Abstract
Intrahepatic cholangiocarcinoma (ICC) is the second commonly-seen liver malignancy and one of the most fatal cancers in Taiwan. Survival after diagnosis of ICC remains poor. This study aimed to investigate the survival and prognostic factors in patients with ICC. All patients with newly diagnosed ICC during 2004 to 2018 were identified from a national cancer database and followed until December 2020. Estimates of overall survival (OS) were conducted using the Kaplan–Meier method and Cox proportional hazards model. Hazard ratios with 95% confidence intervals were calculated. Initially, 7940 patients with ICC disease (stage IV: 55.6%, 4418/7940) were eligible for this study. Only 32.3% (2563/7940) patients with ICC underwent liver resection. After Propensity score matching, 969 pairs (N = 1938) of patients were matched and selected (mean age 62.8 ± 11.0 years, 53.1% were male, 29.7% had cirrhosis). The median follow-up time was 80.0 months (range 25–201 months). The 3-, 5-year OS rates were 44.0%, 36.4% in the surgical group and 26.0%, 23.7% in the non-surgical group, respectively. Surgery, young patients (≤ 54 years), small tumor size, no vascular invasion and chemotherapy were associated with better OS in patients with stages I–III disease. Surgery benefit was maximum in stage I disease followed by stage II. In patients with stage IV disease, factors such as surgery, young patients (≤ 64 years), single tumor, and no vascular invasion were associated with better OS. Chemotherapy was insignificantly associated with better OS. Long-term survival in patients with ICC is very poor. Compared to non-surgical patients, surgery conveys approximately 18% and 12% better OS rates at 3-year and 5-year, respectively. Early detection and surgical intervention may improve OS substantially in patients with ICC.
Similar content being viewed by others
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver malignancy1. It accounts for 10% to 15% of all primary liver malignancies in the United Sates (USA)2 and has a relatively high prevalence in parts of East Asia3. Traditionally, cholangiocarcinoma can be classified into the intrahepatic (5–10%) and the extrahepatic types (80% to 90%, including distal tumors of the common bile duct and perihilar/Klatskin tumor)4,5,6. Although surgery is the mainstay of treatment that offers the possibility for a cure, patients with ICC have an extremely poor prognosis7. Unlike its extrahepatic counterpart, patients with ICC are frequently found to have disease that is beyond the limits of surgical therapy because of multiple intrahepatic metastases, peritoneal carcinomatosis, or extrahepatic metastases at the time of diagnosis1. The outcome after treatment for ICC is far from expectation possibly due to the lack of an effective adjuvant therapy, the aggressive nature of the disease, and the critical location of the tumor in close proximity to vital structures8. The majority of patients have unresectable disease and surgery is effective in only 25% to 30% of patients9.
The effect of adjuvant therapy (chemotherapy/radiotherapy) is inconclusive for patients with ICC who either did or did not undergo surgery10. The probable causes are its poor prognosis and low incidence of ICC, both of which might hinder a conduction of outcomes research. Although the incidence of ICC has significantly increased in many areas, the clinicopathologic features and surgical outcomes of this neoplasm are not fully understood because of the limited number of cases11. Given patients with ICC have a poor prognosis and no adequate treatment options, studies that can clarify their survival and prognostic factors are important and informative. The aim of this study was to investigate long-term survival and to identify the prognostic factors for survival in patients with ICC.
Material and methods
Study population
This study examined patients who were newly diagnosed with ICC from two national databases, the Taiwan Cancer Database (TCDB, 2004 to 2006) and the Taiwan Cancer Registry (TCR, 2007 to 2018). Those who had a diagnosis of liver malignancy (code C22) of International Classification of Diseases for Oncology-3rd edition (ICD-O-3) in these databases during January 2004 to December 2018 were initially identified and their information of demographic, disease specification and treatment profiles were retrieved. National administrative has initiated national cancer registry since 2002 to facilitate comprehensive cancer research. Both data sets are prospective databases that regularly accumulate histopathology, treatment and outcome profiles of cancer patients who have been reported by well-trained registry professionals from accredited hospitals. These databases, endorsed by the government (Health Promotion Administration), cover approximately 85% of the newly-diagnosed liver cancer patients in Taiwan and provide cancer staging, treatment profiles and follow-up status of individual patients12.
Analysis cohort
Patients were excluded from this study for the following criteria: whose age were younger than 25 years or older than 85 years, who had a pathology report that did not indicate ICC (ICD-O-3 histology code 8160 bile duct adenocarcinoma), and who survived less than 3 months.
Independent variables
Related demographic, clinical, pathological and therapeutic data of patients with ICC were extracted from the TCDB and the TCR. Possible prognostic variables included patient characteristics (age, gender, diabetic comorbidity, body mass index, smoking behavior and drinking habit), disease characteristics [tumor size, tumor number, histological grade of cell differentiation, TNM stage, vascular invasion, background hepatitis profile, cirrhosis, preoperative highest bilirubin and alpha-fetoprotein (AFP) level] and therapeutic characteristics [surgery, resection margin, transarterial chemoembolization (TAE), chemo-radiation and nucleoside/nucleotide analogue therapy]. Body mass index (BMI, kg/m2) is derived by dividing bodyweight in kilogram by squared height in meters. Background hepatitis profile (virus-related status) referred to status of no hepatitis B virus (HBV)/hepatitis C virus (HCV) infection, HBV infection, HCV infection, and HBV/HCV co-infection. nucleoside/nucleotide analogue (NA) therapy referred to patients who took at least one of the following drugs as antiviral therapy for chronic HBV infection: lamivudine, entecavir, telbivudine, adefovir dipivoxil, and tenofovir disoproxil13. To obtain details of diabetes, cirrhosis, and antiviral therapy, we needed to connect the TCDB and the TCR databases to a third database, the National Health Insurance Research Database (NHIRD). The NHIRD (from the National Health Insurance Administration) contains claims data of healthcare usage from patients under National Health Insurance (NHI). Because the insurance system in Taiwan is a single-payer system, this database covered healthcare billing services for nearly all citizens. Therefore, data for all beneficiaries could be accurately extracted. Finally, the cohort was divided into the surgery and the no surgery groups, based on initial treatment. Patients without liver resection would be assigned to the no surgery group.
Dependent variables
The primary end point was overall survival (OS), defined as the time from date of initial diagnosis of ICC to date of death from any cause. The secondary end point included cancer-specific survival (CSS, defined as the time from date of initial diagnosis of ICC to date of death from cancer-specific cause), disease-free survival (DFS, or no disease progression, defined as the time from date of initial diagnosis of ICC to date of local recurrence, distant metastasis and any sign of disease progression), and local recurrence. Survival conditions (OS and CSS) were queried from a fourth database, the National Register of Deaths (2004–2020), while the last two (DFS and local recurrence) were obtained from the TCDB and the TCR. The institutional review board at the Taipei City Hospital approved this study and waived informed consent (TCHIRB-11002008-W).
Statistical analysis
Because either assigning to the surgery or the no surgery groups might not be randomized in daily practices (due to general condition, disease severity or doctor’s preference), we employed propensity score matching (PSM) method to decrease this selection bias and make the known prognostic factors balanced14. The individualized propensity score derived from the logistic regression (surgery as dependent variable) was matched via a one-to-one approach (greedy nearest neighbor with caliber matching) and yielded final paired patients who had the closest estimated propensity score within 0.25 standard deviation (SD). Patient-specific demographic, clinical, pathological and therapeutic variables were reported as percentages or means ± SD. Categorical variables were compared by the Chi-square test and continuous variables were compared by Student’s t test to assess the average of a normal distribution. Univariate and multivariate Cox proportional hazards model were fitted using potential prognostic factors. Subgroup multivariate analyses were conducted in stages I-III or stage IV disease. A hazard ratio (HR) with 95% confidence interval (CI) of estimate was reported. Finally, Kaplan–Meier curves were plotted to display the survival of patients in terms of OS, CSS, DFS, and local recurrence and the log-rank tests were used to compare across groups. We used SAS 9.4 software (SAS Institute Inc., Cary, North Carolina) for the initial database merging process and SPSS Statistics 21 software for data management and statistical analyses. All P values were two-sided and the significance level was specified as P < 0.05.
Results
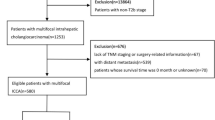
In the beginning, we identified 147,384 patients who were newly diagnosed with liver cancer from January 1, 2004 to December 31, 2018. Only 7.6% of these patients (N = 11,251) had been newly diagnosed with ICC. After exclusion for patients who had not been followed up for at least 3 months (N = 3022), whose age were not between 25 and 85 years (N = 251) and who had unspecified tumor stage (N = 38), a total of 7,940 patients with ICC (mean age 63.8 ± 11.2 years) met the inclusion criteria and were selected (Fig. 1). After PSM algorithm (1:1), final 969 pairs (N = 1938) of patients were matched according to initial surgery or not, and were eligible for this study. The comparison of demographic, clinical and therapeutic characteristics between before matched patients (N = 7940) and after matched patients (969 pairs, N = 1938) are summarized in Table 1. Before PSM, male patients represented 55.8% (4428/7940) of the cohort. A higher incidence of ICC occurred in patients with ages between 65 and 74 years old (mode: 69 years). Only 32.3% (2,563/7,940) of patients had undergone surgery as initial treatment for ICC. Patients with a tumor size measured between 5 and 10 cm (2719/7940, 34.2%) outnumbered those with a tumor size measured less than 5 cm (2410/7940, 30.4%). Most patients had multiple tumors (54.7%, 2891/5281) and known vascular invasion (58.1%, 3881/6685). The majority of patients were diagnosed as stage IV disease (55.6%, 4418/7940), followed by stage III (16.2%, 1283/7940), then by stage I (15.4%, 1221/7940). For those patients who underwent curative intent surgery, pathology showed that 27.4% (240/876) of patients had a safety resection margin of 0.2 cm or less. Underlying disease of chronic infection with HBV and HCV were found in 9.9% (787/7940) and 4.6% (366/7940) of the cohort, respectively. Cirrhosis and diabetes mellitus occurred in 28.1% (2231/7940) and 21.4% (1702/7940) of all cohort. The percentages of patients who received chemotherapy or radiotherapy were 40.3% (3203/7940) and 16.9% (1341/7940), respectively. A total of 1042 patients (13.1%, 1042/7940)) took NAs as an antiviral therapy for chronic HBV infection. Around nineteen percent (18.7% 1485/7940) and 15.5% (1230/7940) of the cohort had history of habitus of smoking and alcohol, respectively. For those whose data were available, 37.9% (1640/4324) of patients had BMI greater than 25 kg/m2. The median follow-up time was 80.8 months (range from 24 to 203 months). The overall mortality rates at the end of study were 69.6% (5530/7940) and 71% (1376/1938) for the unmatched and the matched cohorts, respectively (Table 2). The rates of stable disease at the end of study were 15.3% (1215/7940) and 17.5% (339/1938) for the unmatched and the matched cohorts, respectively.
Before PSM, many baseline characteristics between the two groups (surgery or not) were significantly different. Patients with cirrhosis, older age, higher grade, BMI and higher tumor stage had a lower probability of undergoing surgery (Table 1, left columns). After PSM (based on gender), most baseline characteristics such as age, grade, cirrhosis, BMI and bilirubin level were indifferent between the two groups (Table 1, right columns). Figure 2 shows the surgery group was associated with better OS and CSS than the no surgery group (all P values < 0.001). The OS rates at 1-, 3-, 5-year in the surgery and the no surgery groups (matched cohort) were 72.4%, 44.0%, 36.4% and 48.3%, 26.0%, 23.7%, respectively. However, only the surgery (liver resection) group of the unmatched cohort had median CSS longer than 9 years. Figure 3 shows the difference between the surgery and the no surgery groups regarding disease progression and local recurrence (left: unmatched cohort; right: matched cohort). Curves ran smoothly about 2 years after initial diagnosis. The surgery group was associated with less disease progression and local recurrence than the no surgery group (all P values < 0.001). The magnitude of difference decreased between the two groups in the matched cohort. Figure 4 shows the difference between the surgery and the no surgery groups regarding OS according to tumor stages in the matched cohort. The surgery group was associated with improved OS, in which patients with stage I disease had the best outcome. Favorable 3-, 5-, and 8-year OS rates (77.0%, 70.7%, and 59.8%, respectively) were noted in patients with stage I disease of the surgery group (matched cohort). However, in patients with stages III and IV disease, the differences between the surgery and the no surgery groups were smaller than that for the stage I. The surgery advantage seemed poor in patients with stages III and IV disease except that it was slightly better for patients with stage III 1 to 3 years after initial diagnosis.
Overall survival (OS; upper left: unmatched cohort; upper right: matched cohort) and cancer-specific survival (CSS; lower left: unmatched cohort; lower right: matched cohort). The OS rates at 1-, 3-, 5-year in the surgery and the no surgery groups were 72.4%, 44.0%, 36.4% and 48.3%, 26.0%, 23.7%, respectively (matched cohort). Solid line: surgery group; dotted line: no surgery group.
Overall survivals (matched cohort) by stages (upper left: stage I; upper right: stage II; lower left: stage III; lower right: stage IV). For those patients with stage I disease of the surgery group (matched group), favorable 3-, 5-, and 8-year overall survival rates (77.0%, 70.7%, and 59.8%, respectively) were noted. Solid line: surgery group; dotted line: no surgery group.
In multivariate Cox proportional hazards model (Table 2), the surgery group was significantly associated with better OS than the no surgery group in both the unmatched (HR 0.65, 95% CI 0.60–0.71, P < 0.001) and the matched cohorts (HR 0.69, 95% CI 0.62–0.78, P < 0.001), respectively. The surgery group was significantly associated with better DFS and less local recurrence than those of the no surgery group in both the unmatched and the matched cohorts, either by univariate or multivariate Cox proportional hazards models.
Table 3 summarizes subgroup analysis of patients with stages I–III disease in the matched cohort. Surgery, age of diagnosis, tumor size, preoperative highest AFP level, vascular invasion and chemotherapy were associated with OS. Surgery, age of diagnosis, tumor size, preoperative highest AFP level, vascular invasion, diabetes and chemotherapy were associated with CSS. Patients who had received chemotherapy had better OS (HR 0.39, 95% CI 0.23–0.65, P < 0.001) and CSS (HR 0.35, 95% CI 0.18–0.67, P = 0.002), respectively. Surgery, lower tumor grade (well/moderate differentiated vs. poorly differentiated, HR 0.76, 95% CI 0.64–0.91, P = 0.003), tumor size, preoperative highest AFP level and vascular invasion were associated with prognosis of DFS. Surgery, tumor grade (well/moderate differentiated vs. poorly differentiated, HR 0.78, 95% CI 0.64–0.94, P = 0.010), tumor size, preoperative highest AFP level, tumor number (multiple vs. single, HR 1.75, 95% CI 1.05–2.94, P = 0.032) and vascular invasion were associated with local recurrence. Results of subgroup analysis of patients with stage IV disease are displayed in Table 4. Surgery, age of diagnosis, tumor number and vascular invasion were associated with OS. Surgery, age of diagnosis, tumor number, vascular invasion and BMI (≥ 30.0 kg/m2 vs. < 30.0 kg/m2, HR 1.42, 95% CI 1.06–1.91, P = 0.019) were associated with CSS. Surgery, tumor size, preoperative highest bilirubin level, preoperative highest AFP level and vascular invasion were associated with DFS and local recurrence. Chemotherapy was not associated with improved OS, CSS, DFS and local recurrence in patients with stage IV disease. Radiotherapy was associated with insignificant smaller HR (0.47, 95% CI 0.21–1.08, P = 0.076) in CSS.
Discussion
Long-term outcomes for patients with ICC were poor as observed in the current study. Intrahepatic cholangiocarcinoma accounted for less than 8% of primary liver malignancy, and only 32.3% of patients with ICC could initially be managed with liver resection. Most patients (55.6%) presented with stage IV disease when first diagnosed. About 72% of patients with ICC were found to have severe (stage III or IV) disease initially. This condition inherently contributed to the poor prognosis of patients with ICC. Surgery was associated with better outcomes for patients with all stages, though surgical benefit attenuated as stage aggravated. Chemotherapy might be associated with improved prognosis only in patients with disease of stage III or less. The use of chemotherapy for patients with stage IV did not have significant improvement of OS and CSS. The use of radiotherapy was not associated with improved survival either in patients with stages III or IV disease. Other prognostic factors related to OS included age, perioperative AFP level and vascular invasion.
The incidence of ICC in our study (7.6%) was comparable to other large-scale studies. In Japan, Kudo et al. reported that ICC accounts for 4.4% of primary liver cancer in a population-based study15. In the USA, ICC accounts for 10–15% of primary liver cancer16. Although incidence of cholangiocarcinoma continues to increase, it increases with greater rate than its counterpart, extrahepatic cholangiocarcinoma in the USA17. Compared to 19.1% of our cohort (unmatched cohort, 457/2389), the Japanese reported a smaller positive margin existed in 13.4% of patients undergoing liver resection for ICC15. A surgical margin of less than 0.2 cm was noted in 27.4% of our cohort which was higher than that reported by Ikai et al. as 18.4% in 434 Japanese patients with ICC who underwent partial hepatectomy18. Liver resection and negative surgical margin involved might provide the best opportunity to improve survival in patients with ICC. Yet, Spolverato et al. investigated 584 patients with ICC who underwent surgery with curative intent at multiple centers and found that the overall probability of cure for liver resection was approximately 10%19. They reported 1-, 3-, and 5-year OS rates were 75%, 37%, and 22%, respectively, which were comparable to our results of 72.4%, 44.0%, and 36.4%, respectively (matched cohort). In our study (unmatched cohort), 32.3% (2563/7940) of patients underwent liver resection for treatment of ICC. The figure was also higher than 20.8% (portion of surgery group) from the Surveillance, Epidemiology and End Results (SEER) database20.
Our study also found several prognostic factors in patients with ICC. Prognostic factors for improved OS included surgery, younger age, smaller tumor size, no vascular invasion, while factors relating less DFS and more local recurrence included poor cell differentiation and simultaneous multiple tumors. But in the current study, we failed to prove that gender, cell grade and radiotherapy were associated with OS as those in the multivariate analysis of SEER study20. For prognostic factors, Ali et al. investigated patients with ICC who underwent surgery at a single-center in the USA and showed significantly worse survival in those with microvascular invasion, large tumor size, higher grade disease, multiple tumors and positive lymph nodes, while a negative resection margin was associated with better survival21. Similarly, a study from Uenishi et al. showed that tumor size greater than 5 cm, multifocality, nodal metastases, and HCV infection were predictors of poor prognosis in Japanese patients who underwent curative resection for mass-forming ICC22. Again, our results failed to show that HCV infection was a poor prognostic factor in patients with ICC who underwent liver resection. However, there was only 4.6% of the unmatched cohort of the current study had HCV infection. Another study using an international Eastern and Western multicenter dataset showed that older age at diagnosis, tumor size, multiple tumors, cirrhosis, lymph node metastasis, and macrovascular invasion were factors predicting survival23. In subgroup analysis, Spolverato et al. investigated patients with large or multifocal ICC and revealed that prognostic factors associated with a worse OS included more than three tumor nodules, nodal metastasis, and poor differentiation24.
The prognostic significance of chronic hepatitis virus infection for outcomes in patients with ICC has seldom been investigated due to the small number of patients. Recently, an increasing number of studies have shown that viral hepatitis B and C are statistically related to ICC3. The result of our prognostic significance of HBV for surgical patients with ICC was consistent with studies of Zhou et al., who examined the prognosis of ICC after liver resection in regard to chronic HBV infection and showed HBV infection was a favorable prognostic factor25,26. They suggested HBV-associated ICC might have a better survival rate than those without HBV infection. Another study showed that patients with antiviral therapy had lower 5-year cumulative incidence of ICC than those without antiviral therapy and suggested that antiviral therapy with NA might contribute to preventing HBV-associated ICC27. While there is still no robust evidence indicating that antiviral therapy plays an important role in the prognosis of patients with ICC, our current data did show that patients with stages I-III disease without virus infection history had poor CSS (HR 1.37, 95% CI 1.01–1.86, P = 0.045) than those with HBV infection history.
In Europe, Bektas et al. advocated that surgical resection was still the best treatment option for patients with ICC in regard to long-term survival whereas adjuvant chemotherapy did not significantly improve survival28. In a population-based survey in the USA that included 2,751 patients with ICC who underwent surgery, Miura et al. showed that the use of chemotherapy was associated with a survival benefit only for patients with nodal metastasis, advanced tumor stage, or an inadequate surgical resection, but that patients with N0 disease (i.e., node-negative) derived no benefit from chemotherapy29. The effectiveness of adjuvant therapy for patients with ICC remained inconclusive. As the current study showed, in addition to surgery that was associated with improved OS, chemotherapy was associated with improved outcomes in the stages I-III, but not in the stage IV. Though the benefit of radiotherapy in our study was insignificant, it probably could be reserved as a modality to patients who could not be a candidate for surgery. Recently, a research using National Cancer Data Base from the USA has proposed that ablative liver radiotherapy could be considered for unresected ICC30. A prior expert consensus meeting sponsored by the American Hepato-Pancreato-Biliary Association recommended that patients with high-risk features (lymphovascular invasion, multicentricity or satellitosis, large tumors) should be encouraged to consider adjuvant therapy31. An investigation of 4,595 patients with ICC disease from the USA showed chemotherapy and radiotherapy were associated with better survival in patients without surgery20. Furthermore, combined adjuvant therapy might be applied for certain cases. As the potential radiosensitization effect of 5-fluorouracil, the combination of adjunctive radiotherapy and chemotherapy should theoretically be more effective than either method alone7. Our results did not show similar results probably due to unselected target patients.
We acknowledge that our study has several limitations. First of all, the treatment option depends on surgeons’ discretion and consideration of tumor aggressiveness. To assess whether patients with ICC are suitable for surgery usually depends on three domains including physiologic, anatomic and biologic aspects, sometimes along with surgeons’ discretion32. Thus, it may have impact on the patients’ outcomes. Although we conducted PSM analysis, the treatment algorithm could not be totally balanced. The PSM algorithm cannot overcome any bias that is caused by unmeasurable confounders. Second, because the cancer registry did not request hospital officials to record data of carcinoembryonic antigen (CEA) in patients with liver cancer, we could not control preoperative CEA in the survival analysis. Third, interventions beside surgery were heterogeneous between the surgery and non-surgery groups. In addition, the members of our study cohort had been treated in at least 37 different hospitals or medical centers, where treatment algorithm (upfront surgery or not) and follow-up schemes might not be identical. This somewhat might have an influence on outcomes in patients with ICC. However, the results of the current study represent the treatment effectiveness that is currently available for patients with ICC. Finally, the current study included patients with stage IV disease, which had very different outcomes from those of other stages. But the large percentage of stage IV disease in patients with ICC makes research of such patients cannot be overlooked. Unfortunately, we did not find any modifiable factors that could be employed to significantly improve prognosis in patients with stage IV disease. Modern medicine seemed not improve greatly on the survival of patients with stage IV ICC disease.
In conclusion, the current study investigated the long-term survival of patients with ICC in a national database level and showed poor prognosis for patients with ICC. The dilemma of diagnosis of ICC in the late stage might incur difficulty in treatment and therefore contributed to ominous outcome. Surgery conveyed better improvement for prognosis than those who did not undertake surgery as initial treatment in patients with stage I disease. However, the surgical advantage decreased tremendously in patients with stage II or later stages. In patients with stages III or IV disease, the discernible benefit of surgery confined to a relatively limited improvement of OS. Only those who were diagnosed with stage I disease and underwent upfront surgery had median survival time more than eight years after initial diagnosis. Early detection of ICC and quick surgery, therefore, even in this era of marked improvement of adjuvant chemotherapy and radiation, remain good solutions to combat the poor prognosis of ICC in modern medicine. The long-term survival of patients with ICC is very poor. Even in the surgery group (all stages), the 5- and 8-year OS rates for patients with ICC were only 36.4% and 32.5%, respectively. Prognostic factors included age, surgery, primary tumor size, multiple tumors, vascular invasion, and negative surgical margin. Chemotherapy was associated with improved survival in patients with stages I-III disease, but not with stage IV disease. Hepatitis virus profile and radiotherapy did not seem to affect patients’ long-term outcomes significantly. Based on the fact of dominantly late stage and poor prognosis, early detection and appropriate treatment (surgery or chemotherapy) will increase survival in patients with ICC. Future study may seek to find effective adjuvant therapy (included novel chemotherapy agents and immunotherapy) in patients with stage IV disease.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ICC:
-
Intrahepatic cholangiocarcinoma
- TCDB:
-
Taiwan Cancer Database
- TCR:
-
Taiwan Cancer Registry
- ICD-O-3:
-
International Classification of Diseases for Oncology-3rd edition
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- NHIRD:
-
National Health Insurance Research Database
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Guglielmi, A. et al. Intrahepatic cholangiocarcinoma: Prognostic factors after surgical resection. World J Surg. 33, 1247–1254. https://doi.org/10.1007/s00268-009-9970-0 (2009).
Shaib, Y. H. et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: A case-control study. Gastroenterology. 128, 620–626. https://doi.org/10.1053/j.gastro.2004.12.048 (2005).
Wu, Z. F. et al. Prognosis after resection for hepatitis B virus-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 21, 935–943. https://doi.org/10.3748/wjg.v21.i3.935 (2015).
Cardinale, V. et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol. 2, 407–416. https://doi.org/10.4251/wjgo.v2.i11.407 (2010).
Suarez-Munoz, M. A. et al. Risk factors and classifications of hilar cholangiocarcinoma. World J Gastrointest Oncol. 5, 132–138. https://doi.org/10.4251/wjgo.v5.i7.132 (2013).
Blechacz, B. R. & Gores, G. J. Cholangiocarcinoma. Clin Liver Dis. 12, 131–150. https://doi.org/10.1016/j.cld.2007.11.003 (2008).
Anderson, C. D., Pinson, C. W., Berlin, J. & Chari, R. S. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 9, 43–57. https://doi.org/10.1634/theoncologist.9-1-43 (2004).
Hong, J. C. et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg. 146, 683–689. https://doi.org/10.1001/archsurg.2011.116 (2011).
Singh, M. K. & Facciuto, M. E. Current management of cholangiocarcinoma. Mt Sinai J Med. 79, 232–245. https://doi.org/10.1002/msj.21298 (2012).
Aljiffry, M. et al. Evidence-based approach to cholangiocarcinoma: A systematic review of the current literature. J Am Coll Surg. 208, 134–147. https://doi.org/10.1016/j.jamcollsurg.2008.09.007 (2009).
Nakagohri, T. et al. Surgical outcome and prognostic factors in intrahepatic cholangiocarcinoma. World J Surg. 32, 2675–2680. https://doi.org/10.1007/s00268-008-9778-3 (2008).
Chang, Y. J., Chung, K. P., Chang, Y. J. & Chen, L. J. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg. 103, 1513–1520. https://doi.org/10.1002/bjs.10196 (2016).
Wu, C. Y. et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 308, 1906–1914. https://doi.org/10.1001/2012.jama.11975 (2012).
Chang, Y. J. et al. Application of propensity score model to examine the prognostic significance of lymph node number as a care quality indicator. Surg Oncol. 21, e75-85. https://doi.org/10.1016/j.suronc.2011.12.003 (2012).
Kudo, M. et al. Report of the 19th follow-up survey of primary liver cancer in Japan. Hepatol Res. 46, 372–390. https://doi.org/10.1111/hepr.12697 (2016).
Lubezky, N. et al. Surgical treatment of intrahepatic cholangiocarcinoma in the USA. J Hepatobiliary Pancreat Sci. 22, 124–130. https://doi.org/10.1002/jhbp.157 (2014).
Javle, M. et al. Temporal changes in cholangiocarcinoma incidence and mortality in the United States from 2001 to 2017. Oncologist. 27, 874–883. https://doi.org/10.1093/oncolo/oyac150 (2022).
Ikai, I. et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res. 40, 1043–1059. https://doi.org/10.1111/j.1872-034X.2010.00731.x (2010).
Spolverato, G. et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma?. Cancer. 121, 3998–4006. https://doi.org/10.1002/cncr.29619 (2015).
Yu, T. H. et al. Clinicopathological characteristics and prognostic factors for intrahepatic cholangiocarcinoma: A population-based study. Sci Rep. 11, 3990. https://doi.org/10.1038/s41598-021-83149-5 (2021).
Ali, S. M. et al. Model to predict survival after surgical resection of intrahepatic cholangiocarcinoma: The Mayo Clinic experience. HPB (Oxford). 17, 244–250. https://doi.org/10.1111/hpb.12333 (2015).
Uenishi, T. et al. The long-term outcomes after curative resection for mass-forming intrahepatic cholangiocarcinoma associated with hepatitis C viral infection: A multicenter analysis by Osaka Hepatic Surgery Study Group. J Surg Oncol. 110, 176–181. https://doi.org/10.1002/jso.23611 (2014).
Hyder, O. et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: An Eastern and Western experience. JAMA Surg. 149, 432–438. https://doi.org/10.1001/jamasurg.2013.5168 (2014).
Spolverato, G. et al. Is hepatic resection for large or multifocal intrahepatic cholangiocarcinoma justified? Results from a multi-institutional collaboration. Ann Surg Oncol. 22, 2218–2225. https://doi.org/10.1245/s10434-014-4223-3 (2014).
Zhou, H. B. et al. Hepatitis B virus infection: A favorable prognostic factor for intrahepatic cholangiocarcinoma after resection. World J Gastroenterol. 17, 1292–1303. https://doi.org/10.3748/wjg.v17.i10.1292 (2011).
Zhou, H. B., Hu, J. Y. & Hu, H. P. Hepatitis B virus infection and intrahepatic cholangiocarcinoma. World J Gastroenterol. 20, 5721–5729. https://doi.org/10.3748/wjg.v20.i19.5721 (2014).
Lee, T. Y. et al. Effect of nucleos(t)ide analogue therapy on risk of intrahepatic cholangiocarcinoma in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 16, 947–954. https://doi.org/10.1016/j.cgh.2017.09.031 (2018).
Bektas, H. et al. Surgical treatment for intrahepatic cholangiocarcinoma in Europe: A single center experience. J Hepatobiliary Pancreat Sci. 22, 131–137. https://doi.org/10.1002/jhbp.158 (2014).
Miura, J. T. et al. Chemotherapy for surgically resected intrahepatic cholangiocarcinoma. Ann Surg Oncol. 22, 3716–3723. https://doi.org/10.1245/s10434-015-4501-8 (2015).
De, B. et al. Ablative liver radiotherapy for unresected intrahepatic cholangiocarcinoma: Patterns of care and survival in the United States. Cancer. 128, 2529–2539. https://doi.org/10.1002/cncr.34223 (2022).
Weber, S. M. et al. Intrahepatic cholangiocarcinoma: Expert consensus statement. HPB (Oxford). 17, 669–680. https://doi.org/10.1111/hpb.12441 (2015).
Beal, E. W., Cloyd, J. M. & Pawlik, T. M. Surgical treatment of intrahepatic cholangiocarcinoma: Current and emerging principles. J Clin Med. 10, 104. https://doi.org/10.3390/jcm10010104 (2020).
Acknowledgements
We gratefully thank the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan, for providing the data for analyses.
Funding
The study was supported by Taipei City Hospital, Taipei (TPCH-111-22). The funding agency had no role in study design, data collection and analysis, publication decision, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conception/design: Yun-Jau Chang, Yao-Jen Chang, Provision of study material or patients: Yun-Jau Chang Collection and/or assembly of data: Yun-Jau Chang, Li-Ju Chen Provision data analysis and interpretation: Yun-Jau Chang, Li-Ju Chen Manuscript writing: Yun-Jau Chang, Li-Ju Chen Final approval of manuscript: Yun-Jau Chang, Yao-Jen Chang, Li-Ju Chen.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chang, YJ., Chang, YJ. & Chen, LJ. Prognostic factors in patients with intrahepatic cholangiocarcinoma. Sci Rep 14, 19084 (2024). https://doi.org/10.1038/s41598-024-70124-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70124-z
- Springer Nature Limited