Abstract
Background
Pancreas-sparing duodenectomy (PSD) offers definitive therapy for duodenal polyposis associated with familial adenomatous polyposis (FAP). We reviewed the long-term complications of PSD and evaluated the incidence of high-grade dysplasia (HGD) and cancer in the remaining upper gastrointestinal tract.
Methods
Forty-seven FAP patients with duodenal polyposis undergoing PSD from 1992 to 2019 were reviewed. Long-term was defined as > 30 days from PSD.
Results
All patients were treated with an open technique, and 43 (91.5%) had Spigelman stage III or IV duodenal polyposis. Median follow-up was 107 months (IQR, 26–147). There was no 90-day mortality. Seven patients died at a median of 10.5 years (IQR, 5.4–13.3) after PSD, with one attributed to gastric cancer. Pancreatitis occurred in 10 patients (21.3%), and two required surgical intervention. Seven patients (14.9%) developed an incisional hernia, and all underwent definitive repair. Forty-one patients (87.2%) had postoperative surveillance endoscopy over a median follow-up of 111 months (IQR, 42–138). Three patients (6.4%) developed adenocarcinoma (two gastric, one jejunal), and four (8.5%) had adenomas with HGD (two gastric, two jejunal) with a median of 15 years (IQR, 9–16) from PSD. One patient with gastric adenocarcinoma and all patients with HGD or adenocarcinoma of the jejunum required surgical intervention.
Conclusion
PSD can be performed with a low but definable risk of long-term morbidity. Risk of gastric and jejunal carcinoma rarely occurs and was diagnosed decades after PSD. This demonstrates the need for lifelong endoscopic surveillance and educates us on the risk of carcinoma in the remaining gastrointestinal tract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogenic variants in the adenomatous polyposis coli (APC) gene cause familial adenomatous polyposis (FAP). Colon and rectum adenomas will inevitably develop, and there is a near universal occurrence of duodenal adenomas and an increased incidence of duodenal carcinoma.1,2 While the incidence of colorectal cancer in FAP patients is 100%, duodenal cancer is seen in only 5%.3 The Spigelman criteria for staging of duodenal polyposis were developed to help guide the frequency of endoscopic surveillance and treatment by estimating the duodenal cancer risk. Individuals with stage IV polyposis have been shown to have a 36% risk of cancer.3,4 To complicate matters, the majority of these duodenal adenomas are seen in the second and third portions of the duodenum, making treatment challenging due to the proximity of the pancreas.3
Treatment of duodenal polyposis in FAP consists of endoscopic and surgical modalities. Depending on the degree of involvement within the duodenum and stage of the disease, surgical management involves local resection, organ-preserving resections, or pancreatoduodenectomy. Local resection in FAP-associated duodenal polyposis, as opposed to sporadic disease, has a high recurrence rate, whereas pancreatoduodenectomy or organ-preserving resections, such as pancreas-sparing duodenectomy (PSD) and segmental duodenectomy, offers definitive management of current dominant polyp burden.5 Moreover, PSD can provide definitive management for advanced duodenal polyposis in FAP patients with the added advantage of leaving an intact pancreas to preserve the endocrine and exocrine function as compared with pancreatoduodenectomy.6,7,8
PSD was first reported by Chung et al.9 for treatment in FAP patients with nonmalignant duodenal adenomas not involving the pancreas. We have previously described the early postoperative complications and reoperations along with 30-day mortality for this approach in FAP patients with duodenal polyposis.10 Yet, the long-term complications and mortality of this approach have not been studied. The primary aim of this study was to characterize the long-term complications and mortality of PSD. Additionally, we have previously reported the prevalence of duodenal bulb and jejunal polyposis in FAP patients.11 However, this represented a mixed population of patients undergoing different types of duodenal resection. Similarly, polyp disease in the stomach and common bile duct is not reported. Therefore, the secondary aim of this study was to evaluate the incidence of high-grade dysplasia (HGD) and cancer in the remaining upper gastrointestinal tract, including the stomach, duodenal bulb, jejunum, and common bile duct, after PSD. We hypothesized that there would be a low risk of long-term morbidity and mortality, with a rare incidence of HGD and cancer in the remaining gastrointestinal tract.
Materials and Methods
A retrospective case review was done at a single, quaternary hospital. All FAP patients with duodenal polyposis who underwent PSD at the Cleveland Clinic from 1992 to 2019 were identified from a prospectively maintained database and reviewed. The Cleveland Clinic Institutional Review Board approved the study.
Patient demographics including age and gender along with preoperative body mass index (BMI) and most recent Spigelman stage prior to surgical intervention were collected. Each chart was reviewed to collect information on long-term complications, reoperations, and mortality. Any complication, reoperation, and mortality > 30 days from the date of PSD were considered long-term. Pancreatitis was recorded if there was a documentation of elevated lipase with typical symptoms or a documented diagnosis. All incisional hernias were diagnosed clinically. Small bowel obstruction was recorded if there was a documented diagnosis based on clinical and radiographic findings. As these patients may have multiple abdominal surgeries, small bowel obstruction was only recorded if the cause was determined to be related to the PSD operation. Therefore, obstructions that occurred in the setting of PSD as the most recent surgery were the only ones included. Exclusions consisted of obstructions related to ileostomy site hernias and patients undergoing surgical intervention with findings of only pelvic adhesions. Additionally, admissions for small bowel obstructions following other abdominal surgeries were excluded. Other long-term complications such as cholangitis and marginal ulcer were also recorded. Information regarding post-PSD upper gastrointestinal tract cancer was extracted from the electronic medical record. As we have recently published our results concerning the prevalence of duodenal bulb and jejunal polyposis in FAP patients after duodenectomy,11 this study only reviewed the development of HGD or cancer in the stomach, duodenal bulb, jejunum, or common bile duct in those patients undergoing PSD specifically. Any interventions related to these findings are discussed. Any visit after PSD in our medical system was reviewed for follow-up data.
This study focused on the long-term data for this patient population, and as such, no information regarding the PSD operation (e.g., estimated blood loss and operative time) or outcomes within 30 days from the date of PSD was included. Pancreatic function has been previously studied relative to pancreatoduodenectomy and is not reiterated.8 PSD was performed on FAP patients with diffuse duodenal disease and no suspicion of cancer based on preoperative evaluation.
Surgical Technique
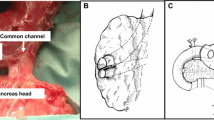
Our surgical technique for PSD has been previously described.6,8 In brief, an upper midline incision is performed, and the jejunum is transected distal to the ligament of Treitz. Moving proximally toward the duodenum, the mesentery of the jejunum is divided, and the distal duodenum is mobilized. If the gallbladder is present, a cholecystectomy is performed. The cystic duct is cannulated with a Fogarty catheter to aid in identification of the ampulla. The duodenum is transected distal to the pylorus, and the proximal duodenum is mobilized. The entire ampullary complex is resected, along with any polyp tissue present in the distal common bile duct or pancreatic duct. The duodenum is then removed, and reconstruction is performed. The common channel of the common bile duct and pancreatic duct is anastomosed in a single layer to the proximal jejunum. Distal to the pancreaticobiliary reconstruction, an end-to-side duodenojejunostomy is performed.
Statistical Analysis
Continuous variables were reported as medians with interquartile ranges or range as indicated. Comparisons were made using analysis of variance (ANOVA). Categorical variables were reported as total occurrences. A p < 0.05 was considered statistically significant. Analysis was performed using JMP (version 14, Cary, NC).
Results
There were 47 patients who underwent PSD with all patients undergoing an open approach. Median follow-up was 107 months (IQR, 26–147) with one patient lost to follow-up.
Study Population
Table 1 represents the demographic characteristics. The majority of the patients were male (61.7%). Three patients (6.4%) had a desmoid tumor removed during their PSD operation with intra-abdominal, retroperitoneal, and abdominal wall locations, respectively. In the patient with the intra-abdominal location, there were multiple mesenteric desmoids. These tumors were identified preoperatively on computed tomography scan, and a small bowel follow through was completed that revealed no obstruction. During PSD, a small bowel resection of the mid-jejunum was performed to remove all but one desmoid. The other desmoid was proximal, non-obstructing, and determined to be unresectable. In the patient with the retroperitoneal location, the desmoid tumor was adherent to and appeared to be arising from the distal duodenum. Therefore, this tumor was included in the resected specimen. In the patient with the abdominal wall location, multiple tumors were removed from the posterior abdominal wall during lysis of adhesions. This patient also had a desmoid in the mesenteric root identified at the time of operation and was not removed. Of the four patients (8.5%) who had a concomitant incisional hernia repair during PSD, only one had repair with mesh, which consisted of biologic material.
Table 2 shows the preoperative endoscopic characteristics along with Spigelman stage. The majority of the patients had Spigelman stages III and IV. One patient with an unknown Spigelman stage had tubulovillous polyps in the duodenum and proximal jejunum with no evidence of cancer, but there was concern for carcinoma as one of the polyps in the jejunum was ulcerated. Therefore, this patient had additional proximal jejunum removed during the operation. Both patients with Spigelman stage II had dominant polyps at the ampulla warranting definitive resection. One patient (2.1%) with a dominant periampullary polyp and Spigelman stage IV (high-grade dysplasia on preoperative biopsy) preoperatively had periampullary duodenal cancer (pT1bN0) on final surgical pathology. No intraoperative biopsy was performed, and one lymph node was evaluated in the pathological specimen. This patient developed a hepatic metastasis 1 year after PSD. It has been 2 years since PSD, and the patient remains alive.
Long-Term Complications and Mortality
Table 3 shows the long-term mortality. There was no mortality within 90 days of the operation. Seven patients (14.9%) died at a median interval of 10.5 years (IQR, 5.4–13.3) after surgery. All of the known causes of mortality were not directly attributed to PSD. One patient developed gastric cancer with hepatic metastasis and peritoneal carcinomatosis and died 187 months after PSD at 76 years of age. One patient who had gastric polyposis associated with multiple episodes of upper gastrointestinal bleeding underwent a subtotal gastrectomy that was complicated by an intra-abdominal abscess. As the patient was being treated for this, they had a massive gastrointestinal bleeding episode leading to hemorrhagic shock and eventual death 97 months after PSD at 56 years of age. Another patient had a hemorrhagic stroke as the cause of their death 126 months after PSD at 61 years of age. The remaining four patients had a cause of death that was unknown due to unclear documentation and died at a median age of 68 years (range, 45–87).
Table 4 shows the long-term complications. Seven patients (14.9%) developed an incisional hernia, with none of these patients having any prior hernia repairs before PSD. Additionally, no patient with a concomitant incisional hernia repair or desmoid tumor removal during PSD developed an incisional hernia. Two patients had an associated surgical site infection (SSI), and one had an associated organ space infection (OSI) within 30 days of PSD. All seven patients underwent surgical repair with a median of 34 months (IQR, 10–113) from PSD. One of the patients needed two hernia repairs due to a separate area of herniation after the first hernia surgery. This patient had an open approach for the first hernia repair, and the second hernia repair was able to be performed laparoscopically. All other hernia repairs were performed with an open approach. One was attempted laparoscopically but had to be converted to open due to extensive adhesions. Four patients had mesh placed, whereas three patients were able to be closed primarily. There was no difference in age, gender, or BMI for those patients that developed incisional hernias.
Pancreatitis developed in ten patients (21.1%) with a median occurrence of 27 months (IQR, 15–53) after PSD. Two of these patients had an episode of acute pancreatitis preoperatively with one related to the gallstones and the other to post-endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy and ampullectomy. Postoperative pancreatitis etiology was attributed to a pancreatic ductal anastomotic stricture seen on ERCP in one patient and pancreas divisum in another. Even after an extensive work-up including ERCP documenting patent pancreatic orifices, the other eight patients had an unclear etiology. However, the pancreatic ductal orifice in one patient was unable to be located on ERCP due to polyps surrounding the expected area of anastomosis. No intervention has been needed for these polyps, and the patient has had no further documented episodes of pancreatitis for over a year. There was no difference in age, gender, or BMI for those patients that developed pancreatitis.
The patient with a pancreatic ductal anastomotic stricture has been treated with balloon dilation and stenting. Only two patients (4.3%) required an operation related to their pancreatitis. One patient had pancreas divisum that was discovered at the time of PSD, and therefore, both pancreatic ducts were reimplanted as separate anastomoses. However, the patient had multiple episodes of ventral pancreatitis postoperatively with failed endoscopic management and underwent a pancreatic head resection 87 months after PSD. The other patient had a Puestow 26 months after PSD due to development of chronic pancreatitis with a dilated main pancreatic duct. Both patients have had no recurrent pancreatitis episodes.
One patient (2.1%) with a duodenojejunostomy developed a marginal ulcer 7 months after PSD. Initial presentation was due to an upper gastrointestinal bleeding which was managed endoscopically. Three years later, the patient presented with pneumoperitoneum from a perforated marginal ulcer and underwent a modified Graham patch repair. Two patients (4.3%) developed a small bowel obstruction with a median of 97 months (range, 30–163) from surgery. Both were managed conservatively with resolution of symptoms. No patients developed cholangitis.
Reoperations
Overall, there was a total of 13 long-term complication-related reoperations in 10 patients (21%)—eight incisional hernia repairs, one pancreatic head resection and one Puestow for pancreatitis, two reoperations for a patient with an enterocutaneous fistula (ECF), and one modified Graham patch repair for a perforated marginal ulcer. Three patients (6.5%) underwent two reoperations. One patient had two hernia repairs, while another patient underwent a pancreatic head resection and hernia repair. The ECF was related to a gastrojejunal anastomotic leak that occurred in the early (≤ 30 days) postoperative period. A distal gastrectomy and Billroth I was subsequently performed 7 months later for definitive management. However, there was recurrence of the ECF followed by repair and takedown of the ECF with an abdominal wall reconstruction.
Postoperative Endoscopic Surveillance
Table 5 shows characteristics of postoperative endoscopic surveillance. Forty patients (87.2%) underwent endoscopic surveillance with a median of 8 upper endoscopies (IQR, 2–12). Median follow-up was 111 months (IQR, 42–138).
Three patients (6.4%) had detection of advanced neoplasia in the jejunum, including two developing HGD and one adenocarcinoma. One patient had a tubulovillous adenoma with low-grade dysplasia (LGD) involving the neo-ampulla on endoscopic biopsy and was unable to undergo complete endoscopic resection. Further work-up with a capsule endoscopy revealed jejunal polyposis, and the patient underwent a pancreatic head and transposed jejunum resection 21 years after PSD. Final pathology showed evidence of HGD with no invasive carcinoma. There has been no further development of HGD in the remaining jejunum 6 years after surgical intervention. Another patient had a dominant polyp with an area of HGD just distal to the duodenojejunostomy not involving the neo-ampulla discovered on endoscopy 15 years after PSD. This lesion was unable to be completely resected by endoscopy. Capsule endoscopy was performed prior to surgical intervention and did not reveal jejunal polyposis. This patient underwent resection of the transposed proximal jejunum involving the dominant polyp and remaining duodenal bulb and pylorus with an end-to-end gastrojejunostomy reconstruction. Final pathology revealed LGD. Lastly, jejunal adenocarcinoma was discovered on postoperative endoscopic surveillance 16 years after PSD in one patient. The dominant polyp did not involve the neo-ampulla, and this patient is currently being worked up for surgical intervention.
Overall, four patients (8.5%) had development of HGD or carcinoma of the stomach. Two patients had HGD 8 and 11 years, respectively, after PSD. Both underwent endoscopic polypectomy and have had no recurrence of HGD. The other two patients had gastric cancer 9 and 15 years, respectively, from PSD. One patient was managed surgically with a total gastrectomy, while the other patient had hepatic metastases with peritoneal carcinomatosis warranting chemotherapy with mortality 6 months after diagnosis.
One patient (2.1%) developed a common bile duct polyp with evidence of LGD 11 years after PSD and underwent resection of the pancreatic head and transposed proximal jejunum. Final surgical pathology revealed a tubular adenoma with LGD. No patients had a development of HGD or carcinoma in the remaining duodenal bulb.
Discussion
This study validates the use of PSD as definitive management in FAP-associated advanced duodenal polyposis. There is a low but definable risk of long-term morbidity of PSD with pancreatitis and incisional hernia being the most common long-term complications. Moreover, the rare occurrence of cancer in the stomach or jejunum speaks to understanding the approach to and utility of postoperative upper endoscopic surveillance. Overall, PSD remains an effective treatment option for FAP-associated duodenal polyposis, and those patients who ultimately need additional surgery for upper gastrointestinal polyp disease is rare.
This study had a long-term mortality rate of 14.9% at a median of 10.5 years, with no mortality within 90 days of the operation. The known causes of mortality were primarily attributable to gastric cancer, stroke, or hemorrhagic shock. This is similar to the reported rates of long-term mortality after PSD in FAP patients with duodenal polyposis.12 We have shown that these patients can survive for years after PSD. More importantly, no deaths were directly attributed to PSD. Although there was a small percentage of patients who developed HGD or adenocarcinoma in the remaining upper gastrointestinal tract, only one patient ultimately had mortality from advanced gastric cancer. Gastric cancer had not previously been considered a risk in FAP but has clearly been documented in our experience.13 Furthermore, only five patients ultimately needed surgical intervention for polyp-related disease. This highlights the benefits and efficacy of postoperative endoscopic surveillance and management, with surgery being reserved for those that are not amenable to complete endoscopic resection or progression to carcinoma. The major advantage in performing PSD in these patients is allowing for a more feasible endoscopic evaluation of the jejunum and bile duct compared with pancreatoduodenectomy due to the differences in gastrointestinal reconstruction.
Incisional hernias are a known complication of laparotomy with an incidence of 9–23% in elective cases, and both SSIs and OSIs are risk factors for the development of incisional hernias after abdominal surgery.14,15,16,17 Our rate of 14.9% for the development of incisional hernias was at the lower end of this range, with approximately half of the patients having an associated SSI or OSI in the early postoperative period. This was interesting as one would hypothesize that these patients would have an increased rate of incisional hernia due to their abdominal surgical history. All of these incisional hernias were clinically diagnosed and not solely by radiography. Nevertheless, this rate of incisional hernias in this patient population is similar to that reported by Al-Sarireh et al..18 They performed the operation with a bilateral subcostal incision and had a much smaller subset of patients with a follow-up of 20 months. Fink et al.,14 however, showed that incisional hernia rates increased significantly from 1 to 3 years of follow-up suggesting that the rate of incisional hernia could be higher than reported by Al-Sarireh et al..18 Even so, the rate of incisional hernia after a subcostal incision is similar to that for a midline laparotomy.19,20 In this patient population, though, a midline laparotomy seems to be the optimal choice given the high rates of previous midline laparotomies for a total colectomy and often need for complete adhesiolysis. Incisional hernias do not occur at an increased rate in this patient population for those who undergo a midline laparotomy, and hence, the risk is similar to other elective midline laparotomy cases. Therefore, a midline laparotomy is the ideal incision for this operation and should be done, barring no other factors preclude its use.
When Chung et al.10 first described PSD, he mentioned that there might be a risk of pancreatitis, yet there have been few studies to assess this risk. Three studies have been performed that report the rate of pancreatitis between 8 and 22% in patients after PSD.12,18,21 Our rate of pancreatitis was similar to these studies. It is of interest that only one of our patients developed an anastomotic stricture involving the pancreatic or common bile duct. One of the patients was unable to have assessment of their pancreatic ductal anastomosis due to polyp growth at the presumed site. Special considerations need to be made in patients with PSDs who present with pancreatitis. In addition to the typical etiologies of pancreatitis, anastomotic strictures and polyps need to be ruled out as potential causes. Another consideration in this patient population is that the pancreatitis could be due to occasional reflux of enteric contents into the pancreatic duct due to the lack of a sphincter. Nevertheless, pancreatitis is a complication of PSD and pancreatoduodenectomy, and reoperation is rarely needed for treatment. Due to the difficulty in the reimplantation of both dorsal and tiny ventral pancreatic ducts, our experience indicates that pancreas divisum found at PSD should require conversion to pancreatoduodenectomy. This serves as a better treatment option for patients with pancreas divisum and can help to reduce the risk of postoperative pancreatitis in this subset of patients.
There are limitations to this study. First, this was a single center review, possibly leading to selection bias. Given that the majority of these patients are referred and the retrospective nature of the study, follow-up data for patients is variable. However, we are a specialized center for FAP, and most patients are followed at our institution postoperatively for endoscopic surveillance. Similarly, if they are not followed at our institution, they are typically referred back to our surgeons and gastroenterologists with any new findings related to their surgery or FAP disease. Lastly, as FAP is a rare disease and those requiring upper gastrointestinal surgery for polyps is a small subset of this patient population, the risk of HGD or cancer in the stomach, duodenal bulb, jejunum, and common bile duct reported here represents a unique population. To estimate the true risk of advanced neoplasia in the upper gastrointestinal tract, patients not undergoing duodenectomy will need to also be studied.
Conclusions
We have the largest single site PSD dataset and hope that our longstanding commitment to these patients aid others interested in the disease. PSD offers definitive management in those with advanced duodenal polyposis and is associated with a low risk of long-term morbidity and mortality. Given the repeated abdominal surgeries in FAP patients and complexity with a PSD in reimplanting the pancreatic duct, pancreatitis and incisional hernia risk are low, but surgeons who perform this procedure should be aware of these risks and have the resources to manage them. Given the risk of carcinoma in the remaining upper gastrointestinal tract, lifelong postoperative endoscopic surveillance is necessary. By providing a direct route to the jejunum along with the bile and pancreatic duct for endoscopy, PSD is the optimal treatment in FAP patients with advanced duodenal polyposis compared with pancreatoduodenectomy. The outcomes from this study can be utilized to help better inform patients in the future on the risks and benefits involved with this operation.
Abbreviations
- PSD:
-
Pancreas-sparing duodenectomy
- FAP:
-
Familial adenomatous polyposis
- APC:
-
Adenomatous polyposis coli
- BMI:
-
Body mass index
- SSI:
-
Surgical site infection
- OSI:
-
Organ space infection
- HGD:
-
High-grade dysplasia
- LGD:
-
Low-grade dysplasia
References
Bulow S, Bjork J, Christensen IJ, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004; 53: 381-86
Offerhaus GJ, Giardello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992; 102(6): 1980-2.
Groves CJ, Saunders BP, Spigelman AD, et al. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002; 50(5): 636-41.
Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989; 2: 783-85.
Johnson MD, Mackey R, Brown N, et al. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg. 2010; 14(2): 229-35.
Mackey R, Walsh RM, Chung R, et al. Pancreas-sparing duodenectomy is effective management for familial adenomatous polyposis. J Gastrointest Surg. 2005; 9(8): 1088-93
Kalady MF, Clary BM, Tyler DS, et al. Pancreas-preserving duodenectomy in the management of duodenal familial adenomatous polyposis. J Gastrointest Surg. 2002: 6(1): 82-7.
Walsh RM, Augustin T, Aleassa EM, et al. Comparison of pancreas-sparing duodenectomy (PSD) and pancreatoduodenectomy (PD) for the management of duodenal polyposis syndromes. Surgery. 2019; 166(4): 496-502.
Chung RS, Church JM, vanStolk R. Pancreas-sparing duodenectomy: indications, surgical technique, and results. Surgery. 1995; 117(3): 254-59.
Augustin T, Moslim MA, Tang A, et al. Tailored surgical treatment of duodenal polyposis in familial adenomatous polyposis syndrome. Surgery. 2018; 163:594-99.
Yoon JY, Mehta N, Burke CA, et al. The Prevalence and Significance of Jejunal and Duodenal Bulb Polyposis After Duodenectomy in Familial Adenomatous Polyposis: Retrospective Cohort Study. Ann Surg. 2019; in press.
Ganschow P, Hackert T, Biegler M, et al. Postoperative outcome and quality of life after surgery for FAP-associated duodenal adenomatosis. Langenbecks Arch Surg. 2018; 403(1): 93-102.
Mankaney G, Leone P, Cruise M, et al. Gastric cancer in FAP: a concerning rise in incidence. Fam Cancer. 2017; 16(3): 371-6.
Fink C, Baumann P, Wente MN, et al. Incisional hernia rate 3 years after midline laparotomy. Br J Surg. 2014; 101(2): 51-4.
Deerenberg EB, Harlaar JJ, Steyerberg EW, et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015; 386(10000): 1254-1260.
Diener MK, Voss S, Jensen K, et al. Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg. 2010; 251(5): 843-56.
Itatsu K, Yokoyama Y, Sugawara G, et al. Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg. 2014; 101(11): 1439-47.
Al-Sarireh B, Ghaneh P, Gardner-Thorpe J, et al. Complications and follow-up after pancreas-preserving total duodenectomy for duodenal polyps. Br J Surg. 2008; 95(12): 1506-11.
D’Angelica M, Maddineni S, Fong Y, et al. Optimal abdominal incision for partial hepatectomy: increased late complications with Mercedes-type incisions compared to extended right subcostal incisions. World J Surg. 2006; 30(3): 410-18.
Donataccio M, Genco B, Donataccio D. Right subcostal incision in liver transplantation: prospective study of feasibility. Transplant Proc. 2006; 38(4): 1109-10.
Nakayama Y, Konishi M, Gotohda N, et al. Comparison of postoperative early and late complications between pancreas-sparing duodenectomy and pancreatoduodenectomy. Surg Today. 2017; 47(6): 705-11.
Author information
Authors and Affiliations
Contributions
Authors meet all 4 criteria for authorship as described by the International Committee of Medical Journal Editors (ICMJE).
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naples, R., Simon, R., Moslim, M. et al. Long-Term Outcomes of Pancreas-Sparing Duodenectomy for Duodenal Polyposis in Familial Adenomatous Polyposis Syndrome. J Gastrointest Surg 25, 1233–1240 (2021). https://doi.org/10.1007/s11605-020-04621-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04621-7