Abstract
Background
Anastomotic leak (AL) is a potentially life-threatening complication after low anterior resection (LAR). This meta-analysis aimed to compare outcomes of LAR with and without diverting stoma and to determine factors associated with AL in non-diverted patients.
Methods
This was a PRISMA-compliant systematic review of electronic databases (PubMed, Scopus, and Web of Science). Randomized controlled trials comparing LAR with and without diverting stoma were included. Main outcome measures were AL, complications, and operation time in the two groups and risk factors of AL in non-diverted patients.
Results
Nine randomized control trials (RCTs) (946 patients; 53.2% male) were included. The diverting stoma group had lower odds of complications (OR: 0.61, 95%CI: 0.461–0.828; p < 0.001), AL (OR: 0.362, 95%CI: 0.236–0.555; p < 0.001, I2 = 0), abscess (OR: 0.392, 95%CI: 0.174–0.883; p < 0.024, I2 = 0), and reoperation (OR: 0.352, 95%CI: 0.222–0.559, p < 0.001, I2 = 0) than the no-diversion group. Both groups had comparable odds of bowel obstruction, surgical site infection, and perioperative mortality. The weighted mean operation time in the diverting stoma group was longer than the no-diversion group (WMD: 34.804, 95%CI: 14.649–54.960, p < 0.001). Factors significantly associated with AL in non-diverted patients were higher body mass index (BMI), ASA ≥ 3, lower tumor height, neoadjuvant therapy, open surgery, end-to-end anastomosis, and longer operation time.
Conclusions
Non-diverted patients with increased body mass index, high American Society of Anesthesiologists scores, low rectal cancers, received neoadjuvant therapy, underwent open surgery, end-to-end anastomosis, and longer operation times were at a higher risk of AL after LAR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total mesorectal excision (TME) is the universally preferred treatment of locally advanced rectal cancer to reduce locoregional recurrence.1 Two techniques are used for the treatment of low-lying rectal cancer, low anterior resection (LAR) and abdominoperineal resection (APR).2 Sphincter-sparing LAR has been reported to be associated with better quality of life than APR with a permanent colostomy;3 however, one of the known hazards of LAR is an anastomotic leak (AL).4 AL is associated with both costly postoperative morbidities and a negative impact on the long-term outcomes, including reduced overall survival.5
Preventive strategies have been devised to reduce the incidence of AL after LAR for rectal cancer including preoperative mechanical bowel preparation, oral antibiotics, intraoperative testing of anastomotic integrity and perfusion, the creation of colonic J-pouch, colonic bypass, anastomotic reinforcement, compression anastomosis, anastomotic buttressing, avoiding crossing staple lines, defunctioning stoma, and other techniques.6,7,8,9,10,11 Defunctioning stoma has been postulated to reduce the incidence of AL after LAR; however, a prospective study found diverting stoma to decrease the serious adverse effects of AL such as fecal peritonitis and septicemia, rather than prevent AL.12
Although previous meta-analyses 10,13 have assessed the protective role of diverting stoma after LAR, unfortunately, these meta-analyses did not discuss the risk factors of AL in patients who did not have a diverting stoma which may have important clinical implications. The majority of previous studies that assessed the risk factors of leak after LAR included patients with or without diversion which can be a confounding factor. Also, the studies were mainly based on retrospective, non-randomized data; therefore the risk of selection bias can be high. In contrast, prospective randomized trials tend to minimize selection bias and entail more complete and accurate data, as compared to reviews of retrospective data. Our meta-analysis had two main objectives. First, to confirm the benefit of diversion in reducing anastomotic leak that has been previously reported. Second, to determine when diversion is necessary after LAR by knowing which patients are at a higher risk of leak when they were not diverted. The main aim was to help select patients who will need diversion after LAR instead of diverting all patients invariably through a meta-regression analysis to help risk-stratify these patients and tailor the use of diversion. We aimed to bridge this gap in the existing knowledge by this meta-analysis, to determine which patients will be at high risk of leak when not diverted based on the pooling of high-quality data.
Methods
Registration and Reporting
The updated guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) were followed when reporting the present systematic review. Registration of the protocol of the current systematic review was made a priori in the prospective register of systematic reviews (PROSPERO) under the special identifier CRD42022319410. There was no deviation from the registered protocol when reporting this review. Ethics approval was not required based on institutional guidelines.
Search Strategy
A systematic organized search of the present literature was independently performed by two authors (S.E., S.K.). Randomized controlled trials (RCTs) comparing the use of a diverting stoma and omission of stoma after LAR of rectal cancer were searched. Cross-checking of the articles found after the literature search was made, addressing any conflicts about article selection by mutual agreement. The senior author (S.D.W.) supervised the literature search and article selection.
We searched three electronic databases (Medline through PubMed, Scopus, and Web of Science) from inception through March 2022. To ensure the inclusion of verified evidence only, non-peer-reviewed publications and gray literature were not included in the search process. To aid in the search for further eligible RCTs, the PubMed function “related articles” was activated, and the reference section of the articles was manually screened.
We excluded duplicate reports and the remaining articles were screened by title/abstract followed by full-text screening. One of two authors (S.E., S.K.) reviewed the full text of the articles to ascertain their eligibility for inclusion. The search outcome and the initial and final lists of articles were reviewed by the senior author (S.D.W.) before approval.
Search Keywords
The following keywords were used in the database search: “rectal cancer”, “rectal carcinoma,” “low anterior resection,” “anterior resection,” “diversion,” “ileostomy,” “colostomy,” “stoma,” “randomized,” “randomised,” “controlled trials,” “leak,” “leakage,” “complications,” and “outcome.” In addition, we used the following medical subject heading (MeSH) terms: (rectal neoplasms), (surgical stomas), (ileostomy), (colostomy), and (randomized controlled trial).
The following syntax combination was used for the literature search: (rectal cancer OR rectal carcinoma) AND (anterior resection OR low anterior resection OR sphincter-saving resection) AND (diversion OR stoma OR ileostomy OR colostomy) AND (leak OR complications OR outcome).
Article Selection Criteria
Only English-language RCTs comparing the use of a diverting stoma and omission of stoma after LAR of rectal cancer were considered for inclusion. We excluded non-randomized cohort studies, case reports, and case series entailing fewer than ten patients, animal studies, editorials, previous reviews, and meta-analyses. On reviewing overlapping RCTs that included the same cohort of patients within similar time periods, only the most recent and complete RCT was included. The studies had to fulfill the following PICO criteria to be included in this network meta-analysis:
P (patients): Patients with rectal cancer undergoing LAR.
I (intervention): Diverting stoma (ileostomy or colostomy)
C (comparator): No diverting stoma.
O (outcome): AL, complications, reoperation, operation time, hospital stay, and risk factors of AL in the non-diversion group.
Assessment of Risk of Bias and Certainty of the Evidence
The risk of bias was independently assessed by two authors (S.E. and Z.G.) with the ROB-2 tool for assessing RCTs.14 Any conflicts of interpretation of the results were resolved by mutual agreement. The certainty of the evidence of each outcome was graded as very low, low, moderate, and high using the GRADE approach 15 which entails five parameters: risk of bias, imprecision, inconsistency, indirectness, and publication bias.
Assessment of Publication Bias
The publication bias was assessed by inspection of a funnel plot of the standard error of the rate of each outcome against the rate of each outcome. The symmetry of the funnel plot confirmed the absence of publication bias.
Data Extraction
Two authors (S.E., S.K.) extracted the following information from each study into an Excel sheet template:
-
Authors, duration, and country of the study.
-
The total number of patients and numbers in each group.
-
Age, sex, body mass index (BMI), comorbidities, and ASA classification.
-
Tumor height and anastomosis height.
-
Neoadjuvant therapy (type and duration), operative approach, splenic flexure mobilization, level of inferior mesenteric vessels ligation, type of anastomosis, method of testing the anastomosis, and type of stoma.
-
Outcome of each procedure in terms of AL, complications, mortality, reoperation, operation time, and blood loss.
Review Outcomes
The primary outcome of this review was the difference between diversion and no-diversion groups in terms of AL, complications, reoperation, and operation time. The secondary outcome was the risk factors associated with complications and AL in non-diverted patients.
Statistical Analysis
An open-source, cross-platform software for advanced meta-analysis “openMeta [Analyst] ™” version 12.11.14 and Cochrane Review Manager 5.4® were used to perform this meta-analysis. A pairwise meta-analysis was conducted to assess the difference in AL, complications, reoperation, and operation time between diverting stoma and no stoma after LAR of rectal cancer odds ratio (OR) and 95% confidence interval (CI) and weighted mean difference (WMD).
Statistical heterogeneity was assessed using the p-value of the Cochrane Q test and the inconsistency (I2) statistics (low if I2 < 25%, moderate if I2 = 25–75%, and high if I2 > 75%). A random-effect meta-regression analysis of the risk factors of AL and complications in the non-diversion group was done weighing the studies by their within-study variance and the degree of heterogeneity. The inter-study heterogeneity was assessed in terms of the differences in the available patient-related and treatment-related factors. The statistical significance of each examined variable was expressed using slope coefficient (SE) and p-value. p-value < 0.1 was considered statistically significant.
Results
Study Characteristics
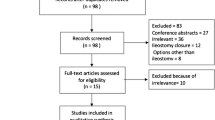
The initial literature search returned 2813 results and after the exclusion of duplicates and irrelevant studies, the full text of 12 studies was reviewed and three studies were further excluded (Fig. 1). Thus, the present meta-analysis included nine RCTs 16,17,18,19,20,21,22,23,24 published between 1983 and 2021. All studies were from European countries except for one study from India. Three trials were multicenter and six were single-center studies. The studies entailed 946 patients who were 503 (53.2%) male and 443 female with a median age of 65 (range, 55.5–68) years and median BMI of 26 (range, 24.9–26.2) kg/m2 (Table 1). Six trials used a diverting ileostomy, two used a diverting colostomy, and one used either.
Characteristics of Diversion and No-Diversion Groups
Overall, 489 (51.7%) patients underwent LAR with a diverting stoma and 457 (48.3%) without a stoma. The two groups were comparable in regards to male:female ratio (1.03:1 vs 0.8:1), median age (64 vs 67.2 years), median BMI (25.9 vs 25.6 kg/m2), tumor height from the anal verge (7.8 vs 7 cm), and receiving neoadjuvant therapy (42.7% vs 37.6%). Neoadjuvant chemoradiation therapy was given except in one study 21 that reported preoperative radiotherapy. Most of the procedures were performed via laparotomy in the two groups (63.4% vs 54.5%). The majority of anastomoses in the two groups were end-to-end (69.3% vs 74.6%), followed by J-pouch construction (26.5% vs 23%) (Table 2). The technical details of LAR performed in the trials are illustrated in Table 3. Treatment details in the two groups are shown in Table 4.
Meta-analysis of Outcomes of Diversion and No-Diversion Groups
Complications and Mortality
The diverting stoma group had lower odds of complications (OR: 0.61, 95%CI: 0.461–0.828; p < 0.001, I2 = 1%) (Fig. 2), AL (OR: 0.362, 95%CI: 0.236–0.555; p < 0.001, I2 = 0) (Fig. 3), and abscess (OR: 0.392, 95%CI: 0.174–0.883; p < 0.024, I2 = 0) (Fig. 4) than the no-diversion group. The definitions of AL across the studies are summarized in Appendix Table S1.
Both groups had comparable odds of SBO (OR: 2.843, 95%CI: 0.696–11.608, p = 0.146, I2 = 28.1%), SSI (OR: 1.231, 95%CI: 0.705–2.149; p = 0.466, I2 = 0), and perioperative mortality (OR: 0.833, 95%CI: 0.255–2.714, p = 0.761, I2 = 0).
Operation Time and Reoperation Rate
The diverting stoma group had lower odds of reoperation than the no-diversion group (OR: 0.352, 95%CI: 0.222–0.559, p < 0.001, I2 = 0) (Fig. 5). The weighted mean operation time in the diverting stoma group was longer than the no-diversion group (WMD: 34.804, 95%CI: 14.649–54.960, p < 0.001, I2 = 70.3%) (Fig. 6). Table 5 illustrates the outcomes of the two groups across the studies reviewed.
Subgroup Meta-analyses
Ileostomy vs Colostomy
Studies that used a diverting ileostomy showed lower odds of complications (OR: 0.648, 95%CI: 0.455–0.923, p = 0.016, I2 = 0) and AL (OR: 0.354, 95%CI: 0.193–0.648, p < 0.001, I2 = 0) in favor of the diversion group whereas studies that used a diverting colostomy showed similar odds of complications (OR: 0.619, 95%CI: 0.320–1.199, p = 0.155, I2 = 30.4%) and AL (OR: 0.638, 95%CI: 0.204–1.991, p = 0.439, I2 = 0).
Before or After the Year 2000
Trials published after the year 2000 showed lower odds of complications (OR: 0.575, 95%CI: 0.41–0.806, p = 0.001, I2 = 11.4) and AL (OR: 0.329, 95%CI: 0.207–0.523, p < 0.001, I2 = 0) in favor of the diversion group whereas trials published before the year 2000 showed similar odds of complications (OR: 0.619, 95%CI: 0.320–1.199, p = 0.155, I2 = 30.4%) and AL (OR: 0.638, 95%CI: 0.204–1.991, p = 0.439, I2 = 0).
Risk Factors of Complications and AL in the No-Diversion Group
According to the random-effect meta-regression analysis, only blood loss (SE = 0.0001, p = 0.09) was significantly associated with higher overall complications in patients who underwent LAR without a diverting stoma. Age (p = 0.9), male sex (p = 0.17), BMI (p = 0.22), comorbidities (p = 0.69), ASA ≥ 3 (p = 0.2), tumor height (p = 0.6), anastomosis height (p = 0.8), neoadjuvant therapy (p = 0.23), open surgery (p = 0.46), end-to-end anastomosis (p = 0.3), J pouch (p = 0.35), and operation time (p = 0.28) were not significantly associated with higher rate of total complications.
Factors significantly associated with higher rates of AL in patients who underwent LAR without a diverting stoma were:
-
Higher BMI (SE: 0.078, p = 0.05).
-
ASA ≥ 3 (SE: 0.016, p = 0.05).
-
Lower tumor height (SE: 0.032, p < 0.001).
-
Neoadjuvant therapy (SE: 0.002, p = 0.002).
-
Open surgery (SE: 0.001, p = 0.02).
-
End-to-end anastomosis (SE: 0.001, p = 0.02).
-
Longer operation time (SE: 0.002, p < 0.001).
Technical factors, including number of stapler firing, level of IMA ligation, splenic flexure mobilization, and testing the anastomotic integrity, were not significantly associated with AL; however, only three studies reported these parameters.
Publication Bias
There was evidence of publication bias in the outcomes: AL, abscess, and reoperation, whereas there was no publication bias in total complications, mortality, and operation time as shown in Fig. 7.
Risk of Bias and Certainty of the Evidence
All studies had some concern of bias as per the ROB-2 tool, except for two studies that had a high risk of bias (Appendix Table S2). All studies had a concern of bias in regards to the randomization process and deviation from the intended intervention due to lack of allocation concealment and awareness of the investigators and patients to the intervention made. According to the GRADE approach, the certainty of the evidence was moderate for total complications, AL, and reoperation; low for abscess and mortality; and very low for operation time (Appendix Table S3).
Discussion
AL remains a very concerning issue after colorectal anastomoses, especially low colorectal and coloanal anastomoses. Different strategies have been devised to reduce the risk of AL after LAR,11 and one effective strategy is the construction of a diverting stoma. Previous collective evidence has shown a significant benefit of a diverting stoma in lowering the incidence and consequences of AL after LAR.10,13 This fact has been confirmed by the results of the present meta-analysis which concluded a lower likelihood of AL and complications overall after LAR when a diverting stoma was used.
A diverting stoma in LAR is usually called “protective” as its main objective is to divert fecal matter and reduce the pressure imposed on the constructed anastomosis, thus protecting against its dehiscence.25 The main benefit of a diverting stoma has been debated as some investigators stated that it can actually decrease the incidence of AL.20,21,26 However, other studies showed that a diverting stoma does not prevent AL, yet it can potentially mitigate against its consequences that necessitate relaparotomy.27 These potential benefits were confirmed by the present meta-analysis since the use of a diverting stoma was associated with lower odds of abscess, which can be secondary to subacute leak, and reoperation needed to manage AL presenting with peritonitis and/or toxemia.
In addition to asserting the conclusions of the former meta-analyses, this meta-analysis might add new information. The subgroup meta-analysis demonstrated that not all diverting stomas confer the same outcome. While a diverting ileostomy managed to reduce the odds of AL and complications significantly as compared to no diversion, a subgroup analysis found that a diverting colostomy did not confer this benefit. While the reason for this finding is not clear, a previous experimental study found that a diverting colostomy may increase the risk of AL in rats by 22%.28 The presumed mechanism of this observation is that diverting colostomy may result in decreased intestinal synthesis of collagen, a fundamental component of the extracellular matrix that plays a critical role in wound healing,29 which subsequently can impair anastomotic healing and promote AL in the unloaded colon.30 Furthermore, a potential compromise of blood flow from the marginal arcade creation may occur during the creation of colostomy and decreases anastomotic perfusion, contributing to AL. A case-matched study 31 compared the morbidity rates of transverse colostomy and ileostomy when used for temporary diversion and reported higher complications in the colostomy group (47.6% vs 36.5%). It has been also reported a diverting colostomy is associated with higher rates of wound infection, parastomal hernias, and other complications compared to a diverting ileostomy.32 This may translate to an increased complication rate in patients who had a diverting colostomy to be comparable to patients without diversion. Perhaps these results contributed to the increased use of ileostomy as the standard method of diversion after LAR, noting that the trials that used a diverting colostomy were published before the year 2000.
Although a diverting stoma is proven to reduce AL and its consequences after LAR, it is not free of adverse events and complications. Although the trials included in this meta-analysis did not report data on complications associated with stoma reversal, it is worthy to note that the reversal of stomas is associated with its own set of complications that may not be encountered in non-diverted patients. A diverting ileostomy is prone to stoma-related complications such as stomal prolapse, stenosis, necrosis, and para-stomal hernia. In addition, skin excoriation, surgical site infection, and negative impact on quality of life have been associated with a diverting ileostomy.33,34 Therefore, the present meta-analysis included a secondary analysis to determine the risk factors of AL and complications in non-diverted patients. Knowing which patients actually need a diversion to protect their anastomoses may help avoid unnecessary stomas in patients who otherwise may not gain the benefit of diversion.
Seven risk factors for AL that indicate the need for a diverting stoma were identified by the meta-regression analysis; three of which were patient-related and four of which were treatment/procedure-related. Patients with obesity, higher ASA scores, and low rectal cancers may benefit of a protective stoma after LAR. Obesity can increase the risk of colorectal AL by one and half times across different populations.35 The impact of obesity on AL is probably multifactorial and includes the effect of excessive peri-visceral adipose tissue and thickened mesentery that increase the technical difficulty of the procedure and make the identification and ligation of mesenteric vessels challenging. In addition, obesity tends to restrict the working space in the pelvis, especially with low rectal tumors.36,37 Higher ASA scores imply patients with comorbidities and have been recognized to be associated with an increased risk of colorectal AL.38
Neoadjuvant therapy was found as a risk factor for AL in non-diverted patients. A previous study showed that patients who achieved complete pathological response after neoadjuvant therapy may be at higher risk of AL after TME than did incomplete responders.39 The negative impact of neoadjuvant therapy on AL was explained in light of its effect on local tissue healing. Radiotherapy activates certain immune signaling pathways to reinforce the antitumor response.40 However, the production of cytokines, chemokines, and reactive oxygen species may also cause endothelial dysfunction and impair tissue perfusion, predisposing to poor tissue healing and a higher risk of AL.41
Furthermore, end-to-end anastomosis was associated with a higher likelihood of AL in non-diverted patients, as compared to colonic J-pouch anastomosis.42 The senior author (SDW) previously reported that the colonic J pouch conferred a significant reduction in the rate of AL from 15 to 2%, as compared to the straight anastomosis. This benefit of a colonic J pouch was subsequently attributed to better anastomotic perfusion than was noted in an end-to-end anastomosis.43 Longer operative time was also found to be associated with a greater chance of AL in non-diverted patients. Prolonged operative time has been linked to higher postoperative complications and approximately triple the odds of developing AL 44.
The three main messages from the present meta-analysis are (1) diversion effectively reduces AL and complications after LAR; (2) not all patients may need diversion after LAR but patients with a higher BMI, higher ASA, lower tumor height, received neoadjuvant therapy, and/or who underwent open surgery are at a higher risk and might benefit of diversion; and (3) a diverting ileostomy would be preferred over a colostomy. To substantiate these conclusions, future randomized trials would be needed to assess the benefit of diverting ileostomy in patients with normal BMI, ASA I, and middle or upper rectal cancers that are planned to undergo laparoscopic LAR without neoadjuvant therapy.
The present meta-analysis has its strengths and limitations. This meta-analysis included randomized trials only to entail a high level of evidence and minimize selection bias which served to have a moderate certainty of the evidence of the main outcomes. It also helped elucidate the potential indications for a stoma. However, the small number of trials, small sample size of some studies, presence of some bias, and the relative heterogenous definition of anastomotic leaks are important limitations of this meta-analysis. Nearly all trials were based in European countries which may hinder the generalizability of the conclusions on the benefits of a diverting stoma in other ethnic and racial backgrounds. Another limitation involves the decision-making and subject exclusion in the trials. Also, most studies did not test for anastomotic integrity with air leak test, donut inspections, or fluorescence angiography. Certain factors may have impacted the incidence of AL such as the level of inferior mesenteric vessels ligation and splenic flexure mobilization which were not reported by many trials. Finally, the cohort analyzed in this study may not be representative of a contemporary patient population given the broad period of the study and the prevalence of open surgery.
Conclusions
The use of a diverting stoma was significantly associated with lower odds of AL, complications, reoperation, and longer operation time as compared to no diverting stoma. Non-diverted patients with increased BMI; high ASA scores; low rectal cancers, who received neoadjuvant therapy and underwent open surgery and end-to-end anastomosis; and with longer operation times were at a higher risk of AL after LAR.
References
Ridgway PF, Darzi AW. The role of total mesorectal excision in the management of rectal cancer. Cancer Control 2003;10 (3):205-211.
Bordeianou L, Maguire LH, Alavi K, Sudan R, Wise PE, Kaiser AM. Sphincter-sparing surgery in patients with low-lying rectal cancer: techniques, oncologic outcomes, and functional results. J Gastrointest Surg 2014;18:1358-1372
Scheele J, Lemke J, Wittau M, Sander S, Henne-Bruns D, Kornmann M. Quality of Life after Rectal Cancer Resection Comparing Anterior Resection, Abdominoperineal Resection, and Complicated Cases. Visc Med 2022;38:138-149.
Caulfield H, Hyman NH. Anastomotic Leak After Low Anterior Resection: A Spectrum of Clinical Entities. JAMA Surg 2013;148:177–182.
Allaix ME, Rebecchi F, Famiglietti F, Arolfo S, Arezzo A, Morino M. Long-term oncologic outcomes following anastomotic leak after anterior resection for rectal cancer: does the leak severity matter? Surg Endosc 2020;34:4166-4176.
Emile SH, Khan SM, Wexner SD. Impact of change in the surgical plan based on indocyanine green fluorescence angiography on the rates of colorectal anastomotic leak: a systematic review and meta-analysis. Surg Endosc 2022;36:2245-2257.
Slim K, Vicaut E, Panis Y, Chipponi J. Meta-analysis of randomized clinical trials of colorectal surgery with or without mechanical bowel preparation. Br J Surg 2004;91:1125-1130.
Roos D, Dijksman LM, Tijssen JG, Gouma DJ, Gerhards MF, Oudemans-van Straaten HM. Systematic review of perioperative selective decontamination of the digestive tract in elective gastrointestinal surgery. Br J Surg 2013;100:1579-1588.
Machado M, Nygren J, Goldman S, Ljungqvist O. Similar outcome after colonic pouch and side-to-end anastomosis in low anterior resection for rectal cancer: a prospective randomized trial. Ann Surg 2003;238:214-220.
Phan K, Oh L, Ctercteko G, Pathma-Nathan N, El Khoury T, Azam H, Wright D, Toh JWT. Does a stoma reduce the risk of anastomotic leak and need for re-operation following low anterior resection for rectal cancer: systematic review and meta-analysis of randomized controlled trials. J Gastrointest Oncol 2019;10:179-187.
Shalaby M, Thabet W, Morshed M, Farid M, Sileri P. Preventive strategies for anastomotic leakage after colorectal resections: A review. World J Meta-Anal 2019;7:389-398.
Wong NY, Eu KW. A defunctioning ileostomy does not prevent clinical anastomotic leak after a low anterior resection: a prospective, comparative study. Dis Colon Rectum 2005;48:2076-2079.
Ahmad NZ, Abbas MH, Khan SU, Parvaiz A. A meta-analysis of the role of diverting ileostomy after rectal cancer surgery. Int J Colorectal Dis 2021;36:445-455.
Sterne JAC, Savović J, Page MJ, et al. RoB 2. A revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898.
Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-406.
Altomare DF, Delrio P, Shelgyn Y, et al. Transanal reinforcement of low rectal anastomosis versus protective ileostomy after total mesorectal excision for rectal cancer. Preliminary results of a randomized clinical trial. Colorectal Dis 2021;23:1814-1823.
Mrak K, Uranitsch S, Pedross F, et al. Diverting ileostomy versus no diversion after low anterior resection for rectal cancer: A prospective, randomized, multicenter trial. Surgery 2016;159:1129-1139.
Thoker M, Wani I, Parray FQ, Khan N, Mir SA, Thoker P. Role of diversion ileostomy in low rectal cancer: a randomized controlled trial. Int J Surg 2014;12:945-951.
Ulrich AB, Seiler C, Rahbari N, Weitz J, Büchler MW. Diverting stoma after low anterior resection: more arguments in favor. Dis Colon Rectum 2009;52:412-418.
Chude GG, Rayate NV, Patris V, Koshariya M, Jagad R, Kawamoto J, Lygidakis NJ. Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: should we make an ileostomy as a routine procedure? A prospective randomized study. Hepatogastroenterology 2008;551562-1567.
Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007;246:207–214.
Pimentel JM, Duarte A, Patricio J. The role of a protecting stoma in low anterior resection with TME and colonic J-pouch for rectal cancer; results of a prospective randomized trial. Colorectal Dis 2003;5:83.
Pakkastie TE, Ovaska JT, Pekkala ES, et al. A randomised study of colostomies in low colorectal anastomoses. Eur J Surg 1997;163:929-933.
Graffner H, Fredlund P, Olsson SA, et al. Protective colostomy in low anterior resection of the rectum using the EEA stapling instrument. A randomized study. Dis Colon Rectum 1983;26:87-90.
Wu, Y., Zheng, H., Guo, T. et al. Temporary Diverting Stoma Improves Recovery of Anastomotic Leakage after Anterior Resection for Rectal Cancer. Sci Rep 2017;7:15930.
Hanna, M. H., Vinci, A. & Pigazzi, A. Diverting ileostomy in colorectal surgery: when is it necessary? Langenbecks Arch Surg 2015;400;145–152.
Shiomi, A. et al. Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg 2015;220:186–194.
Månsson P, Fork T, Blomqvist P, Jeppsson B, Thorlacius H. Diverting colostomy increases anastomotic leakage in the rat colon. Eur Surg Res 2000;32:246-250.
Mathew-Steiner SS, Roy S, Sen CK. Collagen in Wound Healing. Bioengineering (Basel) 2021;8:63.
Uden P, Blomquist P, Jiborn H, Zederfeldt B. Influence of proximal colostomy on the healing of a left colon anastomosis: An experimental study in the rat. Br J Surg 1988;75:325–329.
Sakai Y, Nelson H, Larson D, Maidl L, Young-Fadok T, Ilstrup D. Temporary transverse colostomy vs loop ileostomy in diversion: a case-matched study. Arch Surg 2001;136:338-342.
Wu X, Lin G, Qiu, H. et al. Loop ostomy following laparoscopic low anterior resection for rectal cancer after neoadjuvant chemoradiotherapy. Eur J Med Res 2018;23:24.
Maroney S, Chavez de Paz C, Duldulao M, et al. Complications of Diverting Ileostomy after Low Anterior Resection for Rectal Carcinoma. Am Surg. 2016;82:1033-1037.
Keane C, Sharma P, Yuan L, Bissett I, O'Grady G. Impact of temporary ileostomy on long-term quality of life and bowel function: a systematic review and meta-analysis. ANZ J Surg 2020;90:687-692.
Nugent TS, Kelly ME, Donlon NE, et al. Obesity and anastomotic leak rates in colorectal cancer: a meta-analysis. Int J Colorectal Dis 2021;36:1819-1829.
Panteleimonitis S, Popeskou S, Harper M, Kandala N, Figueiredo N, Qureshi T, Parvaiz A (2018) Minimally invasive colorectal surgery in the morbid obese: does size really matter? Surg Endosc 2018;32:3486–3494.
Akiyoshi T, Ueno M, Fukunaga Y, et al. Effect of body mass index on short-term outcomes of patients undergoing laparoscopic resection for colorectal cancer. Surg Laparosc Endosc Percutan Tech 2011;21:409–414.
Battersby C, Green P, Vyapury V, et al. PTU-227 Statins may modify colorectal anastomotic leak risk in high risk patients Gut 2015;64:A162-A163.
Zaborowski AM, Stakelum A, Winter DC. Anastomotic leak risk in complete responders to neoadjuvant therapy for rectal cancer: a systematic review. Int J Colorectal Dis 2021;36:671–676.
Chajon E, Castelli J, Marsiglia H, de Crevoisier R. The synergistic effect of radiotherapy and immunotherapy: a promising but not simple partnership. Crit Rev Oncol Hematol 2017;111:124–132.
Langley RE, Bump EA, Quartuccio SG, Medeiros D, Braunhut SJ (1997) Radiation-induced apoptosis in microvascular endothelial cells. Br J Cancer 1997;75:666–672.
Person B, Vivas DA, Wexner SD. Totally laparoscopic low anterior resection with transperineal handsewn colonic J-pouch anal anastomosis for low rectal cancer. Surg Endosc 2006;20:700–702.
Hallböök O, Påhlman L, Krog M, Wexner SD, Sjödahl R. Randomized comparison of straight and colonic J pouch anastomosis after low anterior resection. Ann Surg 1996;224:58-65
Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 2010;4:5.
Author information
Authors and Affiliations
Contributions
Sameh Emile designed the study. Sameh Emile and Sualeh Khan searched the literature and screened eligible articles. Sameh Emile and Sualeh Khan extracted the required data. Sameh Emile, Zoe Garoufali, Emanuela Silva-Alvaremga, Nir Horesh, Rachel Gefen, and Michael R. Freund assessed the quality of the studies. Sameh Emile wrote the manuscript. Steven Wexner reviewed the results of the initial and final search and the preliminary and final lists of articles before approving them and revised the manuscript. All authors critically reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial disclosures. SDW is a paid consultant for Medtronic, Intuitive Surgical, Stryker, and Olympus and receives royalties for intellectual property license from Intuitive Surgical, Medtronic, and Karl Storz Endoscopy .
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emile, S.H., Khan, S.M., Garoufalia, Z. et al. When Is a Diverting Stoma Indicated after Low Anterior Resection? A Meta-analysis of Randomized Trials and Meta-Regression of the Risk Factors of Leakage and Complications in Non-Diverted Patients. J Gastrointest Surg 26, 2368–2379 (2022). https://doi.org/10.1007/s11605-022-05427-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05427-5