Abstract
Background
The aim of this study was to define whether procalcitonin (PCT) is an earlier and more accurate predictor than C-reactive protein (CRP) for anastomotic leakage (AL) and major infective complications (MICs).
Methods
This was a prospective multicentric observational study conducted in three Italian centers, including all patients undergoing gastrectomy from May 2016 to April 2021. The endpoint was the assessment of the discrimination and accuracy achieved by the PCT and CRP values measured from POD1 to POD7 for predicting the occurrence of AL and MICs. Accuracy was assessed by calculating the area under the receiver operating curve (AUROC) values and Youden’s statistics. Two charts were created for risk stratification during the postoperative course.
Results
The rate of AL was 4.6%, with a median day of occurrence on POD5 (range 3–26). The overall rate of major infective complications was 19.9%, with a median day of occurrence on POD6 (range 2–30). PCT showed a significant association with AL on POD6 and POD7 and a significant association with MICs on POD2, while CRP values showed a significant association with AL on POD4 and a significant association with MICs on POD1. No difference in the prediction of AL was observed between PCT and CRP, while CRP was found to be a superior predictor of major infective complications on POD5 (p = 0.024) and POD7 (p = 0.035).
Conclusions
PCT was not superior to CRP as an early predictor of AL and major infective complications after gastrectomy. CRP should be used as the reference screening postoperative marker.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anastomotic leakage (AL) is one of the most serious complications after gastric surgery, and it has a severe impact on the postoperative course as well as on functional and oncologic outcomes.1 AL occurs in 2 to 14% of patients undergoing gastrectomy, and the median time for occurrence is 7 days after surgery.2,3 The morbidity and mortality rates of this complication are reported to be as high as 50%, especially when leakage occurs in the thorax.4 An early diagnosis of AL is considered critical to prevent further complications and minimize the high mortality associated with this event. Therefore, an early diagnostic marker of AL would be a valuable instrument to support the postoperative management of patients undergoing gastric surgery. Moreover, given the recent diffusion of the enhanced recovery protocols in esophagogastric surgery, which are aimed at improving patient outcomes and reducing hospital stay,5,6 an accurate and early diagnostic marker of AL could be employed as an adjunctive criterion to determine the safety and feasibility of early discharge.
Biomarkers such as C-reactive protein (CRP) have already been investigated in a few preliminary studies that revealed high sensitivity for the detection of AL as early as postoperative day 2.7,8 Procalcitonin (PCT) is produced by neuroendocrine cells of the thyroid and lungs as the precursor for the hormone calcitonin. It circulates at very low concentrations in the serum of healthy subjects. However, when severe inflammation and infectious complications occur, the serum levels of PCT usually increase faster than CRP, reaching values ranging from tens of times higher to thousands of times higher than normal levels.9 A 2016 study conducted on patients undergoing colorectal surgery identified PCT as an effective biomarker for the early detection of AL.10 With regard to upper GI surgery, some studies have suggested that elevated PCT levels could precede combined surgical/infectious intrabdominal complications after esophagectomy or gastrectomy in a timely manner,11,12 while other studies have regarded PCT as an early predictor of septic complications after sleeve gastrectomy.13 Nevertheless, the use of PCT as an early laboratory marker of anastomotic leakage has not yet been systematically analyzed or validated for use after gastrectomy.
The aim of this study was to determine whether PCT is an earlier and more accurate predictor of AL than CRP after gastric resection for cancer. Moreover, we explored the roles of PCT and CRP in predicting major infective complications (MICs) and conducted an exploratory analysis to enhance the clinical applicability of the results.
Methods
Study Design and Population
This was a prospective multicentric observational study. The participating centers were the Fondazione Policlinico Universitario A. Gemelli IRCCS, the IRCCS Humanitas Research Hospital, and the Istituto Europeo di Oncologia IRCCS. The study was approved by the IRBs of the participating institutions. The study was initially designed to include both an esophagectomy and a gastrectomy arm; however, due to low recruitment in the esophagectomy arm at the end of the study (n = 35 patients), only the results for the gastrectomy arm are reported herein. The study included all patients > 18 years undergoing gastrectomy in the elective setting from May 2016 to April 2021. The following patients were excluded: patients undergoing surgery without an anastomosis being performed (i.e., wedge resections); patients with ongoing infection or systemic inflammation at the time of surgery (bacterial, viral, parasitic infection, or systemic inflammation due to inhalational injury; pulmonary aspiration; pancreatitis; mesenteric infarction or heat stroke); patients with trauma other than surgery (burns, mechanical injuries); patients undergoing emergency procedures; and those with an ASA score > 3.
Data Collection
For all the patients included in his study, the following data were prospectively collected: age, sex, sex performance status, comorbidities according to the Charlson comorbidity index (CCI),17 ASA score, preoperative oncologic treatment, type of operation, surgical approach (open or minimally invasive), extent of lymphadenectomy, tumor location, tumor stage, postoperative complications (within 30 days after surgery or during the same hospitalization) classified according to the Clavien–Dindo classification, length of postoperative stay and 30-day unplanned readmission. In all cases, the white blood cell count (WBC), PCT, and CRP levels were measured before surgery and during the first seven postoperative days (PODs).
Surgical Technique and Postoperative Management
Total gastrectomy was performed with Roux-en-Y reconstruction and circular stapled anastomosis. Subtotal gastrectomy was performed with Roux-en-Y or Billroth 2 reconstruction and manual or linear mechanic stapled anastomosis. One abdominal drain was placed routinely for subtotal gastrectomy, and two abdominal drains were placed routinely for total gastrectomy. Blood samples were obtained on postoperative days 1 to 7. Contrast studies were planned according to the surgeon’s preference. The postoperative management consisted of mobilization and removal of the urinary catheter on POD1. Patients undergoing subtotal gastrectomy started water intake on POD3, and the diet composition was progressively increased from liquid to soft diet. For patients undergoing subtotal gastrectomy, abdominal drainage tubes were removed on POD4 if the drainage fluid was clear. For patients undergoing total gastrectomy, drainages were removed after the contrast study. Patients were discharged under the decision of the attending surgeon after diet tolerance had been assessed and in the absence of other complications. After 1 week, they attended an outpatient clinic appointment and were then followed via telephone.
PCT and CRP Measurement
Serum PCT and CRP levels were measured using endpoint nephelometry with an autoanalyzer UniCel® DxC 800 (Beckman Coulter, Brea, CA, USA), and the normal range was 0–5 mg/L.
Definition of AL and Other Complications
AL was defined as a full thickness gastrointestinal defect involving the esophagus or esophagojejunal anastomosis, the staple lines, or the gastric stump irrespective of presentation or method of identification.14 AL and other postoperative complications were classified according to the Clavien–Dindo classification. We considered major complications those needing reoperation or interventional radiology procedures (Clavien–Dindo grades III to V).
Endpoints
The primary endpoint of this study was the assessment of the discrimination and accuracy achieved by the PCT and CRP values measured on every POD (from 1st to 7th) to determine the occurrence of anastomotic leakage and the comparison among the predictive values of these two biomarkers. The secondary endpoint was to assess the discrimination and accuracy achieved by the PCT and CRP values for predicting a composite major infective surgical complication outcome (anastomotic leak + duodenal leak + intrabdominal collections).
Statistics
Data Analysis
Values were recorded as absolute values and percentages, means and standard deviations and medians and ranges, as appropriate. Regarding the clinicopathologic characteristics, patients were divided into three groups: patients who developed AL (with or without other complications), patients who developed other complications, and patients who had neither AL nor other complications. For the statistical analysis, patients who developed AL (with or without other complications) or major infective complications and patients who did not develop AL or other major infective complications were considered.
Discrimination was assessed with the area under the curve (AUC) of receiver operating characteristic (ROC) curves, and accuracy was assessed with the creation of calibration plots. The p value was calculated with De Long’s method. The cutoff values of PCT, CRP and WBC were calculated by Youden’s J statistics and employed to calculate sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV). Comparison of the ROC curves was performed with De Long’s method.
A post hoc analysis was designed to enhance the clinical applicability of the results. Based on the mean and 95% CI (confidence interval) for the values of CRP from POD1 to POD7 in patients with AL and major infective complications, two charts were created for risk stratification during the postoperative course (Figs. 1 and 2). Moreover, an exploratory multivariable backward logistic regression for variables associated with major infective complications among the CRP and WBC values from POD2 to POD5 and from POD2 to POD4 was used to select an optimal laboratory panel for the early prediction of MICs. Based on the selected variables, a neural network multilayer perception analysis (one hidden layer, 70%/30% training/testing set ratio) was used to evaluate the performance of the composite models using ROC curves for the composite model, a predicted-by-observed-value chart for each dependent variable, and an independent variable performance analysis. The advantage of the neural network analysis in this case was the ability to control for possible collinearity (e.g., the laboratory values during different PODs) and to rate the importance of each predictor variables after detecting all their possible interactions.15
Performance of the composite model including CRP based on a neural network multilayer perception analysis. The AUC was 0.842 for the first model based on Tables 4 and 5 and 0.772 for the second based on Table 5. The graph shows the pseudoprobability of being assigned to a certain cathegory: a in the leftmost boxplot, for cases in the observed category No, the predicted pseudoprobability of category No according to the model; b in the next boxplot to the right, for cases in the observed category No, the predicted pseudoprobability of category Yes; c in the third boxplot, for cases in the observed category Yes, the predicted pseudoprobability of category No; d the last boxplot shows, for cases in the observed category Yes, the predicted pseudoprobability of category Yes. In both figures, at the bottom right, a bar graph shows the normalized importance of the variable within the model. The distance of the pseudoprobabilities from 0.5 for both models further confirms the good performance of the model
The analysis was performed using SPSS v.22 for Windows XP (SPSS, Chicago, IL, USA) and MedCalc statistical software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018). All frequentist statistical tests were two‐sided with significance set at P ≤ 0.05.
Results
Population of the Study
A total of 282 patients undergoing gastrectomy were recruited. The clinicopathologic characteristics of these patients are presented in Table 1. The overall rates of morbidity, major morbidity and mortality were 47.9%, 22.7%, and 3.2%, respectively. The rate of AL was 4.6%. The median day of AL diagnosis was POD5 (range 3–26), 92.9% were major complications, and the mortality rate was 28.6%. The rate of duodenal leak/paraduodenal abscess was 10.6%. The median day of diagnosis was POD8 (range 3–30), 83.3% of complications were major complications, and the mortality rate was 9.7%. The overall rate of major infective complications was 19.9%, the median day of diagnosis was POD6 (range 2–30), and the mortality rate was 12.7%.
Performance of PCT and CRP
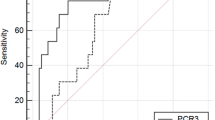
For the detection of AL, a significant discrimination was identified from POD4 for CRP (AUC 0.709, CI95% 0.647–0.765, cut-off 156 mg/ml, p = 0.015) and from POD6 for PCT (AUC 0.676, 0.603–0.743, cut-off 0.13 mg/ml, p = 0.039) (Table 2) with the better discrimination achieved in POD7 for both CRP (AUC 0.772, CI95% 0.697–0.836, cut-off 94, Se 100%, Sp 54%, PPV 10%, NPV 99%, p < 0.001), and PCT (AUC 0.763, CI95% 0.684–0.831, cut-off 0.4, Se 67%, Sp 79%, PPV 18%, NPV 97%, p = 0.002). The results for the ROC curve analysis for WBC showed a significant and better discrimination from POD5 (AUC 0.705, CI95% 0.644–0.761, cut-off 10.6 × 109, Se 54%, Sp 88%, PPV 20%, NPV 97%, p = 0.014) (Supplementary Materials).
For the detection of major infective complications, a significant discrimination was identified from POD1 for CRP (AUC 650, CI95% 0.577–0.719, cut-off 68 mg/ml, p = 0.005) and from POD2 for PCT (AUC 0.643, CI95% 0.577–0.705, cut-off 0.18 mg/ml, p = 0.002) with the better discrimination achieved in POD5 for CRP (AUC 0.780, CI95% 0.719–0.833, cut-off 117 mg/ml, Se 80%, Sp 67%, PPV 38%, NPV 92%) and in POD6 for PCT (AUC 0.708, CI 95% 0.637–0.772, cut-off 0.09 mg/ml, Se 95%, Sp 43%, PPV 31%, NPV 97%) (Table 3). The results for the ROC curve analysis for WBC showed a significant discrimination from POD3 (AUC 0.570, CI95% 0.508–0.630, cut-off 12.4 × 109, p = 0.036) with the better discrimination achieved in POD6 (AUC 0.735, CI95% 0.668–0.795, cut-off 8.7 × 109, Se 64, Sp 76, PPV 47, NPV 86, p < 0.001) (Supplementary Materials).
The trend of CRP, PCT, and WBC based on the postoperative day is depicted in Fig. 1s.
Comparison Among ROC Curves for AL and MICs
In the comparison among the AUCs, no difference in the prediction of AL was observed between PCT and CRP, while CRP was superior in predicting major infective complications on POD5 (p = 0.024) and POD7 (p = 0.035).
Development of Charts for Risk Stratification for AL and Mics and of Models for the Prediction of Major Infective Complications After Gastrectomy
The two charts for risk stratification of AL and MICs during the postoperative course are shown in Fig. 1.
The results of the multivariable analysis for AL selected CRP in POD6 as the only independent variable predictive of AL (Supplementary Materials). The results of the multivariable logistic regression for MICs selected CRP on POD2 and POD5; CCI and age as independently associated with the risk of major complications when considering biomarkers available from POD1 to POD5; and CRP on POD3 and CCI when considering biomarkers available from POD1 to POD4 (Tables 4 and 5). The subsequent ANN analysis (Fig. 2) evaluated the performance of the composite models, yielding an AUC value of 0.842 for the first model based on CRP on POD2 and POD as well as CCI and age. The AUC value for the second model based on CRP on POD3 and CCI was 0.772.
Discussion
In this study, we analyzed and compared the predictive values of PCT and CRP in determining the occurrence of postoperative anastomotic leakage and major infective complications after gastrectomy. According to our results, CRP performed better than PCT in predicting both AL and MIC, even though there was no significant difference in the performance of these two biomarkers. Given the mean occurrence of most cases of AL and MIC in this study (POD5 and POD6), the most effective strategy to stratify patients’ risk of complications seems reasonable to dose CRP from days 2 to 5–6.
Our results can be interpreted based on the different etiologies underlying the increase in PCT and CRP values. PCT is produced by the neuroendocrine cells of the thyroid and the lungs as the precursor for the hormone calcitonin. Serum PCT levels have been found to increase as soon as 3–4 h after exposure to bacterial products, reaching a peak within 6–24 h. PCT has been regarded as having higher sensitivity and specificity for predicting bacterial infection than other biomarkers and to correlate with the severity of the infection.16 The composition of the pathogen agents involved in systemic infection, as well as the location of the infection (i.e., abdominal, urinary tract, and upper and lower respiratory tract infections), correlate with the rise in PCT. Indeed, the PCT rise seems to be specifically triggered by Gram-negative bacteria, while it is less sensitive during Gram-positive or fungal sepsis.17 Moreover, it could be influenced by other factors, such as the use of antibiotics,18 and false-positives due to impairments in renal function, neutropenia, or major stressors that cause systemic inflammation (including surgery) have been reported, as well as false negatives, especially when infections are localized as in mediastinitis or abscesses, or if procalcitonin is dosed too early.19
CRP, instead, is an acute phase protein synthetized in the liver. Its secretion starts 4 to 6 h after an inflammatory insult and peaks at 36–50 h. Its production and elimination are not influenced by renal replacement therapy or immunosuppression (both systemic steroids and neutropenia), and it has a lower sensitivity and specificity than PCT in studies on critically ill patients.16,20
Common pathogens identified in the abdominal cultures and bloodstream after abdominal gastric surgery complications have often included Candida species and Klebsiella pneumoniae, followed by Streptococcus, Pseudomonas, and Staphylococcus species.21,22,23 Instead, when lower GI surgery complications occur, the rate of fungal infection is extremely low, and the role of Gram-negative bacteria seems to be prominent.24 This is the most plausible explanation for why PCT proved to be a superior predictive biomarker for anastomotic leakage in colorectal surgery in previous studies10 but did not outperform CRP as a marker of AL and major infective complications in our study. In previous studies, PCT proved to be more accurate than CRP in predicting AL after colorectal surgery,10 and it was shown to be promising for the early detection of intrabdominal complications in esophagogastric surgery.11,12 However, in our study, it was not superior to CRP for detecting AL of major infective complications.
There are notable implications for the results of this study. The first is that PCT should not be routinely used during the postoperative course of esophagogastric resection to exclude AL or other infective complications. The main reason is that the mean cost for this laboratory exam is €14–30 per patient in Europe, compared to the €4 of most CRP assays.16 Previous cost-efficacy studies focused on critically ill patients found PCT guidance to be overall cost-effective for antibiotic management in the intensive care unit.25 In our study, data on the cost-effectiveness of the different biomarkers were not available for analysis even though a reliable estimate of the adjunctive cost of PCT for this study, according to the mean cost of 15€ for PCT and 4€ for PCR among the participating institutions, is 21,714€. Due to the superior performance of CRP observed herein, we can conclude that there is likely an unfavorable cost/effectiveness balance for PCT. For patients undergoing gastrectomy, PCT should continue to have a role in detecting and monitoring sepsis derived from specific postoperative complications (i.e., sepsis from Gram-negative bacteria as in some abdominal infections, urinary sepsis), as well as in patients with severe complications needing intensive care unit admission.
A second implication follows the assessment of the accuracy of CRP in predicting AL and MIC. Our results are consistent with those of other studies that have identified CRP as a biomarker superior to WBC in detecting postoperative infective complications.26 The routine monitoring of CRP could lead to an improvement in the risk stratification of patients, favoring timely decisions to detect and treat postoperative complications and the identification of low-risk patients eligible for early discharge. In the inpatient setting, it could favor a prompt diagnosis, the targeting of radiologic imaging (i.e., postoperative oral contrast exams) and a timely and appropriate use of antibiotic therapy. Identifying patients at risk of complications also aids decision-making on the timing for discharge to minimize readmissions and the dreaded “failure to rescue” phenomenon.27 On the other hand, a possible disadvantage related to the use of biomarkers is that they could lead to increased blood sampling, longer monitoring, and increased length of hospital stay.28 However, the possible advantages in terms of postoperative management of a biomarker-based strategy are particularly resonant in gastric surgery, a field in which hospitalization is usually long, and fast-track/enhanced recovery protocols and early discharge plans have not been widely applied to date due to concerns for patient safety and unclear benefits in terms of readmission rate.29 For patients with low and minimal risk of complications, these protocols could be pursued with more confidence, leading to a notable change in postoperative management and a reduction in costs.
Based on our results, we provided Fig. 1a and b to aid clinicians in stratifying the risk of anastomotic leak and postoperative complications. Given the high NPV of the single values of CRP on different postoperative days, we believe the best use of the models would be to aid in selecting patients with a low risk of postoperative complications for early discharge. Nevertheless, it should be pointed out that most complications are diagnosed between POD5 and POD8, and given that CRP alone did not have a high accuracy until day 5, it may not be considered a very early predictor of AL and MICs. In the future, based on the promising results of the post hoc analysis (where CRP in POD3 combined with the CCI had a satisfactory performance), the CRP values could be integrated with other clinicopathological variables (e.g., the Charlson comorbidity score) or biomarkers (e.g., laboratory ratios) to improve the accuracy of the predictive models, thereby facilitating the planning for early diagnostic and therapeutic measures as well as the application of fast-track principles to selected patients.
This study has some limitations, including the lack of external validation for the results. Moreover, the choice of AL as the primary outcome could have led to biased interpretation of the results, as other infective and/or abdominal complications (such as pancreatic fistula and/or periduodenal collections) also have a prominent role in determining the need for intervention, intensive care, or readmission. Future studies should consider creating a composite outcome measure including anastomotic and duodenal leaks and peripancreatic collections as the primary outcome to avoid misinterpretation of the results or outcome reporting bias.30,31 Notwithstanding its limitations, this is the first prospective study with a large sample size that systematically investigated the use of PCT compared to PCR in predicting AL and MIC after gastric surgery. The results were valuable as they suggest excluding the use of PCT for the early detection of complications after gastrectomy. Additionally, the post hoc exploratory analysis revealed possible practical applications, such as the postoperative CRP charts and composite models, for use in the postoperative setting.
Conclusions
In this study, PCT was not superior to CRP as a predictor of AL and major infective complications after gastrectomy. CRP should be used as the reference screening postoperative marker. In the future, models based on CRP as well as on other patient-related variables and biomarkers could further optimize postoperative management.
References
Kamarajah SK, Navidi M, Griffin SM, Phillips AW (2020) Impact of anastomotic leak on long-term survival in patients undergoing gastrectomy for gastric cancer. Br J Surg. https://doi.org/10.1002/bjs.11749
Kim KM, An JY, Kim HI, et al (2012) Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg 99:1681–1687. https://doi.org/10.1002/BJS.8924
Oh SJ, Choi WB, Song J, et al (2009) Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J GastrointestSurg 13:239–245. https://doi.org/10.1007/S11605-008-0716-3
Makuuchi R, Irino T, Tanizawa Y, et al (2019) Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg Today. https://doi.org/10.1007/s00595-018-1726-8
Low DE, Allum W, De Manzoni G, et al (2019) Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. https://doi.org/10.1007/s00268-018-4786-4
Mortensen K, Nilsson M, Slim K, et al (2014) Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. https://doi.org/10.1002/bjs.9582
Gordon AC, Cross AJ, Foo EW, Roberts RH (2018) C-reactive protein is a useful negative predictor of anastomotic leak in oesophago-gastric resection. ANZ J Surg. https://doi.org/10.1111/ans.13681
Ji L, Wang T, Tian L, Gao M (2016) The early diagnostic value of C-reactive protein for anastomotic leakage post radical gastrectomy for esophagogastric junction carcinoma: A retrospective study of 97 patients. Int J Surg. https://doi.org/10.1016/j.ijsu.2016.02.021
Castelli GP, Pognani C, Meisner M, et al (2004) Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care. https://doi.org/10.1186/cc2877
Giaccaglia V, Salvi PF, Antonelli MS, et al (2016) Procalcitonin reveals early dehiscence in colorectal surgery. the PREDICS study. Ann Surg. https://doi.org/10.1097/SLA.0000000000001365
Hoeboer SH, Groeneveld ABJ, Engels N, et al (2015) Rising C-Reactive Protein and Procalcitonin Levels Precede Early Complications After Esophagectomy. J Gastrointest Surg. https://doi.org/10.1007/s11605-015-2745-z
Yang W, Chen X, Zhang P, et al (2021) Procalcitonin as an Early Predictor of Intra-abdominal Infections Following Gastric Cancer Resection. J Surg Res. https://doi.org/10.1016/j.jss.2020.08.037
Ruiz-Tovar J, Muñoz JL, Gonzalez J, et al (2017) C-reactive protein, fibrinogen, and procalcitonin levels as early markers of staple line leak after laparoscopic sleeve gastrectomy in morbidly obese patients within an Enhanced Recovery After Surgery (ERAS) program. Surg Endosc. https://doi.org/10.1007/s00464-017-5602-1
de Mooij CM, Maassen van den Brink M, Merry A, et al (2019) Systematic Review of the Role of Biomarkers in Predicting Anastomotic Leakage Following Gastroesophageal Cancer Surgery. J Clin Med 8:2005. https://doi.org/10.3390/JCM8112005
Tu JV (1996) Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 49:1225–1231. https://doi.org/10.1016/S0895-4356(96)00002-9
Nora D, Salluh J, Martin-Loeches I, Póvoa P (2017) Biomarker-guided antibiotic therapy—strengths and limitations. Ann Transl Med 5. https://doi.org/10.21037/ATM.2017.04.04
Li S, Rong H, Guo Q, et al (2016) Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J Res Med Sci 21. https://doi.org/10.4103/1735-1995.183996
Leng Y, Chen C, Zhang Y, et al (2019) Ability of serum procalcitonin to distinguish focus of infection and pathogen types in patients with bloodstream infection. Ann Transl Med 7:135–135. https://doi.org/10.21037/ATM.2019.03.24
Rhee C (2017) Using Procalcitonin to Guide Antibiotic Therapy. Open Forum Infect Dis 4. https://doi.org/10.1093/OFID/OFW249
Cho SY, Choi JH (2014) Biomarkers of sepsis. Infect Chemother 46:1–12. https://doi.org/10.3947/IC.2014.46.1.1
Althuwaini S, Bamehriz F, Alobaid O, et al (2018) Identification of Bacterial and Fungal Pathogens in Patients with Post-Laparoscopic Sleeve Gastrectomy Leakage. Obes Surg 28:3965–3968. https://doi.org/10.1007/S11695-018-3442-2
Zappella N, Desmard M, Chochillon C, et al (2015) Positive peritoneal fluid fungal cultures in postoperative peritonitis after bariatric surgery. Clin Microbiol Infect 21:853.e1-853.e3. https://doi.org/10.1016/J.CMI.2015.05.024
Migita K, Takayama T, Matsumoto S, et al (2014) Impact of bacterial culture positivity of the drainage fluid during the early postoperative period on the development of intra-abdominal abscesses after gastrectomy. Surg Today 44:2138–2145. https://doi.org/10.1007/S00595-014-0881-9
Lee DS, Ryu JA, Chung CR, et al (2015) Risk factors for acquisition of multidrug-resistant bacteria in patients with anastomotic leakage after colorectal cancer surgery. Int J Colorectal Dis 30:497–504. https://doi.org/10.1007/S00384-015-2161-6
Kip MM, Kusters R, IJzerman MJ, Steuten LM (2015) A PCT algorithm for discontinuation of antibiotic therapy is a cost-effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. J Med Econ 18:944–953. https://doi.org/10.3111/13696998.2015.1064934
Zhang K, Xi H, Wu X, et al (2016) Ability of Serum C-Reactive Protein Concentrations to Predict Complications After Laparoscopy-Assisted Gastrectomy: A Prospective Cohort Study. Medicine (Baltimore) 95. https://doi.org/10.1097/MD.0000000000003798
Silber JH, Williams SV, Krakauer H, Schwartz JS (1992) Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care 30:615–627. https://doi.org/10.1097/00005650-199207000-00004
Eschborn S, Weitkamp J-H (2019) Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol 2019 397 39:893–903. https://doi.org/10.1038/s41372-019-0363-4
D’Ugo D, Agnes A, Grieco M, et al (2020) Global updates in the treatment of gastric cancer: a systematic review. Part 2: perioperative management, multimodal therapies, new technologies, standardization of the surgical treatment and educational aspects. Updates Surg. https://doi.org/10.1007/s13304-020-00771-0
Kirkham JJ, Altman DG, Williamson PR (2010) Bias Due to Changes in Specified Outcomes during the Systematic Review Process. PLoS One 5. https://doi.org/10.1371/JOURNAL.PONE.0009810
Catalogue of Bias Collaboration, Thomas ET, Heneghan C. Outcome reporting bias. In: Catalogue Of Biases 2017. www.catalogueofbiases.org/outcomereportingbias
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Figure 1s
Graphs depicting the mean values of CRP, PCT and WBC on each postoperative day from 1 to 7 for patients with or without major infective complications. The aim of this figure is to depict the timing of the variations among the different biomarkers according to the postoperative day. (PNG 424 kb)
Table 1s
(DOCX 15 kb)
Table 2s
(DOCX 15 kb)
Table 3s
(DOCX 13 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cananzi, F.C.M., Biondi, A., Agnes, A. et al. Optimal Predictors of Postoperative Complications After Gastrectomy: Results from the Procalcitonin and C-reactive Protein for the Early Diagnosis of Anastomotic Leakage in Esophagogastric Surgery (PEDALES) Study. J Gastrointest Surg 27, 478–488 (2023). https://doi.org/10.1007/s11605-022-05547-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05547-y