for continuous variables, while categorical variables were presented as frequencies (%). To compare continuous variables, the Mann-Whitney U test was employed, whereas categorical variables were assessed using the chi-square test or Fisher’s exact test, depending on appropriateness. The trend of LT, resection, and non-CIT cases for very early ICC patients was assessed by the Mantel-Haenszel trend test. Survival probabilities were estimated utilizing Kaplan-Meier curves and compared using the log-rank test. The Cox regression model was employed to evaluate the association between relevant clinicopathologic factors and overall survival (OS). Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were reported for variables found to be statistically significant. Hazard rates were estimated using the local regression-smoothing method. Aalen’s linear hazards models were employed to examine the hypothesis that LT may exert distinct effects on the hazard function across different time intervals.20 When the time-varying coefficient of a covariate in Aalen’s model remains constant, the plot of the cumulative hazard ratio versus time appears as a straight line. An elevated hazard of death is indicated by an increased slope, while a reduced hazard is reflected by a decreased slope in the cumulative regression coefficient line. For ease of interpretation, results from the Aalen’s linear hazard models were represented by a plot of the cumulative hazard ratio against time from surgery. Additionally, 5-year conditional survival (CS) estimates were analyzed, considering the age groups (i.e., <60 years old vs. ≥60 years old) to examine the long-term survival advantages of LT compared with resection. CS estimates are used to assess the likelihood of patients surviving for a specific number of additional years, based on the duration of time they have already survived after the surgical procedure.21 Specifically, the 5-year CS refers to the conditional probability of surviving 5+t years, given that the patient is still alive t years after the surgery.21
To address the substantial imbalance between LT and non-CIT groups, a propensity score weighting using overlapped weights was performed to adjust the imbalances between the two groups regarding demographic information including social- and tumor-related factors, as well as background liver condition.22 An overlap weight propensity score for treatment allocation was estimated from a multivariate model containing the patient’s age, gender, year of diagnosis, race, median income, insurance status, education levels, facility location and types, Charlson-Deyo comorbidity score, and fibrosis. Results from the comparison between covariate subgroups were reported as standardized mean differences (SMDs). SMDs lower than 0.1 indicated very small differences between means, whereas values between 0.1 and 0.3, between 0.3 and 0.5, and greater than 0.5 indicated small, moderate, and large differences, respectively.23 A sensitivity analysis was conducted to identify patients who received treatment during the period of modern chemotherapies, in order to capture the impact of advancements in systemic chemotherapy. All statistical tests were conducted as two-sided, and a p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.2.0 (R Project for Statistical Computing, Vienna, Austria).
Results
Patients’ Demographics
A total of 863 patients were identified for inclusion in the study. The median age was 67.0 years (IQR, 60.0–75.0) and 365 (42.3%) patients were female. Among the patients, 54 (6.3%) underwent LT, while 342 (39.6%) underwent surgical resection, and 467 (54.1%) received non-CIT. The vast majority of patients (n=683, 79.1%) were treated during 2010 to 2018. Most patients resided in metropolitan areas (n=683, 79.1%) and were treated at academic institutions (532, n=61.6%). Among patients with an available fibrosis score (n=121, 14.0%), only a small subset of patients had fibrosis of the liver (n=50, 5.8%). Approximately 10% of patients had poor or undifferentiated histological differentiation (n=109, 12.6%) (Table 1).
Chronological Trend for LT, Resection, and Non-CIT
Figure 1 illustrates the trend over time in the proportion of LT, resection, and non-CIT for patients with very early ICC. Notably, the percentage of patients undergoing resection for very early ICC exhibited a slight but significant decline throughout the study period, decreasing from 62.5% in 2004 to 28.9% in 2018 (Mantel-Haenszel test of trend p<0.01). Conversely, the proportion of patients receiving non-CIT steadily increased over time, more than doubling from 31.3% in 2004 to 66.7% in 2018 (Mantel-Haenszel test of trend p<0.01). In contrast, the percentage of LT remained relatively stable at around 5–7% throughout the study period (Mantel-Haenszel test of trend p=0.71).
Survival Benefits of LT and Surgical Resection
Compared with patients who underwent liver resection, individuals who had LT were more likely to be younger (LT, 61.0 years, IQR 54.0–64.0 vs. resection, 65.0 years, IQR 59.0–72.0), male (LT, n=14, 25.9% vs. resection, n=143, 41.8%) and treated at an academic hospital (LT, n=48, 88.9% vs. resection, n=244, 71.3%). In addition, patients who underwent LT had an increased likelihood of having an advanced Charlson-Deyo comorbidity score (≥2: LT, n=18, 33.3% vs. resection, n=40, 11.7%) and fibrosis of the liver (LT, n=15, 27.8% vs. resection, n=15, 4.4%) (all p<0.05) (Supplementary Table 1).
With a median follow-up of 35.6 months (IQR 19.2-65.7 months), LT patients had similar 5-year OS to individuals who underwent resection (5-year OS, LT 60.5% vs. resection 53.9%; 10-year OS, LT 53.9% vs. resection 33.1%; p=0.25) (Fig. 2). On multivariable Cox regression analysis, LT was not associated with worse OS compared with hepatic resection (referent, resection: LT, HR 1.06, 95%CI 0.63–1.79, p=0.82) (Table 2). To further examine the time-varying effect of LT versus resection on survival, Aalen’s linear hazard models were utilized. While not statistically significant, LT patients exhibited a higher risk of mortality versus individuals who underwent resection for approximately 36 months following surgery (Fig. 3a).
CS estimates were analyzed relative to LT versus hepatectomy with stratification according to age. Notably, among patients under the age of 60 (n=27), the 5-year CS following LT was higher than that observed after resection during the initial 0 to 5 years after surgery. In contrast, the 5-year CS for patients aged 60 or older who underwent LT (n=29) was initially comparable to that of LT patients under 60 years old for up to 3 years after surgery, yet beyond that point, CS declined and fell below the 5-year CS of patients aged 60 or older who underwent resection (Fig. 3b).
Survival Benefits of LT and Non-CIT
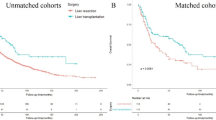
To compare the survival advantages among patients who underwent LT, and individuals who were unable to undergo curative intent resection, patients aged ≤ 70 years old who had LT versus non-CIT were analyzed, as elderly patients aged >70 years old are normally not considered candidates for LT. The total number of elderly patients undergoing LT and non-CIT was 49 and 219, respectively. Before adjustment, there were several clinicodemographic imbalances between the two groups including gender, income level, insurance status, hospital location, and hospital type, as well as Charlson-Deyo comorbidity score and the level of fibrosis. After adjustment, all measured variables were balanced (all SMDs <0.001) (Table 3). In the propensity score overlapped weighting-adjusted cohort, 5-year OS was better among patients who had LT versus non-CIT (5-year OS, LT 54.8% vs. LR 27.9%, p=0.005) (Fig. 4). A sensitivity analysis of patients between 2010 and 2018 demonstrated similar results with LT patients having a more favorable OS versus patients in the non-CIT cohort (5-year OS, LT 52.8% vs. LR 21.9%, p<0.001) (Supplementary Figure 1).
Discussion
With advances in chemotherapy, surgical techniques, and immunological therapies, there has been an extension of oncologic indications for liver transplantation in recent years.24 Though relatively well established for perihilar CCA, the role of LT for ICC remains controversial.25 Initial reports on the survival outcomes of LT for ICC were poor, however, with a reported 2-year OS of only 30%.26 More recently, there has been renewed interest in LT for ICC based on revised, more limited selection criteria.27, 28 The current study was important because it demonstrated an upward trend in the utilization of non-CIT for patients with very early ICC, while the use of LT remained constant over the study period. Although there was no overall difference in OS among patients who underwent LT versus resection in the overall cohort, among patients under the age of 60, the 5-year CS following LT was higher than that observed after resection during the initial 0 to 5 years after surgery. In addition, LT was associated with a survival advantage over non-CIT after controlling confounding factors. This finding was further supported by sensitivity analysis, which specifically examined patients treated with more recent chemotherapeutic regimens.
With growing interest in transplant oncology over the last decade, there is renewed interest in comparing long-term outcomes of LT versus surgical resection. Hong et al. published initial data over a decade ago that suggested LT combined with neoadjuvant and adjuvant systemic therapy could provide reasonable long-term outcomes compared with hepatectomy for patients with early ICC.29 More recently, other reports have noted similar median survival times following LT versus resection for ICC in the setting of the modern era with systemic chemotherapeutic agents.15 Specifically, using propensity score matching, Hue et al. reported a median survival of 36.1 months for patients undergoing LT versus 33.6 months among individuals who underwent hepatic resection.15 Most previous studies evaluated survival differences for all ICC patients and failed to stratify patients with “very early” ICC. In the current study, we specifically analyzed only patients with very early ICC defined as a single tumor and ≤ 2cm. Of note, utilization of LT for very early ICC was associated with comparable overall long-term outcomes versus hepatectomy. In particular, 5-year OS following LT for very early ICC was 60.5%, which was similar to the 5-year OS of 53.9% among patients who underwent hepatic resection (Fig. 2). While these findings suggest that LT should not prioritized as a treatment option for all patients with ICC, certain subsets of individuals with very early HCC may benefit from LT. For example, among patients under the age of 60, 5-year CS following LT was higher than after resection during the initial 0 to 5 years after surgery (Fig. 3b). As such, patient age at the time of LT may be one factor to consider when considering LT versus resection, although further validation is warranted.
Patients with very early ICC who have cirrhosis or other contraindications to resection are often only considered for alternative non-CIT options such as chemotherapy or radiotherapy.30 To date, there has been a dearth of literature examining the differences in outcomes among patients who underwent LT versus non-CIT. In the current study, we noted a survival benefit among patients undergoing LT versus individuals who were treated with non-CIT, after propensity score matching to control for competing variables between the two groups (Fig. 4). Of note, the improvement in outcomes among patients undergoing LT persisted in the era of modern chemotherapies (2010–2018). In this sense, the survival advantage associated with LT versus non-CIT suggests that clinicians should consider LT in determining the optimal course of treatment for patients with early ICC who are not candidates for resection. While there have been marked advancements in non-surgical treatment options for ICC, these treatments are not curative in nature.31 As such, findings from the current study suggest that LT may be a viable treatment option for patients who are not suitable candidates for surgical resection. LT is, however, a highly specialized procedure that requires substantial human and medical resources.32 Furthermore, socioeconomic disparities pose challenges to the widespread implementation of LT.33, 34 Overcoming these barriers is essential to expand the utilization of LT for early-stage ICC, as well as to determine the optimal LT protocol for patients with very early ICC.10
Treatment of early ICC is particularly important as there is an increasing proportion of patients being diagnosed with early-stage disease.16 In fact, Lee et al. demonstrated that the proportion of patients with ICC ≤ 3 cm has increased to 10% in 2018.16 The observed increase in early-stage ICC detection can be attributed to the broader adoption of liver cancer surveillance among patients with cirrhosis or chronic hepatitis infection, as well as advancements in imaging techniques for ICC diagnosis.35 Additionally, policy changes that have enhanced access to healthcare may contribute to the early detection of ICC.3 Interestingly, despite an increase in the proportion of patients diagnosed with early-stage ICC, there was a decrease in the number of patients undergoing liver resection, while the percentage of patients receiving non-CIT increased (Fig. 1). The criteria of resectability for non-metastatic ICC should be based on background liver condition and the extent of liver resection, since the remnant liver function has a profound role in postoperative management.36 Hence, our findings may be explained, in part, by an increase in patients with impaired liver function who were unable to undergo resection. Another possibility may be related to social determinants of health as socioeconomic and environmental factors have been associated with disparities in receipt of LT.37 Interestingly, the proportion of LT was stable around 5–7%, which may be related to LT not being an established standard of care for resectable ICC.38, 5, 39 Data from the current study suggest that clinicians should consider LT more often as a potential treatment option for appropriately selected patients with early ICC. As 5-year survival following LT for early-stage ICC did not reach the 75% “benchmark,” prioritizing LT for ICC over other nonmalignant diseases might not be advisable.27 Measures to expand the liver donor pool, such as donor after cardiac death, living liver transplantation, and xenotransplantation, may hold potential in expanding the indications for liver transplantation in cases involving malignancies in the future.40, 41
Several limitations should be considered when interpreting the results. As the NCDB did not include information on the waiting list for LT, analyses on waitlist mortality and morbidity could not be performed. Furthermore, the comparison between LT and hepatic resection was not conducted in an intention-to-treat manner, resulting in a possible selection bias for LT patients.42 In addition, selection bias and residual confounding may have affected the patient selection of each treatment option due to the retrospective nature of the study. To mitigate this, propensity score overlapped weight analyses were utilized to minimize residual bias and confounding. The NCDB also did not provide information on the reasons why patients did not undergo resection or LT. Furthermore, the NCDB did not provide detailed information on the preoperative diagnosis or the utilization of portal lymphadenectomy. Potential coding errors related to data input and coding of population-based registry data may have also impacted the results; however, this effect was likely not to have biased the results in a particular direction.
In conclusion, very early ICC patients who had LT had comparable outcomes to individuals who underwent hepatectomy, yet a markedly better prognosis than individuals treated with non-CIT. LT should be carefully considered a potential treatment for patients with very early ICC who are not suitable candidates for resection. Surgeons should evaluate each patient’s individual circumstances and determine whether LT may be a viable and appropriate treatment option. Data on patients undergoing LT for ICC should be monitored in prospective protocol-driven registries to better define selection criteria and long-term outcomes.
References
Singal AK, Vauthey JN, Grady JJ, Stroehlein JR. Intra-hepatic cholangiocarcinoma--frequency and demographic patterns: thirty-year data from the M.D. Anderson Cancer Center. J Cancer Res Clin Oncol. 2011;137(7):1071-8. https://doi.org/10.1007/s00432-010-0971-z.
Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37(6):806-13. https://doi.org/10.1016/s0168-8278(02)00297-0.
Lima HA, Moazzam Z, Endo Y, Alaimo L, Diaz A, Woldesenbet S et al. Impact of the Affordable Care Act on Presentation, Treatment, and Outcomes of Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2022. https://doi.org/10.1007/s11605-022-05496-6.
Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69-76. https://doi.org/10.1016/j.jhep.2012.02.022.
Kubo S, Shinkawa H, Asaoka Y, Ioka T, Igaki H, Izumi N et al. Liver Cancer Study Group of Japan Clinical Practice Guidelines for Intrahepatic Cholangiocarcinoma. Liver Cancer. 2022;11(4):290-314. https://doi.org/10.1159/000522403.
de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140-5. https://doi.org/10.1200/JCO.2011.35.6519.
Alaimo L, Endo Y, Lima HA, Moazzam Z, Shaikh CF, Ruzzenente A et al. A comprehensive preoperative predictive score for post-hepatectomy liver failure after hepatocellular carcinoma resection based on patient comorbidities, tumor burden, and liver function: the CTF score. J Gastrointest Surg. 2022;26:2486–95. https://doi.org/10.1007/s11605-022-05451-5.
Thakral N, Gonzalez T, Nano O, Shin SH, Samuels S, Hussein A. Cirrhosis in intrahepatic cholangiocarcinoma: prognostic importance and impact on survival. BMC gastroenterology. 2023;23(1):151. https://doi.org/10.1186/s12876-023-02710-w.
Sapisochin G, Rodriguez de Lope C, Gastaca M, Ortiz de Urbina J, Suarez MA, Santoyo J et al. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant. 2014;14(3):660-7. https://doi.org/10.1111/ajt.12591.
Eguchi S, Hidaka M, Hara T, Matsushima H, Soyama A. Liver transplantation for intrahepatic and hilar cholangiocellular carcinoma: Most recent updates in the literature. Ann Gastroenterol Surg. 2022;6(5):616-22. https://doi.org/10.1002/ags3.12567.
Pichlmayr R, Weimann A, Tusch G, Schlitt HJ. Indications and Role of Liver Transplantation for Malignant Tumors. Oncologist. 1997;2(3):164-70.
Hara T, Eguchi S, Yoshizumi T, Akamatsu N, Kaido T, Hamada T et al. Incidental intrahepatic cholangiocarcinoma in patients undergoing liver transplantation: A multi-center study in Japan. J Hepatobiliary Pancreat Sci. 2021;28(4):346-52. https://doi.org/10.1002/jhbp.896.
Ziogas IA, Giannis D, Economopoulos KP, Hayat MH, Montenovo MI, Matsuoka LK et al. Liver Transplantation for Intrahepatic Cholangiocarcinoma: A Meta-analysis and Meta-regression of Survival Rates. Transplantation. 2021;105(10):2263-71. https://doi.org/10.1097/TP.0000000000003539.
Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43. https://doi.org/10.1016/s1470-2045(08)70284-5.
Hue JJ, Rocha FG, Ammori JB, Hardacre JM, Rothermel LD, Chavin KD et al. A comparison of surgical resection and liver transplantation in the treatment of intrahepatic cholangiocarcinoma in the era of modern chemotherapy: An analysis of the National Cancer Database. J Surg Oncol. 2021;123(4):949-56. https://doi.org/10.1002/jso.26370.
Lee YT, Singal AG, Lauzon M, Agopian VG, Luu M, Noureddin M et al. Disparities in curative treatments and outcomes for early stage intrahepatic cholangiocarcinoma in the United States. Cancer. 2022;128(20):3610-9. https://doi.org/10.1002/cncr.34436.
De Martin E, Rayar M, Golse N, Dupeux M, Gelli M, Gnemmi V et al. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transpl. 2020;26(6):785-98. https://doi.org/10.1002/lt.25737.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-9. https://doi.org/10.3322/caac.21388.
Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer. 2009;101(4):621-7. https://doi.org/10.1038/sj.bjc.6605211.
Aalen OO. A linear regression model for the analysis of life times. Stat Med. 1989;8(8):907-25. https://doi.org/10.1002/sim.4780080803.
Hieke S, Kleber M, Konig C, Engelhardt M, Schumacher M. Conditional Survival: A Useful Concept to Provide Information on How Prognosis Evolves over Time. Clin Cancer Res. 2015;21(7):1530-6. https://doi.org/10.1158/1078-0432.CCR-14-2154.
Li F, Thomas LE, Li F. Addressing Extreme Propensity Scores via the Overlap Weights. Am J Epidemiol. 2019;188(1):250-7. https://doi.org/10.1093/aje/kwy201.
Burnand B, Kernan WN, Feinstein AR. Indexes and boundaries for "quantitative significance" in statistical decisions. J Clin Epidemiol. 1990;43(12):1273-84. https://doi.org/10.1016/0895-4356(90)90093-5.
Quaresima S, Melandro F, Giovanardi F, Shah K, De Peppo V, Mennini G et al. New Insights in the Setting of Transplant Oncology. Medicina (Kaunas). 2023;59(3). https://doi.org/10.3390/medicina59030568.
Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23(7):692-7. https://doi.org/10.1111/j.1432-2277.2010.01108.x.
Becker NS, Rodriguez JA, Barshes NR, O'Mahony CA, Goss JA, Aloia TA. Outcomes analysis for 280 patients with cholangiocarcinoma treated with liver transplantation over an 18-year period. J Gastrointest Surg. 2008;12(1):117-22. https://doi.org/10.1007/s11605-007-0335-4.
Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY et al. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64(4):1178-88. https://doi.org/10.1002/hep.28744.
Sapisochin G, Ivanics T, Heimbach J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: Ready for Prime Time? Hepatology. 2022;75(2):455-72. https://doi.org/10.1002/hep.32258.
Hong JC, Jones CM, Duffy JP, Petrowsky H, Farmer DG, French S et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg. 2011;146(6):683-9. https://doi.org/10.1001/archsurg.2011.116.
Jesper D, Heyn SG, Schellhaas B, Pfeifer L, Goertz RS, Zopf S et al. Effects of liver cirrhosis and patient condition on clinical outcomes in intrahepatic cholangiocarcinoma: a retrospective analysis of 156 cases in a single center. Eur J Gastroenterol Hepatol. 2018;30(5):552-6. https://doi.org/10.1097/MEG.0000000000001036.
Mauro E, Ferrer-Fabrega J, Sauri T, Soler A, Cobo A, Burrel M et al. New Challenges in the Management of Cholangiocarcinoma: The Role of Liver Transplantation, Locoregional Therapies, and Systemic Therapy. Cancers (Basel). 2023;15(4). https://doi.org/10.3390/cancers15041244.
Endo Y, Sasaki K, Moazzam Z, Woldesenbet S, Yang J, Lima HA et al. The Impact of a Liver Transplant Program on the Outcomes of Hepatocellular Carcinoma. Ann Surg. 2023. https://doi.org/10.1097/SLA.0000000000005849.
Flemming JA, Muaddi H, Djerboua M, Neves P, Sapisochin G, Selzner N. Association between social determinants of health and rates of liver transplantation in individuals with cirrhosis. Hepatology. 2022;76(4):1079-89. https://doi.org/10.1002/hep.32469.
Chan NW, Moya-Mendez M, Henson JB, Zaribafzadeh H, Sendak MP, Bhavsar NA et al. Social determinants of health data in solid organ transplantation: National data sources and future directions. Am J Transplant. 2022;22(10):2293-301. https://doi.org/10.1111/ajt.17096.
Tovoli F, Guerra P, Iavarone M, Veronese L, Renzulli M, De Lorenzo S et al. Surveillance for Hepatocellular Carcinoma Also Improves Survival of Incidentally Detected Intrahepatic Cholangiocarcinoma Arisen in Liver Cirrhosis. Liver Cancer. 2020;9(6):744-55. https://doi.org/10.1159/000509059.
Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):669-80. https://doi.org/10.1111/hpb.12441.
Quillin RC, 3rd, Wilson GC, Wima K, Hohmann SF, Sutton JM, Shaw JJ et al. Neighborhood level effects of socioeconomic status on liver transplant selection and recipient survival. Clin Gastroenterol Hepatol. 2014;12(11):1934-41. https://doi.org/10.1016/j.cgh.2014.05.020.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL-ILCA Clinical Practice Guidelines on Intrahepatic Cholangiocarcinoma. J Hepatol. 2023. https://doi.org/10.1016/j.jhep.2023.03.010.
Biliary tract cancer. 2023. https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf.
Simoneau E, D'Angelica M, Halazun KJ. Liver transplantation for colorectal liver metastasis. Curr Opin Organ Transplant. 2019;24(2):175-81. https://doi.org/10.1097/MOT.0000000000000623.
Cross-Najafi AA, Lopez K, Isidan A, Park Y, Zhang W, Li P et al. Current Barriers to Clinical Liver Xenotransplantation. Front Immunol. 2022;13:827535. https://doi.org/10.3389/fimmu.2022.827535.
Menahem B, Lubrano J, Duvoux C, Mulliri A, Alves A, Costentin C et al. Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: An attempt to perform an ideal meta-analysis. Liver Transpl. 2017;23(6):836-44. https://doi.org/10.1002/lt.24758.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplementary file 1
Supplementary figure 1: Kaplan-Meier curve for liver transplantation and non-curative intent treatment groups in the era of modern chemotherapies (i.e., 2010-2018) after propensity score overlap weighting adjustment. (PNG 79 kb)
Supplementary file 2
(DOCX 30 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Endo, Y., Sasaki, K., Munir, M.M. et al. Survival Benefit Relative to Treatment Modalities Among Patients with Very Early Intrahepatic Cholangiocarcinoma: an Analysis of the National Cancer Database. J Gastrointest Surg 27, 2103–2113 (2023). https://doi.org/10.1007/s11605-023-05821-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-023-05821-7