Abstract
Background
Anastomotic complications are among the most devastating consequences of gastrointestinal surgery. Despite its high morbidity, the factors responsible for anastomotic regeneration following surgical construction remain poorly understood. The aim of this review is to provide an overview of the typical and atypical factors that have been implicated in anastomotic healing.
Methods
A review and analysis of select literature on anastomotic healing was performed.
Results
The healing of an anastomotic wound mirrors the phases of cutaneous wound healing- inflammation, proliferation, and remodeling. The evidence supporting much of the traditional dogma for optimal anastomotic healing (ischemia, tension, nutrition) is sparse. More recent research has implicated atypical factors that influence anastomotic healing, including the microbiome, the mesentery, and geometry. As technology evolves, endoscopic approaches may improve anastomotic healing and in some cases may eliminate the anastomosis altogether.
Discussion
Much remains unknown regarding the mechanisms of anastomotic healing, and research should focus on elucidating the dynamics of healing at a molecular level. Doing so may help facilitate the transition from traditional surgical dogma to evidence-based medicine in the operating room.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biology is a balancing act—a constant struggle to maintain equilibrium through upregulation and downregulation of physiologic, molecular, and biochemical processes. When the balance tips, disease develops. The healing gastrointestinal anastomosis, a fundamental tenet of surgery, is no different. Anastomotic regeneration involves a finely tuned balance between too little healing (anastomotic leak) and excessive healing (anastomotic stricture). These frequently encountered complications are the Achilles heel of surgery, given that they are morbid, costly, and disabling.1
In the auditorium of virtually every surgical morbidity and mortality conference, the common culprits implicated in anastomotic leak (tension, ischemia, and technique) are echoed by conventional wisdom. Yet, despite the legacy of this wisdom, proof that these factors actually contribute to anastomotic leak in a given individual remains specious. In fact, still today, the precise mechanisms that govern the process of anastomotic regeneration remain poorly understood.
In this article, we will highlight general principles of wound healing and review both common and atypical factors that influence anastomotic healing. Finally, we will discuss advances in endoscopic surgery, a rapidly evolving field where anastomoses are avoided altogether.
Physiology of Wound Healing
The mechanics of wound healing have been previously well characterized in the skin, as cutaneous models of wound healing are easy to generate and visualize in real time. Regardless, these fundamentals of healing are applicable to almost every organ system, including the gastrointestinal tract. In brief, the body must achieve hemostasis, decontaminate the site of injury, cover the wound, and induce cellular differentiation to form all layers of the prior tissue to fully regain integrity, strength, and function. These physiologic responses of wound healing have classically been divided into three successive and overlapping phases—inflammation, proliferation, and remodeling.
Inflammation
The objectives of the inflammatory phase of wound healing are to attain hemostasis, clear potentially invading microbes, and signal for the recruitment of additional cell types. Following injury, platelets localize to the wound where they catalyze fibrin plug formation. This plug both stops bleeding and simultaneously acts as a scaffold for deposition of matrix proteins and the migration of cells.2 Platelets also release chemotactic proteins stored within granules at the injury site, most notably platelet-derived growth factors (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), and various cytokines.2 Shortly after platelet arrival and activation of the coagulation cascade, neutrophils and monocytes (later macrophages) migrate to the site of injury to clear any potential pathogens contaminating the wound via phagocytosis.
Proliferation
The objective of the proliferative phase of wound healing is to cover the exposed wound and replete the multiple layers of injured tissue. Chemoattractants released by macrophages attract fibroblasts, which deposit collagen (of various isotypes including type I–V) to add strength to the healing tissue, and myofibroblasts, which contract to shrink the wound.3 In the skin, keratinocytes at the leading edge of the migration front deposit laminin over the wound surface, and stem cells in the epidermis mobilize and proliferate over a regenerated basement membrane to cover the exposed wound.4 This migration occurs concurrently with differentiation of the keratinocytes to generate stratified layers of the epidermis.
Remodeling
Beginning near the end of the proliferative phase and lasting for several weeks after injury, the remodeling phase matures the wound through rearrangement of both collagen and vasculature. Matrix metalloproteases break down type 3 collagen and replace it with type 1 collagen, the primary collagen subtype in scar tissue. Concurrently, the initially disorganized vasculature network remodels and becomes more arranged. Even at the conclusion of the process of remodeling, tissue integrity is not equivalent to that pre-injury—the average healed wound reaches only approximately 80% of the strength of uninjured tissue.
Common Factors Implicated in Anastomotic Healing
A surgical anastomosis weakens almost immediately following its construction—during this early period, the staples or sutures are critical for maintaining the integrity of the connection. The extent to which integrity is maintained by surgical technique alone is debated, with some arguing that “all anastomoses leak,” yet most remain asymptomatic because the adjacent tissues contain and seal further spread of the process. With time, tissue layers regenerate and the submucosa becomes the primary strength layer of the anastomosis, as this area develops both a rich collagen network and an extended vasculature. Many studies have examined factors known to impact anastomotic healing using animal models. The most common factors implicated in anastomotic healing include tissue perfusion/ischemia, tissue tension, and patient nutritional status.
As the area of anastomosis is highly metabolically active, traditional dogma suggests that the regenerating tissue has a requisite blood flow to provide both oxygen and nutrients to the healing wound. Although the precise level of blood flow or oxygen needed at the anastomosis to properly heal has not been defined, ischemia has been commonly implicated as a cause of anastomotic breakdown in the conducting esophagus, small intestine, and colon/rectum.5,6,7 A possible mechanism for this is diminished oxygen tension, as hypoxia has been shown to impair collagen synthesis, thereby impeding collagen reinforcement of the anastomosis as surgical suture/staple strength diminishes over time8 However, in animal models where the gastrointestinal anastomoses is devascularized, no difference in dehiscence and leak rates are observed, suggesting that low vascular flow states and hypoxia are not predictors of anastomotic failure.9,10 In fact, methods to detect hypoxia/hypoperfusion during surgery ignore the observation that most hypoxia in surgical patients occurs postoperatively, which remains unaccounted for in these analyses.11 These conflicting results highlight that the extent to which ischemia plays a role in anastomotic leak remains unclear.
Traditional teaching also notes that tension increases the risk of surgical failure. While surgical training and practice constantly emphasize the need to be vigilant to create “tension-free” physiology within anatomical reconstructions, the claim that tension plays a role in anastomotic leak remains unsubstantiated at the individual patient level. There are no definitive animal studies or convincing clinical studies implicating tension as a putative cause of anastomotic healing, perhaps with the exception of esophageal atresia surgery.12 This may be a result of limited methods to model and measure tension. In an animal model of tension at esophagogastric anastomoses, increased tension (measured by a tension meter) significantly increased the propensity of the anastomosis to break down.13 A retrospective review of intestinal anastomoses revealed a × 10.1 increased odds of anastomotic leak with increased anastomotic tension; however, this study was powered by a sample of 5 patients having tension on the anastomosis, 2 of which leaked.14 Furthering the counterargument against tension, Katory et al. demonstrated no difference in anastomotic outcomes in high colorectal anterior resections when comparing operations with and without splenic flexure mobilization, a common procedure to relieve tension at the anastomosis.15 Therefore, while considered surgical dogma, the role of tension in anastomotic healing also remains unclear. Much of the legacy of thought around the mechanisms of anastomotic leak is a result of the fact that, under proper study conditions, data demonstrate that surgeons are not able to predict which patients will leak and which will not, although, a priori, most are certain that they can.16
Another frequently cited cause for anastomotic failure is poor healing secondary due to inadequate nutritional status. Severely malnourished patients are often started on enteral or parental nutrition prior to surgery, with this nutritional optimization frequently extending postoperatively as well. Enteral supplementation prior to surgery has been shown to significantly reduce (4% vs 25%) septic complications including anastomotic leak in patients with Crohn’s disease.17 Multiple studies have also demonstrated that lower preoperative albumin and prealbumin levels correlate to significantly higher risks of anastomotic leak in gastric, small intestinal, and colonic anastomoses.18,19,20,21 However, while the decrease in albumin levels between leak and no leak groups is statistically significant, the absolute values of these differences are often mild (less than 10%) and many times border on normal physiologic levels. Thus, unless the patient is in extreme states of malnutrition, it is ambiguous how these preoperative nutritional assessments can alter clinical decision-making when differences between groups occur at such a small scale. Finally, recent studies examining the effect of supplemental products to improve outcomes from elective surgery in otherwise healthy patient undergoing gastrointestinal surgery have failed to show any benefit.22
Uncommon Factors Implicated in Anastomotic Healing
Microbiome in Anastomotic Healing
A major disordering agent across all healing wounds is the presence of bacteria. As mentioned above, during tissue healing, cells such as macrophages clear invading pathogens that can disrupt the fragile balance of wound regeneration, which involves repeated and fine-tuned iterations of protein synthesis and protein breakdown to refine and remodel the wound to its greatest level of integrity and strength. As a result, excess protease activity is the hallmark of abnormal healing whereby breakdown exceeds synthetic capacity.23 Bacteria are among those agents that tip the scales of protease activity, shifting from fine-tuning collagen breakdown and remodeling to excessive degradation of collagen and wound integrity.24 Certain strains of bacteria can directly induce, accelerate, and enhance the enzymatic activity of key tissue proteases such as matrix metalloproteinase (MMP) 9 and plasminogen, such that healing becomes pathoadaptive to maintain full integrity and function.24 This fragile mechanism may be especially relevant in the gastrointestinal track given that it normally harbors a diverse population of microbes, collectively referred to as the microbiome. In addition, with surgeons’ different antibiotic prescribing practices, precisely which pathogens are eliminated, which remain, and which colonize anastomotic tissues as they heal vary tremendously between cases.25 These practices may play an unrecognized yet critical role in the molecular pathogenesis of anastomotic healing.
As a result, emerging lines of evidence have begun to shed light on the role of the microbiome on gastrointestinal surgical healing. Commensal bacteria modulate many host genes, including those in maintaining mucosal barrier integrity. The physiologic stress of surgery has been known to dramatically alter the ecology and composition of the gut microbiome, sometimes increasing the relative abundance of Enterococcus species 500-fold.26 The presence of specific phenotypes of Enterococcus faecalis at the site of a surgical anastomosis (i.e., those that produce the protease enzyme collagenase) can activate host tissue proteases such as MMP9, resulting in impaired healing at these sites leading to a significant risk of anastomotic dehiscence.24
The implication of maintaining the beneficial microbiome ecology challenges traditional dogma of preoperative antibiotic prophylaxis. In patients undergoing colon resection, preoperative administration of intravenous cefoxitin, a common prophylactic for gastrointestinal surgery, decreases levels of commensal Escherichia species, resulting in a bloom of cefoxitin-resistant Enterococci.27 In contrast, administration of topical antibiotics at the anastomosis decreases the abundance of collagenase-producing organisms and decreases anastomotic leak rates.24 Furthermore, locally applying tranexamic acid to the surgical anastomosis may provide a novel solution to prevent anastomotic dehiscence by inhibiting gut microbes from activating another key protease system present in the healing anastomotic wound, the plasminogen pathway.28
Diet may also play a key role in anastomotic healing as it can directly modulate the composition and function of the microbiome. For example, feeding mice a high fat western diet has been shown to alter the normal gut microbiome, resulting in a state of persistent low-level intestinal inflammation.29 Interestingly, Hyoju et al. recently demonstrated that administering a short course of dietary prehabilitation (2 days of a high fiber/low fat diet) not only reverses the microbiota disruption associated with a western high fat diet, but also reverses the increased risk of anastomotic dehiscence.30 Thus, a better understanding of the dynamic interactions between diet, use of antibiotics, and their effect on the microbiome may shed light on how to optimally prepare the bowel (either through diet, selective antibiotics, or both) prior to surgery.
The Mesentery in Anastomotic Healing
While anastomotic dehiscence sits at one end of the spectrum of anastomotic healing, at the other end sits anastomotic stricture, a product of excessive healing. This concept is best demonstrated in patients with Crohn’s disease. Following stricturoplasty, a high percentage of patients develop disease recurrence at the anastomotic site.31 A common hypothesis for the etiology of stricture formation is proposed to involve the mesentery, given that stricture recurrence frequently occurs on the mesenteric side of the anastomosis. With this in mind, different anastomotic techniques have been proposed in an attempt to minimize these recurrence rates.
The Kono-S anastomosis, first described in 2003, is one of the best-known anastomotic techniques that provides some evidence that recurrence rates of anastomotic stricture may be based on technique alone. In the Kono-S anastomosis, the stricture bowel is excised 90 degrees to the mesentery, the stumps are sutured together to create a supporting column, and then an anti-mesenteric functional end-to-end anastomosis is created. In effect, the diseased mesentery is excluded from the anastomosis and instead constructed adjacent to the supporting column. The group’s initial study showed a surgical recurrence free survival of 100% at 5 years in the Kono-S group, compared with 85% in the historical stapled anastomosis group.32 In a follow-up randomized control trial, the Kono-S anastomosis group had a significant reduction in postoperative endoscopic and clinical recurrence rates for Crohn’s disease.33 Further studies have also implicated the effect of the mesentery on anastomotic healing. A cohort study comparing mesentery excision with limited resection in ileocolic resection for Crohn’s demonstrated that mesentery excision was associated with decreased rates of recurrence of stricture at the anastomosis.34 Therefore, the mesentery, now considered a metabolically active organ,35 most likely alters anastomotic healing in a manner independent of ischemia. However, further research is needed to fully elucidate the mechanisms by which the mesentery influences anastomotic regeneration, including how the anastomotic microbiome, flow characteristics, and healing parameters are affected by this construction versus more conventional approaches.
Geometry in Anastomotic Healing
While dogmatic teaching passed on to trainees over decades has focused on common themes of anastomotic construction (tension-free, blood supply, technical aspects), there are several factors that remain to be considered and are only now beginning to be studied. Technological advances now allow researchers to study the specific effects of anastomotic geometry on a healing surgical anastomosis.36 Although native luminal anatomy, whether it is intestinal, ureteral, or vascular, exists in a linear conformation, surgeons commonly create structural continuity using non-linear anastomoses (e.g., side-to-side and end-to-side anastomoses). Therefore, a major question exists, i.e. to what extent does the geometry of anastomotic construction itself influence outcome? The answers to this question and others are complicated and rooted in a plethora of confounding factors, but extrapolating from the behavior of non-biological materials under similar conformations and loads may provide some insight to these questions.37,38 Simple computer simulations can be used to analyze hyperelastic neo-Hookean material responses, and introducing Ogden-Holzapfel parameter modeling can attribute physiologic relevance to anastomotic geometry.39 In studying linear and non-linear geometries, it quickly becomes apparent that non-linear constructions result in manipulations of the tissue to which the tissue must adapt. However, it is not clear how these new stresses may affect the tissue response to injury, including and involving the flow of materials and microbes across anastomotic tissues, and their interaction with residual internal stresses. Thus, there is much that remains to be learned about how geometry can influence anastomotic healing across multiple domains of knowledge.
Novel Approaches for Anastomotic Construction and Methods to Eliminate the Anastomosis Altogether
Given the high costs and morbidity associated with anastomotic complications, an optimal approach in surgical resections may be to avoid the anastomosis altogether. This is the promise of endoscopic surgery. Over the past decade, endoscopic surgery has increased in prevalence, initially with the advent of endoscopic mucosal resection (EMR) and subsequently endoscopic submucosal dissection (ESD). Lesions greater than 20 mm can only be removed piecemeal in EMR, thereby limiting histological confirmation of complete tumor excision. ESD, in contrast, allows for deeper en bloc resections. Advances in technology though have minimized these rates of adverse events and also generated novel methods to manage these complications non-operatively, including with endoscopic clips.40
Complications such as bleeding and perforation remain minimal with ESD, with reported rates of 3.3%, 3.5%, and 4.6% in the esophagus, stomach, and colon, respectively.41 Despite the risk of perforation and the exposure of a large submucosal defect to endogenous microflora, use of prophylactic antibiotics with EMR/ESD is not a standard practice. Rates of bacteremia following ESD have been reported between 1 and 2.5%, and among the patients that develop bacteremia, no patients manifested clinical symptoms.42,43,44 These results suggest that the native commensal bacteria of the gastrointestinal tract are nonpathogenic when contained intraluminally, even in the setting of a mucosal or submucosal wound. If the case, then the prophylactic administration of antibiotics would only perturb the normal gut microbiota and predispose the individual to the growth of pathogenic strains.
A prior limitation of endoscopic resections has been its inability to reach the majority of the small intestine. However, gastroenterologists and surgeons are now able to interrogate the entire small bowel with double-balloon endoscopy. The technique involves consecutive inflation/deflation of balloons at the tips of an endoscope and an over-the-scope tube (overtube) to provide points of fixation as the scope is advanced further into the small intestine. Coupling this with EMR/ESD platforms thereby creates the opportunity to endoscopically remove superficial lesions throughout the entirety of the gastrointestinal tract.45 Another cited limitation of endoscopic surgery is the inability to resect non-lifting lesions or neoplasms beyond the superficial layers of the gastrointestinal wall. Therefore, there is great interest in endoscopic full thickness resections (EFTR), with early prospective trials showing a reasonable technical efficacy in successfully removing 89.5% in colorectal adenomas.46 Training and early adoption of these techniques is paramount for surgeons to stay at the forefront of best practices of caring for patients.
Similar to the surgical anastomosis, the precise mechanisms governing regeneration after endoscopic resections remains poorly understood. Most likely in a manner similar to keratinocyte healing, the surrounding mucosal epithelium migrates over the exposed wound and differentiates to form the specific cell types of the mucosa. Development of an ex vivo platform to study mucosal regeneration offers the promise of dissecting the molecular patterns associated with mucosal healing in real time. A better fundamental understanding of the mucosal repair process would shed light on novel targets that can accelerate or alter the wound healing process.
Conclusion
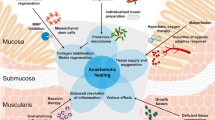
A potentially unanticipated effect of the legacy of applying traditional surgical dogma to explain poor anastomotic healing is that it may dampen enthusiasm to consider other mechanisms of this complex and devastating complication. Further research should focus beyond the classic factors of ischemia, tension, and technique, and investigate atypical causes of anastomotic complications, including the microbiome, the role of the mesentery, and the influence of geometry (Fig. 1). Aside from this, one of the most promising areas of innovation lies in endoscopic surgery, where anastomotic geometry, technique, and flow may be improved, or where an anastomosis is avoided altogether. Prompt adoption of these techniques represents one of the next areas of innovation in gastrointestinal surgery with promise to decrease complications and improve patient outcomes. The first step in this process is to fully understand, at the most detailed molecular and physical-geometric level, what we are doing right and what we are doing wrong.
References
Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal A et al. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg. 2015;220(2):195-206. https://doi.org/10.1016/j.jamcollsurg.2014.11.002.
Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532-48.
Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9-18. https://doi.org/10.1016/j.clindermatol.2006.09.007.
Nguyen BP, Ryan MC, Gil SG, Carter WG. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol. 2000;12(5):554-62. https://doi.org/10.1016/s0955-0674(00)00131-9.
Reavis KM. The esophageal anastomosis: how improving blood supply affects leak rate. J Gastrointest Surg. 2009;13(9):1558-60. https://doi.org/10.1007/s11605-009-0906-7.
Kologlu M, Yorganci K, Renda N, Sayek I. Effect of local and remote ischemia-reperfusion injury on healing of colonic anastomoses. Surgery. 2000;128(1):99-104. https://doi.org/10.1067/msy.2000.107414.
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. 2000;43(1):76-82. https://doi.org/10.1007/bf02237248.
Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186(3):259-63. https://doi.org/10.1016/s0002-9610(03)00211-3.
Shakhsheer BA, Lec B, Zaborin A, Guyton K, Defnet AM, Bagrodia N et al. Lack of evidence for tissue hypoxia as a contributing factor in anastomotic leak following colon anastomosis and segmental devascularization in rats. Int J Colorectal Dis. 2017;32(4):539-47. https://doi.org/10.1007/s00384-016-2737-9.
Posma LA, Hendriks T, Verhofstad AA, de Man BM, Lomme RM, Bleichrodt RP. Reduction of oxygenation and blood flow in pedicled bowel segments in the rat and its consequences for anastomotic healing. Dis Colon Rectum. 2010;53(1):93-100. https://doi.org/10.1007/DCR.0b013e3181bc05a2.
Sun Z, Sessler DI, Dalton JE, Devereaux PJ, Shahinyan A, Naylor AJ et al. Postoperative Hypoxemia Is Common and Persistent: A Prospective Blinded Observational Study. Anesth Analg. 2015;121(3):709-15. https://doi.org/10.1213/ANE.0000000000000836.
Campos J, Tan Tanny SP, Kuyruk S, Sekaran P, Hawley A, Brooks JA et al. The burden of esophageal dilatations following repair of esophageal atresia. J Pediatr Surg. 2020. https://doi.org/10.1016/j.jpedsurg.2020.02.018.
Cui Y, Chen H. The effect of tension on esophagogastric anastomotic wound healing in rats. J Cardiovasc Surg (Torino). 2003;44(6):775-8.
Morse BC, Simpson JP, Jones YR, Johnson BL, Knott BM, Kotrady JA. Determination of independent predictive factors for anastomotic leak: analysis of 682 intestinal anastomoses. Am J Surg. 2013;206(6):950-5; discussion 5-6. https://doi.org/10.1016/j.amjsurg.2013.07.017.
Katory M, Tang CL, Koh WL, Fook-Chong SM, Loi TT, Ooi BS et al. A 6-year review of surgical morbidity and oncological outcome after high anterior resection for colorectal malignancy with and without splenic flexure mobilization. Colorectal Dis. 2008;10(2):165-9. https://doi.org/10.1111/j.1463-1318.2007.01265.x.
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis. 2009;24(5):569-76. https://doi.org/10.1007/s00384-009-0658-6.
Yamamoto T, Nakahigashi M, Shimoyama T, Umegae S. Does preoperative enteral nutrition reduce the incidence of surgical complications in patients with Crohn’s disease? A case-matched study. Colorectal Dis. 2020;22(5):554-61. https://doi.org/10.1111/codi.14922.
Golda T, Lazzara C, Zerpa C, Sobrino L, Fico V, Kreisler E et al. Risk factors for ileocolic anastomosis dehiscence; a cohort study. Am J Surg. 2019. https://doi.org/10.1016/j.amjsurg.2019.11.020.
Zhou J, Hiki N, Mine S, Kumagai K, Ida S, Jiang X et al. Role of Prealbumin as a Powerful and Simple Index for Predicting Postoperative Complications After Gastric Cancer Surgery. Ann Surg Oncol. 2017;24(2):510-7. https://doi.org/10.1245/s10434-016-5548-x.
Telem DA, Chin EH, Nguyen SQ, Divino CM. Risk factors for anastomotic leak following colorectal surgery: a case-control study. Arch Surg. 2010;145(4):371-6;discussion 6. https://doi.org/10.1001/archsurg.2010.40.
Frasson M, Granero-Castro P, Ramos Rodriguez JL, Flor-Lorente B, Braithwaite M, Marti Martinez E et al. Risk factors for anastomotic leak and postoperative morbidity and mortality after elective right colectomy for cancer: results from a prospective, multicentric study of 1102 patients. Int J Colorectal Dis. 2016;31(1):105-14. https://doi.org/10.1007/s00384-015-2376-6.
Takagi K, Domagala P, Hartog H, van Eijck C, Groot Koerkamp B. Current evidence of nutritional therapy in pancreatoduodenectomy: Systematic review of randomized controlled trials. Ann Gastroenterol Surg. 2019;3(6):620-9. https://doi.org/10.1002/ags3.12287.
Edwards JV, Howley PS. Human neutrophil elastase and collagenase sequestration with phosphorylated cotton wound dressings. J Biomed Mater Res A. 2007;83(2):446-54. https://doi.org/10.1002/jbm.a.31171.
Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 2015;7(286):286ra68. 10.1126/scitranslmed.3010658.
Alverdy JC, Hyman N. Bowel preparation under siege. Br J Surg. 2020;107(3):167-70. https://doi.org/10.1002/bjs.11454.
Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome. 2014;2:35. https://doi.org/10.1186/2049-2618-2-35.
Kager L, Malmborg AS, Nord CE, Pieper R. The effect of short-term cefoxitin prophylaxis on the colonic microflora in patients undergoing colorectal surgery. Infection. 1982;10(6):338-40. https://doi.org/10.1007/BF01642294.
Jacobsen PB, Prasad R, Villani J, Lee CM, Rochlin D, Scheuter C et al. The role of economic analyses in promoting adoption of behavioral and psychosocial interventions in clinical settings. Health Psychol. 2019;38(8):680-8. https://doi.org/10.1037/hea0000774.
de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G440-8. https://doi.org/10.1152/ajpgi.00098.2010.
Hyoju SK, Adriaansens C, Wienholts K, Sharma A, Keskey R, Arnold W et al. Low-fat/high-fibre diet prehabilitation improves anastomotic healing via the microbiome: an experimental model. Br J Surg. 2020;107(6):743-55. https://doi.org/10.1002/bjs.11388.
Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11(2):135-49. https://doi.org/10.1093/ecco-jcc/jjw169.
Kono T, Ashida T, Ebisawa Y, Chisato N, Okamoto K, Katsuno H et al. A new antimesenteric functional end-to-end handsewn anastomosis: surgical prevention of anastomotic recurrence in Crohn’s disease. Dis Colon Rectum. 2011;54(5):586-92. https://doi.org/10.1007/DCR.0b013e318208b90f.
Luglio G, Rispo A, Imperatore N, Giglio MC, Amendola A, Tropeano FP et al. Surgical Prevention of Anastomotic Recurrence by Excluding Mesentery in Crohn’s Disease: The SuPREMe-CD Study - A Randomized Clinical Trial. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000003821.
Coffey CJ, Kiernan MG, Sahebally SM, Jarrar A, Burke JP, Kiely PA et al. Inclusion of the Mesentery in Ileocolic Resection for Crohn’s Disease is Associated With Reduced Surgical Recurrence. J Crohns Colitis. 2018;12(10):1139-50. https://doi.org/10.1093/ecco-jcc/jjx187.
Coffey JC, O’Leary DP. The mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol. 2016;1(3):238-47. https://doi.org/10.1016/S2468-1253(16)30026-7.
Fontanella CG, Salmaso C, Toniolo I, de Cesare N, Rubini A, De Benedictis GM et al. Computational Models for the Mechanical Investigation of Stomach Tissues and Structure. Ann Biomed Eng. 2019;47(5):1237-49. https://doi.org/10.1007/s10439-019-02229-w.
Witten TA. Stress Focusing in Elastic Sheets. Reviews of Modern Physics. 2007;79:643-75.
Seffen KA. Fundamental conical defects: The d-cone, its e-cone, and its p-cone. Phys Rev E. 2016;94(1-1):013002. https://doi.org/10.1103/PhysRevE.94.013002.
Ciarletta P, Dario P, Tendick F, Micera S. Hyperelastic Model of Anisotropic Fiber Reinforcements within Intestinal Walls for Applications in Medical Robotics. The International Journal of Robotics Research. 2009;28(10):1279-88.
Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006;63(4):596-601. https://doi.org/10.1016/j.gie.2005.07.029.
Odagiri H, Yasunaga H. Complications following endoscopic submucosal dissection for gastric, esophageal, and colorectal cancer: a review of studies based on nationwide large-scale databases. Ann Transl Med. 2017;5(8):189. https://doi.org/10.21037/atm.2017.02.12.
Kawata N, Tanaka M, Kakushima N, Takizawa K, Imai K, Hotta K et al. The low incidence of bacteremia after esophageal endoscopic submucosal dissection (ESD) obviates the need for prophylactic antibiotics in esophageal ESD. Surg Endosc. 2016;30(11):5084-90. https://doi.org/10.1007/s00464-016-4857-2.
Kato M, Kaise M, Obata T, Yonezawa J, Toyoizumi H, Yoshimura N et al. Bacteremia and endotoxemia after endoscopic submucosal dissection for gastric neoplasia: pilot study. Gastric Cancer. 2012;15(1):15-20. https://doi.org/10.1007/s10120-011-0050-4.
Min BH, Chang DK, Kim DU, Kim YH, Rhee PL, Kim JJ et al. Low frequency of bacteremia after an endoscopic resection for large colorectal tumors in spite of extensive submucosal exposure. Gastrointest Endosc. 2008;68(1):105-10. https://doi.org/10.1016/j.gie.2007.11.051.
Rahmi G, Vinet MA, Perrod G, Saurin JC, Samaha E, Ponchon T et al. Efficacy of double-balloon enteroscopy for small-bowel polypectomy: clinical and economic evaluation. Therap Adv Gastroenterol. 2017;10(6):465-72. https://doi.org/10.1177/1756283X17696232.
Schmidt A, Beyna T, Schumacher B, Meining A, Richter-Schrag HJ, Messmann H et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018;67(7):1280-9. https://doi.org/10.1136/gutjnl-2016-313677.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lam, A., Fleischer, B. & Alverdy, J. The Biology of Anastomotic Healing—the Unknown Overwhelms the Known. J Gastrointest Surg 24, 2160–2166 (2020). https://doi.org/10.1007/s11605-020-04680-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04680-w