Abstract
Purpose
Anastomotic leak is a feared complication of rectal cancer surgery. A diverting stoma is believed to act as a safety mechanism against this undesirable outcome. This meta-analysis aimed to examine the role of loop ileostomy in the prevention of this complication.
Methods
The Medline, Embase and Cochrane databases were searched for randomized controlled trials (RCTs) comparing anastomotic complications after rectal cancer surgery in the presence or absence of diverting ileostomy. The need for reoperation and postoperative complications were also analysed. The length of hospital stay, intraoperative blood loss and operating time were analysed as secondary endpoints.
Results
A significantly higher number of anastomotic leaks was detected in patients with no diverting ileostomies than in those with diversion (odds ratio (OR) 0.292 and 95% confidence interval (CI) 0.177–0.481), and more patients required reoperations in this group (OR 0.219 and 95% CI 0.114–0.422). The rate of complications other than anastomotic leak was significantly higher in patients with diverting ileostomies than in those without (OR 3.337 and 95% CI of 1.570–7.093). The operating time was longer in the ileostomy group than in the no ileostomy group (P 0.001), but no significant differences in the intraoperative blood loss or postoperative hospital stay length were observed between the two groups(P 0.199 and 0.191 respectively).
Conclusion
A lower leak rate in the presence of diverting ileostomy is supported by relatively weak evidence. While mitigating the consequences of leakage, diverting ileostomies lead to numerous other complications. High-quality RCTs are needed before routine ileostomy diversions can be recommended after rectal cancer surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oncological outcomes of rectal cancer surgery have changed since the advent of total mesorectal excision (TME), which was first described by Richard Heald [1], and the incidence of local disease recurrence has significant decreased. However, the incidence rates of postoperative surgical complications, especially anastomotic leak, remain almost unchanged. Anastomotic leak is the most feared complication after local recurrence, as it can lead to a chain of both local and systemic septic events and is strongly associated with the risk of local recurrence if not managed promptly. The overall risk of anastomotic leakage may be as high as 25% [2, 3].
The consequences of a leak are commonly thought to be minimized by diverting the intestinal contents away from the newly constructed anastomosis [4]. A transverse colostomy or loop ileostomy is commonly fashioned as a diverting stoma. The advantages and disadvantages of both types of stomas in terms of construction, function and reversal have been described. Colostomies have a higher risk of complications, particularly related to their closure, whereas ileostomies lead to more electrolyte disturbances [5], and some convincing evidence supports the use of ileostomies as diverting stomas [6,7,8].
A previously published meta-analysis has confirmed the effectiveness of diverting stomas and has recommended routine diversion after rectal cancer surgery [4]. Most of the publications included in the meta-analysis had reported colostomies as diverting stomas. In recent years, an inclination towards ileostomies as diverting stomas has been observed. This preference is probably due to the higher leakage rates associated with colostomy closures when compared with closure of ileostomies. Newly published literature investigating the use of ileostomies as diverting stomas has also become available in the last decade or so. This systematic review and meta-analysis of randomized controlled trials focused on the role of loop ileostomies after rectal cancer surgery.
Methods
A search of the Medline, Embase and Cochrane databases using the keywords “Defunctioning” OR “Diverting” AND “Ileostomy” OR “Stoma” AND “Rectum” OR “Rectal” AND “Cancer” OR “Neoplasm” AND “Surgery” OR “Anterior resection” was performed. The search was limited to randomized controlled trials (RCTs) in the English language only. Data extraction was carried out by one author and counterchecked by the other authors for the accuracy of information. All the data were displayed on Microsoft Excel sheets. Any disagreement or discrepancy in the data extraction or interpretation was resolved via discussion. The PRISMA guidelines were followed in the literature search and study selection process [9].
Endpoints
The primary endpoint of this meta-analysis was anastomotic leak in the two groups with or without diverting ileostomy. There was significant heterogeneity among the studies, and the primary endpoint of anastomotic leak was defined differently in the studies. The secondary endpoints included reoperations and postoperative complications. Reoperations for any cause after primary anastomotic leak and after stoma formation or stoma closure surgery were included in the analysis. Only the surgical complications were compared between the two groups, and general complications, such as cardiopulmonary, haematological and biochemical complications, were not compared. Perioperative continuous variables, including the operative time, intraoperative blood loss and total length of hospital stay, were also analysed as secondary outcomes.
Quality assessment
Quality assessment of the RCTs was carried out independently by two authors using Cochrane’s risk of bias tool [10]. The risk of bias tool comprises seven variables to assess selection bias (random sequence generation and allocation concealment), reporting bias, performance bias, detection bias and attrition bias. Because of the nature of the intervention, no participants or assessing personnel were blinded, and the outcome measure was assumed to be unlikely influenced by the lack of blinding. The sources of bias other than the abovementioned variables were also assessed individually for the included studies. The quality of a trial was considered good only if it met all the criteria. If one criterion was not met or if two of the criteria were unclear, the trial was considered to have either fair or poor quality depending on the likelihood of its influence on the outcomes. If two or more criteria were not met or were unclear, the trial was considered to have poor quality (Table 1).
Statistics
The data collected on the study characteristics and endpoints were displayed and harmonized on Microsoft Excel sheets for meta-analysis. The study by Matthiessen included four patients who had transverse colostomies fashioned as diverting stomas [11]. The data from this group were not reported separately and for the purpose of calculations, these four patients were excluded from the final analysis. The heterogeneity among the studies was checked, and a fixed effects or random effects model was used for meta-analysis accordingly. If the heterogeneity was significant (P < 0.1), a random effects model was used for meta-analysis, and vice versa. For the continuous variables, the standard difference in means (SDM) along with the 95% confidence interval (CI) was calculated. For the dichotomous data, odds ratios (ORs) and 95% CIs were analysed. The means and standard deviations (SDs) were estimated according to the formulas described by Hozo et al. from the given median and range values [12]. Forest plots were generated for the variables of interest, and publication bias was checked for the primary endpoint both graphically using the funnel plot of standard error by Log odds ratio and mathematically using the classic fail-safe N method. Finally, sensitivity analysis was carried out by checking the impact on the overall results after the exclusion of individual studies. Comprehensive Meta-Analysis Version 2 (Biostat, 14 North Street, Englewood, NJ, 07631, USA) was used for the statistical analysis.
Results
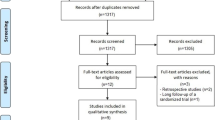
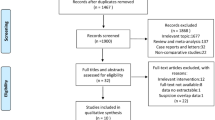
The initial literature search of the Medline and Embase databases on the role of diverting ileostomies after rectal cancer surgery revealed 722 and 1165 publications, respectively. The limits in search strategy for randomized controlled trials (RCTs) in English language were applied which resulted in 36 and 25 RCTs from the Medline and Embase databases, respectively. Further searching of the Cochrane database added another 92 publications. All the abstracts were screened and assessed for the suitability of inclusion by the authors. Four RCTs in each database were found to be suitable for the meta-analysis [11, 13,14,15]. One of the RCTs was a randomized pilot study and was considered suitable for inclusion because of the endpoints addressed [15]. Further manual searching identified another RCT relevant to our endpoints that was included in this meta-analysis [16] (Fig. 1).
PRISMA flow chart [9]
There were a total of 764 patients, 394 in the ileostomy group and 370 in the group without a stoma, with an average patient age of 60.6 years in the ileostomy group and 60.2 in the no ileostomy group. The rest of the study characteristics are shown in Tables 2, 3 and 4.
The primary endpoint of anastomotic leak was reported by all five RCTs. There were 24 leaks in the ileostomy group and 68 in the group without ileostomy. The reported leak rate ranged from 2 to 11% in the ileostomy group and from 10 to 28% in the no ileostomy group. The test of heterogeneity was not significant, and a fixed effects model was used for the meta-analysis, which favoured the ileostomy group. The overall anastomotic leak rate was significantly high in the group not covered with diverting ileostomy, with an OR of 0.292, a 95% CI of 0.177–0.481 and a P value of 0.001 (Fig. 2).
Understandably, because of the high anastomotic leak, the reoperation rate was higher in the group not covered with diverting ileostomy. Most of the operations in the no ileostomy group were carried out to limit the extent of sepsis, whereas stoma-related complications were common causes of reoperation in the group covered with ileostomy. There were 12 reoperations in the ileostomy group and 49 in the no ileostomy groups with a reoperation rate of 3.22% and 15.03% respectively. A fixed effects model was used for meta-analysis, as there was no significant heterogeneity among the studies. This revealed a significant difference in favour of the group with ileostomies, with an OR of 0.219, a CI of 0.114–0.422 and a P value of 0.001 (Fig. 3).
Complications other than anastomotic leak and those not related to the consequences of leakage were analysed separately. Postoperative ileus and electrolyte imbalance secondary to a high-output stoma were not considered or analysed as complications of the procedure. The risk of postoperative complications was higher in the group receiving diverting ileostomy, and there was a significant difference between the groups in favour of the no ileostomy group. Totals of 89 and 41 episodes (23.67% and 11.58%) were observed in the ileostomy and no ileostomy groups, respectively. The heterogeneity among the studies was significant, with a P value of < 0.1; therefore, a random effects model was used for meta-analysis. The results showed an OR of 3.337, a CI of 1.570–7.093 and a P value of 0.002 (Fig. 4).
Among the continuous variables, the operating time was significantly longer in the group with diverting ileostomies. There was significant heterogeneity among the studies, and a random effects model was used for meta-analysis. The results revealed an SDM of 0.614, a CI of 0.306–0.922 and a P value of 0.001. While construction of a diverting stoma adds more time to an already complex and lengthy operation, the amount of blood loss remained similar between the two groups. A random effects model for the meta-analysis of blood loss showed an SDM of 0.242 with a CI of − 0.127–0.612 and a P value of 0.199 (Table 5).
A random effects model was used for meta-analysis of the hospital stay length because of the significant heterogeneity among the studies. The results showed an SDM of − 0.887 with a CI of − 2.216–0.442 and a P value of 0.191. Although there was no significant difference between the two groups, it is strongly believed that in the absence of anastomotic leakage, the total length of hospital stay would have been much longer in the group covered with diverting ileostomy. This result was indicated in one of the RCTs where the initial hospital stay with no leakage in the groups without and with diversion was reported to be 11.5 (6–60) days and 9 (5–21) days, respectively (Table 5).
Publication bias
The primary outcome in the included RCTs was anastomotic leak. Therefore, the publication bias related to this outcome was assessed using the funnel plot of standard error by Log odds ratio and the classic fail-safe N method. The asymmetrical funnel plot suggested a strong possibility of publication bias, and the finding was also supported by the statistical method (Fig. 5). The sensitivity analysis related to the principal outcome of anastomotic leakage showed no impact on the overall results after exclusion of individual studies, suggesting a consistent pattern in the results of the RCTs included in the meta-analysis.
Discussion
The risk of anastomotic leak remains high despite numerous developments in colorectal cancer surgery. A diverting stoma after rectal cancer resection is made with the intent to protect the distal anastomosis and facilitate the healing process. The presence of a stoma is thought to mitigate the consequences of anastomotic leak, and these stomas are deemed suitable for reversal once the integrity and patency of the anastomosis are confirmed by appropriate investigations. The current meta-analysis of RCTs showed that the incidence of anastomotic leak was significantly higher in the group not covered with a stoma than in the stoma-covered group, but the quality of the included RCTs may limit the clinical implications of this finding. The concept of a diverting stoma is supported by previously published non-randomized studies [17, 18] and a previously published meta-analysis [19].
The evidence in support of and against the use of a diverting stoma runs in parallel in the literature, and a diverting stoma has also been blamed for poor quality of life, which is likely secondary to postoperative complications [20,21,22]. Moreover, some large-volume studies have failed to show a significant benefit of a diverting stoma in reducing the risk of anastomotic leak. However, it has been established that the septic consequences of a leak are lessened by use of a diverting stoma [23]. Because of these conflicting views, no real consensus exists for the use of a diverting stoma in rectal cancer surgery.
The postoperative complication rate was seemingly higher in the stoma group than in the group without a stoma, which was probably related to the more prolonged and relatively complex procedure involved in stoma formation. Moreover, a stoma itself is the basis of many surgical and physiological complications. In this meta-analysis, the physiological complications related to high-output ileostomies and electrolyte imbalance were not included in the group comparison to minimize bias, as the group without ileostomy was unlikely to have any of these complications. The complexity of an already demanding procedure is further increased by the addition of a diverting stoma, which leads to a higher rate of postoperative complications [24].
There is a general trend to defunction the patients who received neoadjuvant radiotherapy and in patients where the anastomosis was closer to the anal margin. This practice sounds very reasonable, but regrettably, radiotherapy was not found to be a risk factor for anastomotic leak in the RCTs included in this meta-analysis. Matthiessen et al. found no difference in the rates of anastomotic leak in patients with and without radiotherapy. One publication reported less symptomatic leaks in the group which contained more patients after neoadjuvant radiotherapy than in the group with fewer patients [15]. Univariate and multivariate analyses by Mrak et al. did not show chemoradiotherapy (CRT) as a predictor of anastomotic leak, and a further meta-analysis confirmed these results [25].
The definition of a low anastomosis that could warrant a loop ileostomy in most previous studies was averaged 5 cm from the anal verge [26]. Mrak et al. reported a higher anastomotic leak rate in anastomoses < 6 cm from the anal verge than in higher anastomoses. Low anastomoses are at a higher risk of leak potentially because they require proper mobilization of the mesentery for tension-free construction, and other possibilities involve issues related to access and technical considerations [27,28,29]. The issues of access and technical difficulty encountered for low rectal resection using a stapler or other devices have been reported in the literature. Access issues apply to the male pelvis, which is narrow and results in difficulty of stapler fire use [30,31,32,33]. In cases of low rectal cancer, access is relatively difficult compared to that of ultra-low cancers, for which resection and coloanal anastomosis are performed from the perineal approach. A coloanal hand-sewn anastomosis in these situations may not require a diverting stoma as the technical difficulties, when constructing an anastomosis from above, are fully bypassed [34]. Similarly, perineal rectosigmoidectomy and coloanal anastomosis for rectal prolapse seldom require a diverting stoma.
Adverse intraoperative events were a consistent indication of a diverting stoma in the RCTs included in this meta-analysis. An adverse intraoperative event could be a technical failure and may include anastomosis under tension, a positive air leak test, sub-optimally perfused ends or a spillage of enteric contents. The use of a diverting stoma in these situations is similar to the diversion of traffic away from a bridge that is at risk of collapsing. Subsequent ileostomy closure resembles the resumption of traffic following necessary maintenance work. Endoscopic vacuum therapy may play a role in improving the healing of a leak provided that a diverting ileostomy is already fashioned. This confirms that a diverting ileostomy in these cases does not inhibit leakage but reduces the need for additional surgery in the case of a leak. A diverting stoma would merely prevent the faecal stream from reaching the anastomosis and prevent a subclinical leak from becoming symptomatic. A stoma may delay the presentation of leak, but it will not prevent any delayed complications of leak, such as low anterior resection syndrome (LARS) and other functional issues [35]. Prior radiotherapy in these patients and possibly delayed stoma closure may also impact the functional outcomes commonly seen in clinical practice [36].
The issues related to anastomotic leak are sepsis and the need for reoperation or radiological intervention to limit the severity of infection. The rate of reoperation in this meta-analysis was significantly higher in the group not covered with a stoma than in the stoma-covered group simply because of the absence of a diverting stoma to limit the septic process. In addition to sepsis, the risk of local rectal cancer recurrence is a real concern after anastomotic leak regardless of whether a diverting stoma is used. Theoretically, patients without a diverting stoma would undergo surgical intervention sooner for an anastomotic leak than patients covered with a stoma, which may provide some protection against local recurrence at the cost of permanent colostomy. Only one of the RCTs reported recurrence of the disease in both groups [16]. These issues have never been addressed in large studies and have not been compared in patients with and without a diverting stomas [37, 38].
The economic implications of a diverting stoma are worth mentioning, as health economics along with patient safety have become pivotal factors in the provision of health services. Apparently, patients who require a stoma need to stay in the hospital longer to receive some training for managing their stomas. Additionally, they must be readmitted to have their stomas reversed at a later stage after confirmation of anastomotic integrity by appropriate measures. Moreover, the visits to and by the stoma therapist and the cost of stoma appliances further add to the costs of the procedure. A very selective approach for diverting stomas, on the other hand, would certainly lead to significant cost savings.
It is believed by the authors that instead of covering every distal anastomosis with a loop ileostomy, a change in the strategy and a judicial intraoperative approach may curtail the risks of anastomotic leak. The timing of the anastomosis is a crucial factor that can easily be addressed with minimal effort. Construction of anastomoses in colorectal surgery is usually the last step of a long and exhausting surgical procedure. Surgeons are commonly tired at the end of a complex operation and tend to lose their concentration, thus potentially making compromises on limits of satisfaction and leaving nature to take its course in a favourable way. A short break just before construction of an anastomosis may lower the rate of this complication and could be explored in future trials.
Although the RCTs included in this meta-analysis unanimously supported the use of a diverting stoma, their findings may need further validation. In addition to the small number of randomized patients, especially in one of the multicentre trials, there was a significant cross-over of patients from one group to the other [14]. In another multicentre trial, more than 70% of the total patients were found unsuitable for inclusion in the trial, which potentially reduced the external validity of the findings. A high anastomotic leak rate in general and in 40% of those detected on readmission raises concerns pertaining to the validity of the trial protocol. The precise definition of anastomotic leak was different across the included trials. Two of the trials included only clinical leaks [11, 13], two of the trials considered both radiological and clinical leaks to be significant [14, 15] and one of the trials provided no definition of anastomotic leak [16]. The trials not considering a radiological leak did not report the proportion of radiological leaks in their results. Different types of anastomosis were constructed across the studies but the investigations carried out for the confirmation of leak were identical among all the trials. A routine gastrografin enema was carried out by only Mrak et al. for the detection of leak before discharge from the hospital. The absence of a screening tool for leak detection in the early postoperative period may have led to an underestimation of leaks in the patients with ileostomies. The two multicentre trials and a pilot trial followed both preoperative and intraoperative inclusion criteria, and patients with the adverse intraoperative events of a positive air leak test, incomplete anastomotic rings or a level of anastomosis other than that described in the study protocol, were withdrawn from the study. Patients with stage IV disease were not considered for inclusion in three of the trials, whereas Thoker et al. mentioned only patients requiring APR as being excluded. Patients with radiotherapy and immunosuppression were excluded by Chude et al. Complications related to stomas and stoma closures were not reported by Ulrich et al., and the sequelae of anastomotic leaks were not detailed in another study [16]. The differences in the study characteristics are highlighted in the tables above, and it is believed that because of the inherent flaws in their methodology and the incoherent study protocols and follow-ups, the recommendations of the included RCTs should be followed with caution.
The strong possibility of publication bias, the inclusion of studies from apparently low-volume centres and the relatively low quality of the RCTs investigating the usefulness of diverting ileostomy limit the findings of this meta-analysis. High-quality RCTs from high-volume centres with uniform inclusion/exclusion criteria and one type of anastomosis are needed to further investigate the impact of diverting ileostomy on anastomotic leak. Until then, the decision of whether to divert or not should be left entirely to the operating surgeon. A routine diversion procedure may be discouraged and avoided where possible because of the high complication rate and cost implications.
References
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 69:613–616
Karanjia ND, Corder AP, Bearn P, Heald RJ (1994) Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg 81:1224–1226
Enker WE, Merchant N, Cohen AM, Lanouette NM, Swallow C, Guillem J, Paty P, Minsky B, Weyrauch K, Quan SHQ (1999) Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg 230:544–552 discussion 552-4
Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, Friess H (2008) Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 248:52–60
Gavriilidis P, Azoulay D, Taflampas P (2019) Loop transverse colostomy versus loop ileostomy for defunctioning of colorectal anastomosis: a systematic review, updated conventional meta-analysis, and cumulative meta-analysis. Surg Today 49:108–117
Güenaga KF et al (2007) Ileostomy or colostomy for temporary decompression of colorectal anastomosis. Cochrane Database Syst Rev:Cd004647
Rondelli F, Reboldi P, Rulli A, Barberini F, Guerrisi A, Izzo L, Bolognese A, Covarelli P, Boselli C, Becattini C, Noya G (2009) Loop ileostomy versus loop colostomy for fecal diversion after colorectal or coloanal anastomosis: a meta-analysis. Int J Colorectal Dis 24:479–488
Geng HZ, Nasier D, Liu B, Gao H, Xu YK (2015) Meta-analysis of elective surgical complications related to defunctioning loop ileostomy compared with loop colostomy after low anterior resection for rectal carcinoma. Ann R Coll Surg Engl 97:494–501
Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 339:b2535
Higgins JP et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343:d5928
Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R (2007) Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 246:207–214
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Chude GG, Rayate NV, Patris V, Koshariya M, Jagad R, Kawamoto J, Lygidakis NJ (2008) Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: should we make an ileostomy as a routine procedure? A prospective randomized study. Hepatogastroenterology 55:1562–1567
Mrak K, Uranitsch S, Pedross F, Heuberger A, Klingler A, Jagoditsch M, Weihs D, Eberl T, Tschmelitsch J (2016) Diverting ileostomy versus no diversion after low anterior resection for rectal cancer: a prospective, randomized, multicenter trial. Surgery 159:1129–1139
Ulrich AB, Seiler C, Rahbari N, Weitz J, Büchler MW (2009) Diverting stoma after low anterior resection: more arguments in favor. Dis Colon Rectum 52:412–418
Thoker M, Wani I, Parray FQ, Khan N, Mir SA, Thoker P (2014) Role of diversion ileostomy in low rectal cancer: a randomized controlled trial. Int J Surg 12:945–951
Pakkastie TE, Ovaska JT, Pekkala ES, Luukkonen PE, Järvinen HJ (1997) A randomised study of colostomies in low colorectal anastomoses. Eur J Surg 163:929–933
Graffner H, Fredlund P, Olsson SÅ, Oscarson J, Petersson BG (1983) Protective colostomy in low anterior resection of the rectum using the EEA stapling instrument. A randomized study. Dis Colon Rectum 26:87–90
Tan WS, Tang CL, Shi L, Eu KW (2009) Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg 96:462–472
Cong ZJ, Hu LH, Zhong M, Chen L (2015) Diverting stoma with anterior resection for rectal cancer: does it reduce overall anastomotic leakage and leaks requiring laparotomy? Int J Clin Exp Med 8:13045–13055
Anderin K, Gustafsson UO, Thorell A, Nygren J (2016) The effect of diverting stoma on long-term morbidity and risk for permanent stoma after low anterior resection for rectal cancer. Eur J Surg Oncol 42:788–793
Ihnát P, Guňková P, Peteja M, Vávra P, Pelikán A, Zonča P (2016) Diverting ileostomy in laparoscopic rectal cancer surgery: high price of protection. Surg Endosc 30:4809–4816
Shiomi A, Ito M, Maeda K, Kinugasa Y, Ota M, Yamaue H, Shiozawa M, Horie H, Kuriu Y, Saito N (2015) Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg 220:186–194
Emmanuel A, Chohda E, Lapa C, Miles A, Haji A, Ellul J (2018) Defunctioning stomas result in significantly more short-term complications following low anterior resection for rectal cancer. World J Surg 42:3755–3764
Hu MH, Huang RK, Zhao RS, Yang KL, Wang H (2017) Does neoadjuvant therapy increase the incidence of anastomotic leakage after anterior resection for mid and low rectal cancer? A systematic review and meta-analysis. Colorectal Dis 19:16–26
Shiomi A, Ito M, Saito N, Hirai T, Ohue M, Kubo Y, Takii Y, Sudo T, Kotake M, Moriya Y (2011) The indications for a diverting stoma in low anterior resection for rectal cancer: a prospective multicentre study of 222 patients from Japanese cancer centers. Colorectal Dis 13:1384–1389
Zhou S, Zhou H, Zheng Z, Liang J, Zhou Z, Wang X (2019) Predictive risk factors for anastomotic leakage after anterior resection of rectal cancer in elderly patients over 80 years old: an analysis of 288 consecutive patients. World J Surg Oncol 17:112
Braunschmid T, Hartig N, Baumann L, Dauser B, Herbst F (2017) Influence of multiple stapler firings used for rectal division on colorectal anastomotic leak rate. Surg Endosc 31:5318–5326
Otsuka K et al (2019) Laparoscopic low anterior resection with two planned stapler fires. Jsls 23:e2018
Lipska MA, Bissett IP, Parry BR, Merrie AEH (2006) Anastomotic leakage after lower gastrointestinal anastomosis: men are at a higher risk. ANZ J Surg 76:579–585
Kim JS, Cho SY, Min BS, Kim NK (2009) Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg 209:694–701
Balciscueta Z, Uribe N, Caubet L, López M, Torrijo I, Tabet J, Martín MC (2020) Impact of the number of stapler firings on anastomotic leakage in laparoscopic rectal surgery: a systematic review and meta-analysis. Tech Coloproctol 24:919–925
Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N (2008) Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis 23:703–707
Huh JW, Park YA, Sohn SK (2007) A diverting stoma is not necessary when performing a handsewn coloanal anastomosis for lower rectal cancer. Dis Colon Rectum 50:1040–1046
Hain E, Manceau G, Maggiori L, Mongin C, Prost à la Denise J, Panis Y (2017) Bowel dysfunction after anastomotic leakage in laparoscopic sphincter-saving operative intervention for rectal cancer: a case-matched study in 46 patients using the Low Anterior Resection Score. Surgery 161:1028–1039
Hughes DL, Cornish J, Morris C (2017) Functional outcome following rectal surgery-predisposing factors for low anterior resection syndrome. Int J Colorectal Dis 32:691–697
Wang S, Liu J, Wang S, Zhao H, Ge S, Wang W (2017) Adverse effects of anastomotic leakage on local recurrence and survival after curative anterior resection for rectal cancer: a systematic review and meta-analysis. World J Surg 41:277–284
Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK (2016) Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum 59:236–244
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, N.Z., Abbas, M.H., Khan, S.U. et al. A meta-analysis of the role of diverting ileostomy after rectal cancer surgery. Int J Colorectal Dis 36, 445–455 (2021). https://doi.org/10.1007/s00384-020-03771-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03771-z