Abstract
Background
Surgical and oncological outcomes in ruptured hepatocellular carcinoma (HCC) are not well known. The objective of this study was to review and compare survival outcomes and recurrence rates between ruptured and unruptured HCC.
Methods
Data of patients with ruptured HCC who underwent curative surgical resection between January 2000 and December 2016 were retrospectively reviewed. To compare survival outcomes between ruptured and unruptured HCC, 1:2 individual matching was conducted.
Results
The 1-, 3-, and 5-year overall survival (OS) rates were 88.8%, 67.0%, and 51.9%, respectively. The 1-, 3-, and 5-year disease-free survival (DFS) rates were 51.7%, 32.8%, and 25.0%, respectively. OS and DFS rates were significantly lower in the ruptured HCC group than the matched unruptured HCC group. HCC recurred in 63 patients (70.8%), 33 (52.4%) of whom presented with both intrahepatic and extrahepatic recurrences. Mean recurrence interval was 12.6 ± 13.8 months. The 1-, 3-, and 5-year survival rates after recurrence were 61.6%, 40.2%, and 33.6%, respectively. Mean survival time after recurrence was 26.4 ± 29.5 months. Incidence of peritoneal seeding (PS) was 18.0%, and eight of them demonstrated solitary lesion. Mean recurrence interval was 5.9 ± 8.2 months. The 1-, 3-, and 5-year OS rates after recurrence were significantly lower in patients with PS (49.7%, 18.7%, and 9.3%, respectively) than in patients without PS.
Conclusions
Hepatectomy in ruptured HCC did show worse survival outcome compared with unruptured HCC and bear a high risk of PS. However, surgical resection combined with transcatheter arterial chemoembolization could help in achieving acceptable oncological outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous rupture of hepatocellular carcinoma (HCC) occurs occasionally. Recently, the reported incidence rate of spontaneous rupture of HCC varied from 2.3 to 5.9%1,2,3,4, which has decreased compared with its previously reported incidence of up to 15%, owing to advances in the surveillance system for high-risk group and diagnostic imaging modality5,6,7. Rapid tumor growth leading to intra-tumoral necrosis and tumor hypervascularity with friable feeder artery associated with degeneration of elastin and type IV collagen was the suggested causes of tumor rupture in HCC, although the exact pathogenesis of rupture remains unclear.
HCC rupture management requires a stepwise, multidisciplinary approach that considers hemostasis for bleeding from the ruptured tumor and HCC treatment. A recent review suggested that transarterial embolization followed by elective hepatectomy is effective in patients with ruptured HCC8.
With the development of surgical techniques (hepatic resection, angiographic intervention, and critical care medicine), various treatment modalities for ruptured HCC have been introduced. However, mortality rates in ruptured HCC are still high9 and oncological outcomes are still insufficient. Moreover, whether tumor rupture is a poor prognostic factor after hepatectomy for ruptured HCC remains controversial. Several studies showed that ruptured HCC had inferior outcomes compared with unruptured HCC1,10,11,12,13,14. In selected cases, HCC has low recurrence rate and showed oncological outcome comparable to that of unruptured HCC15,16,17,18,19,20.
Although the TNM staging system of the 8th edition of American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) classifies tumor rupture as T4, the general rules of the 6th edition of the Liver Cancer Study Group of Japan (LCSGJ) do not consider tumor rupture for T staging21,22,23, indicating that the actual HCC rupture effect on oncological outcomes still needs to be elucidated. Several studies reported the actual outcomes of ruptured HCC. However, most previous studies are limited by small study population, short duration of follow-up period, and non-comparative design.
This study aimed to retrospectively review and compare survival outcomes and recurrence rates in patients with ruptured HCC who underwent surgical resection at a single center in hepatitis B virus (HBV)-endemic area to elucidate the actual oncological outcome and analyze the recurrence pattern of ruptured HCC with a focus on peritoneal seeding (PS).
Materials and Methods
Study Population
Clinical data of adult patients (≥ 18 years) who underwent hepatectomy for spontaneously ruptured HCC from January 2000 to December 2016 at Asan Medical Center, Seoul, South Korea, were retrospectively reviewed.
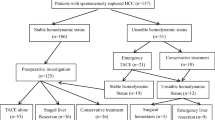
Between January 2000 and December 2016, 7602 patients with HCC underwent liver resection. Among them, 115 had ruptured HCC at the time of diagnosis, of which 26 cases of non-curative resection were excluded. Finally, 89 patients were retrospectively reviewed (Fig. 1).
We performed 1:2 individual matching between the ruptured HCC group and unruptured HCC group and included 85 patient pairs. Matching variables were the number and maximal size of tumors, preoperative alpha fetoprotein (AFP) value, hypermetabolic activity on preoperative positron emission tomography (PET), and presence of macro- and microvascular invasion on pathology.
Diagnosis
Diagnosis of ruptured HCC was made based on computed tomography (CT) or magnetic resonance imaging (MRI) findings and was confirmed through laparotomy and gross findings (hemoperitoneum or hematoma around the tumor accompanied by disruption of tumor integrity). CT is a valuable imaging modality in ruptured HCC diagnosis as it can detect tumor and free intraperitoneal fluid containing high attenuation or blood clot close to tumor24,25,26. In patients with unruptured HCC, chest CT, PET, and bone scan were performed to identify extrahepatic metastasis and assess the disease extent in patients with ruptured HCC with stable vital signs. The assessment of hepatic functional reservoir was focused on the indirect signs of portal hypertension, including thrombocytopenia (< 100,000/uL), prolonged prothrombin time, varix on endoscopy, ascites, splenomegaly, or surface nodularity on imaging studies. Total bilirubin, albumin, and indocyanine green retention tests were taken together. Liver lobar or segmental volume was measured to estimate the remnant liver volume after hepatectomy using CT volumetry software Picture Archiving and Communication System (PetaVision for Clinics, South Korea).
Staging System
The staging system followed the TNM classification (8th Edition) developed by AJCC/UICC and LCSGJ (6th Edition, in Japanese): “General Rules for the Clinical and Pathological Study of Primary Liver Cancer”21, 23.
Treatment Algorithm
Transcatheter arterial chemoembolization (TACE) was considered first to control bleeding from tumor when a patient presented with overt symptoms and signs of significant bleeding (altered hemodynamic profiles, intolerable pain, and abrupt change in serum hemoglobin). After the patient’s status had stabilized, hepatic functional reservoir and extrahepatic metastasis were evaluated, followed by hepatectomy. In principle, the timing of the hepatectomy for ruptured HCC was as early as possible after the evaluation for HCC and functional reservoir of the liver were completed. The assessment on HCC extent was performed through CT scan with 3 weeks interval after TACE and the timing for the hepatectomy was determined accordingly.
Criteria to determine the eligibility for hepatectomy were basically same as in case with unruptured HCC; without any evidence of indirect signs portal hypertension including thrombocytopenia (< 100,000/uL), prolonged prothrombin time, varix on endoscopy, ascites, splenomegaly, or surface nodularity on imaging studies, normal ranged liver function test including total bilirubin and indocyanine green test, and acceptable general condition without any contraindication in general anesthesia for major operation.
One-stage surgical resection of ruptured HCC was considered the primary treatment when the extent of the hematoma or hemoperitoneum was minimal, and the patient had stable condition for full evaluation of tumor extent and liver function.
TACE Protocol
Cisplatin (2 mg/kg body weight) was used in patients who underwent TACE. A microcatheter with a diameter of < 2.4 Fr was used, and cisplatin was infused for 15 minutes into the segmental, lobar, or proper hepatic artery, depending on the location and volume of the tumor. Before the 15-min cisplatin infusion, a certain amount of cisplatin was set aside and mixed at a 1:1 ratio in an emulsion of iodized oil (lipiodol; Guerbet, Roissy, France), which was infused (dose, 3–20 mL according to the tumor size) into the segmental feeding artery, followed by embolization with Gelfoam slurry (Upjohn, Kalamazoo, MI, USA) until stasis of arterial flow was confirmed.
Surgical Technique
Likewise in unruptured HCC, the fundamental principle in hepatectomy for ruptured HCC also follows the anatomical resection. The extent of resection was determined through the remnant liver volume and liver functional reservoir. Anatomical hepatectomy was the primary choice, and the extent of hepatectomy was individualized according to the estimated future remnant liver volume on CT volumetry and hepatic functional reserve. Various standard techniques for liver resection were implemented depending on the conditions of the operative field (Pringle maneuver and hanging maneuver). The Glissonean or individual approach and parenchymal transection with Cavitron ultrasonic surgical aspirator, energy device, or crush–clamp were chosen on a case-by-case basis and surgeon’s preference.
Response to Treatment (mRECIST)
The Modified Response Evaluation Criteria in Solid Tumors (mRECIST) was incorporated in assessing response to the TACE prior to hepatic resection27, 28. The mRECIST criteria only focus on HCC and only consider the viable portion (i.e., the area enhanced after injection during the arterial phase).
Statistical Analysis
All statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) and SAS (SAS version 9.3, Cary, NC). Descriptive statistics for numerical variables are recorded as mean ± standard deviation, and categorical variables are presented as relative frequencies (percentages). We used chi-squared or Fisher’s exact test for comparing categorical data and Student’s t test or Mann–Whitney test for numerical data. A logistic regression model was used for univariate and multivariate analyses. To compare the outcomes of unruptured HCC, the 85 patients in the ruptured HCC group were individually matched 1:2 to an unruptured HCC group using the greedy method, by the size and number of tumors, preoperative AFP value, hypermetabolic activity on preoperative PET, and presence of macro- and microvascular invasion on pathology. The adequacy of the individual matching was described with standardized difference. Patient survival was analyzed using the Kaplan–Meier method and compared by log-rank test. p < 0.05 was considered to indicate significant difference.
Ethical Considerations
The study was approved by the Institutional Review Board of Asan Medical Center, University of Ulsan, Seoul, South Korea (approval number 2017-0477), which waived the requirement for informed consent due to the retrospective nature of the analyses.
Results
Demographics and Clinicopathological Features
Demographic characteristics and clinicopathological features of patients are described in Table 1. The most common primary liver disease and symptom were HBV and pain, respectively. For all 10 asymptomatic patients, ruptured HCC was incidentally identified during diagnostic evaluation for liver tumor.
Treatment
In 38 patients with stable condition, surgical resection was conducted as a primary treatment. TACE was initially performed in the other 51 patients to control bleeding or stabilize the patient’s condition to progress to the staged hepatic resection. In this group, the mean interval between the TACE and hepatectomy was 89 ± 137 days. Among patients who underwent TACE prior to hepatectomy, response to TACE was evaluated through mRECIST. Complete response was achieved in 19.6% of patients (n = 10), partial response was demonstrated in 56.9% (n = 29), stable disease was presented in 17.6% (n = 9), and progressive disease occurred in 5.9% (n = 3).
Right hepatectomy was performed in 23.6% (n = 21) of patients, followed by left hepatectomy in 14.6% (n = 13), monosegmentectomy in 12.4% (n = 11), right anterior sectionectomy in 11.2% (n = 10), right posterior sectionectomy in 10.1% (n = 9), left lateral sectionectomy in 9.0% (n = 8), partial hepatectomy in 7.9% (n = 7), and others (including right or left trisectionectomy, central bisectionectomy, segment 5 and 6 bisegmentectomy) in 11.2% (n = 10). The perioperative mean amount of red blood cell transfusion was 2.1 ± 2.8 units (range 0–16 units). A total of 42 patients (47.2%) did not require transfusion. The mean length of hospital stay was 23.1 ± 10.0 days (range 9–47 days). Neither in-hospital mortality nor post-hepatectomy liver failure occurred.
Survival Outcomes
The 1-, 2-, and 5-year overall survival (OS) rates of the patients were 88.8%, 67.0%, and 51.9%, respectively. The mean survival time was 43.6 ± 34.3 months (Fig. 2a). In the univariate and multivariate analyses for OS in the ruptured HCC group, the gross feature of the tumor (infiltrative type) and variant HCC (sarcomatoid type) were independent significant risk factors (Table 2). HCC recurred in 63 of the 89 patients with ruptured HCC. The actual recurrence rate of ruptured HCC was 70.8%. The 1-, 3-, and 5-year disease-free survival (DFS) rates were 51.7%, 32.8%, and 25.0%, respectively (Fig. 2b). The mean DFS time was 24.5 ± 27.8 months. On the multivariate analysis, AFP > 1000 ng/mL and microvascular invasion were associated with recurrence (Table 2).
a Overall survival. The 1-, 3-, and 5-year overall patient survival rates were 88.8%, 67.0%, and 51.9%, respectively. The mean overall survival time was 43.6 ± 34.3 months. b Disease-free survival. The 1-, 3-, and 5-year disease-free survival rates were 51.7%, 32.8%, and 25.0%, respectively. The mean disease-free survival time was 24.5 ± 27.8 months
Comparison of Survival Outcomes between Ruptured and Unruptured HCC
Prior to the 1:2 individual matching, a significant difference was found between the ruptured and unruptured HCC group in terms of tumor size, hypermetabolism on PET, and macrovascular and microvascular invasion (Table 3). To compare survival outcomes of patients with ruptured HCC with 1:2 matched patients with unruptured HCC, 85 patients with ruptured HCC and 168 matched patients of unruptured HCC were finally included in each group. After 1:2 matching, matching variables showed a standardized difference < 0.1, verifying the adequacy of the matching result (Table 3).
OS and DFS rates of the ruptured HCC group were significantly lower than those of the matched unruptured HCC group (p = 0.041 and p = 0.011, respectively) (Fig. 3a, b). In the ruptured HCC group, the 1-, 3-, and 5-year OS rates were 87.1%, 65.4%, and 48.4%, respectively. In addition, the 1-, 3-, and 5-year OS rates in the matched unruptured HCC group were 84.5%, 72.9%, and 68.7%, respectively. The 1-, 3-, and 5-year DFS rates in the ruptured HCC group were 48.2%, 31.7%, and 25.2%, respectively. In the matched unruptured HCC group, the 1-, 3-, and 5-year DFS rates were 65.8%, 46.3%, and 42.6%, respectively. In the matched set, the rupture per se was an independent significant risk factor for patient death and recurrence of HCC (Table 4).
a Overall survival of patients with ruptured HCC and matched unruptured HCC (p = 0.041). The 1-, 3-, and 5-year patient survival rates in the ruptured HCC group were 87.1%, 65.4%, and 48.4%, respectively. The 1-, 3-, and 5-year patient survival rates in the unruptured HCC group were 84.5%, 72.9%, and 68.7%, respectively. b Disease-free survival of patients with ruptured HCC and matched unruptured HCC (p = 0.011). The 1-, 3-, and 5-year disease-free survival rates in the ruptured HCC group were 48.2%, 31.7%, and 25.2%, respectively. The 1-, 3-, and 5-year disease-free survival rates in the unruptured HCC group were 65.8%, 46.3%, and 42.6%, respectively
Recurrence Pattern and Survival Outcomes after Recurrence in Ruptured HCC
Of the 63 patients with recurrence in ruptured HCC group, 52.4% (n = 33) showed both intrahepatic and extrahepatic recurrences, followed by intrahepatic recurrences only in 19 patients (30.2%) and extrahepatic metastases only in 11 patients (17.5%). The mean interval time to recurrence was 15.1 ± 12.7 months for intrahepatic recurrence only, 4.4 ± 2.4 months for extrahepatic recurrence only, and 13.5 ± 15.8 months for both intrahepatic and extrahepatic metastases.
The lung was the most common single extrahepatic recurrence site (43.2%, n = 19), followed by PS (13.6%) and bone (6.8%). Multiple extrahepatic metastases occurred in 15 patients (34.1%), with the lung (n = 15) and PS (n = 10) as the most common sites of recurrence. In regard to risk factors for extrahepatic metastasis, age > 50 years, infiltrative HCC, and presence of microvascular invasion were associated with extrahepatic metastasis in the univariate analysis. LCSGJ stage IVA and HCC other than nodular or infiltrative were associated with extrahepatic metastasis on the multivariate analysis.
The 1-, 3-, and 5-year survival rates after recurrence were 61.6%, 40.2%, and 33.6%, respectively. The mean survival time after recurrence was 26.4 ± 29.5 months (Fig. 4a). Variant HCC, recurrence sites including extrahepatic recurrence, and presence of PS were associated with poor survival after recurrence in the multivariate analysis. Survival after recurrence in patients with only intrahepatic recurrence was significantly higher than in patients with extrahepatic recurrence (p < 0.001) (Fig. 4b).
a Overall survival after recurrence. The 1-, 3-, and 5-year survival rates after recurrence were 61.6%, 40.2%, and 33.6%, respectively. The mean survival time after recurrence was 26.4 ± 29.5 months. b Overall survival after recurrence according to the pattern of recurrence (p < 0.001 between intrahepatic and extrahepatic and both). The 1-, 3-, and 5-year survival rates after recurrence in patients with only intrahepatic recurrence were 89.5%, 89.5%, and 89.5%, respectively. The mean survival time after recurrence was 40.5 ± 34.7 months. The 1-, 3-, and 5-year survival rates after recurrence in patients with only extrahepatic recurrence were 63.6%, 18.2%, and 18.2%, respectively. The mean survival time after recurrence was 21.3 ± 19.4 months. The 1-, 3-, and 5-year survival rates after recurrence in patients with both intrahepatic and extrahepatic recurrences were 45.5%, 21.9%, and 11.7%, respectively. The mean survival time after recurrence was 20.1 ± 26.9 months. c Overall survival after recurrence according to the presence of PS (p = 0.026). The 1-, 3-, and 5-year survival rates after recurrence in patients without PS were 65.3%, 48.4%, and 43.0%, respectively. The mean survival time after recurrence was 48.0 ± 35.8 months. The 1-, 3-, and 5-year survival rates after recurrence in patients with PS were 49.7%, 18.7%, and 9.3%, respectively. The mean survival time after recurrence was 19.1 ± 18.3 months. d Overall survival after recurrence in patients with PS according to the surgical excision (p = 0.027). The mean survival time after recurrence in patients who received surgical excision for seeding mass was 28.4 ± 21.9 months, and those who did not was 9.9 ± 6.5 months
PS occurred in 16 patients (18.0%) after surgical resection of ruptured HCC. Of 16 patients with PS, 9 (56.3%) demonstrated simultaneous intrahepatic recurrences and 7 (43.7%) presented with extrahepatic metastases only. Concurrent lung metastases were found in 10 patients (62.5%). In patients with PS, the mean interval between the hepatectomy and recurrence was 5.9 ± 8.2 months, which is significantly shorter than in those without PS (14.6 ± 14.7 months, p = 0.005). On the multivariate analysis, risk factors associated with PS were AFP > 1000 ng/mL and tumor size > 5 cm. The survival after recurrence was significantly lower in patients with PS than in patients without PS (p = 0.026) (Fig. 4c).
Except in only one patient with PS who manifested a disseminated pattern, peritoneal metastases were solitary lesion (n = 8) or multiple but countable lesions (n = 7). Eight patients who were eligible for excision underwent surgical resection for PS nodules. Patients who underwent surgical resection for PS mass demonstrated better survival outcome than those who did not undergo excision (p = 0.027) (Fig. 4d).
Discussion
Management of ruptured HCC comprises a two-step approach, i.e., TACE followed by surgical resection, to acquire hemostasis and stabilization of the patient first and then complete treatment with oncological surgery thereafter1,7,8,29. The Korean Liver Cancer Association (KLCA)-National Cancer Center recommended in their practice guidelines that hepatectomy should be considered as the primary treatment for patients with ruptured HCC with stable hemodynamic state, even though several studies reported poorer long-term survival in these patients compared with those with unruptured HCC30. In the management of ruptured HCC, acute phase management dealing with bleeding issue and long-term oncological outcome should be both considered in the decision for treatment modalities.
Through advances in diagnostic imaging modalities and surveillance system for high-risk group (chronic viral hepatitis or liver cirrhosis), earlier diagnosis of HCC with stable hemodynamics in patients with ruptured HCC is now attainable. In these patients, we can consider primary hepatectomy without any previous treatment for hemostasis. In this study, patients who underwent primary hepatic resection first or staged hepatectomy after TACE showed similar OS (p = 0.503) (data not shown). This result implies that elective primary hepatectomy for ruptured HCC after full evaluation of the patient with stable hemodynamic is a feasible treatment option.
Several studies reported high in-hospital mortality in patients with ruptured HCC, widely ranging from 23.1 to 77.8% according to (emergency) treatment modalities31. Recent reports have presented continued decreasing trend of 30-day or in-hospital mortality in patients with ruptured HCC17. Moreover, in patients who were eligible for hepatectomy, a 30-day mortality rate was 7.3%, which is better than the overall mortality rate of 35.6% in patients receiving conservative management, TACE, and surgical hemostasis32. Studies on short-term outcome of patients with ruptured HCC revealed that poor liver reserve, advanced liver disease, severe hemorrhage, and shock on admission were associated with hospital mortality17,31,33. In this study, no in-hospital mortality or post-hepatectomy liver failure occurred. With the development of diagnostic modality and widespread use of CT as surveillance method, earlier diagnosis of HCC is now possible, and this can explain the indolent or stable condition of patients in the present study. Altogether, these results suggest that tailored management of acute phase depending on the degree of emergency, followed by complete evaluation of the tumor extent and liver functional reserve, improves short-term survival outcome.
Similar to short-term outcome, a wide spectrum of reported long-term survival outcome for ruptured HCC is found. Even for the prognosis after surgical resection for ruptured HCC, survival outcomes varied from 37.7 to 90.0% for 1-year OS rate and from 14.7 to 67.5% for 5-year OS rate1,11,34. In the present study, OS rates were also within these previously reported ranges. This might be attributed to variations in operative techniques, heterogeneity in patient characteristics, and tumor characteristics included in studies.
Several studies compared the oncological outcomes between patients with ruptured and unruptured HCC. Moreover, whether tumor rupture itself affects the OS and recurrence is controversial. In our study, patients with ruptured HCC showed inferior survival outcome compared with matched patients with unruptured HCC. As in the present study, several studies reported inferior outcomes of ruptured HCC than unruptured HCC1,10,11,12,13,14. Conversely, a number of studies verified that HCC rupture had low recurrence rate and showed comparable oncological outcome to that of unruptured HCC15,16,17,18,19,20. Based on recently reported comparable survival outcome in patients with ruptured HCC, the general rules of the 6th edition of LCSGJ no longer take tumor rupture into consideration for T staging, where the rupture was classified as T4 in the 5th edition21, 22. Aoki et al. suggested through a nationwide survey of the survival for patients with ruptured HCC that tumor rupture itself had an additional negative effect on patient survival which was correspondent to the addition of 0.5 to 2.0 TNM stage (both LCSGJ classification and AJCC/UICC staging system)1. Chan et al. also indicated the effect of tumor rupture as increasing T stage by 1 for otherwise T1–T2 tumors13. Adverse effect on the prognosis for patients with ruptured HCC was limited to early-stage tumor and was not substantially strong so as to outweigh other tumor-related parameters. Nonetheless, aggressive surgical resection for ruptured HCC is still valid to prolong survival outcome just as in treatment strategy for unruptured HCC.
Liu et al. demonstrated that extrahepatic recurrence after hepatic resection in the ruptured HCC group (45.5%) was significantly more common than in the unruptured group (25.8%), and although not significant, intraperitoneal extrahepatic recurrence rate in the ruptured group (20%) tended to be higher than that in the unruptured HCC group (14%)14. Chan et al. suggested that patients with ruptured HCC were more likely to develop tumor recurrence after hepatectomy, and the pattern of recurrence was more likely to be extrahepatic or concomitant intrahepatic and extrahepatic metastasis13.
PS from HCC is uncommon. Recently reported PS rate of HCC after hepatectomy was 3.0%–5.6%13,35,36. However, when it comes to ruptured HCC, the reported PS rate was greater, ranging from 11.1 to 20%19,34. In accordance with previous reports, the present study showed a PS rate of 18%. Previous studies suggested that tumor cell seeding in the peritoneum derived from HCC rupture resulted in increased incidence of PS in patients with ruptured HCC37,38,39. The mechanism of PS in ruptured HCC is hypothesized as a direct implantation, which is different from adenocarcinoma in intra-abdominal organs demonstrating hematogenous or lymphatic dissemination to peritoneum. In the present study, considering the pattern and timing of recurrence in PS, “implantation” of tumor cells through rupture rather than “hematogenous spread” of tumor is suggested as a mechanism for PS.
In patients with recurrence after hepatectomy for ruptured HCC, we could expect to prolong survival through multidisciplinary treatments, including surgical excision of recurrent disease while controlling intrahepatic recurrence through TACE. In the present study, patients with PS showed earlier recurrence and worse survival outcome than patients without PS. However, as shown in our study result, patients with PS who were suitable for surgical excision for seeding mass manifested prolonged survival after recurrence. Kwak et al. suggested that HCC rupture itself was an independent risk factor for PS, but not an independent risk factor for OS4. On constant vigilance against the increased risk of PS, the result of this study suggests that follow-up surveillance CT or MRI should include the whole abdomen (pelvis) after resection of ruptured HCC, which could enable earlier detection of PS nodules.
Limited effective treatment options are available for HCC with disseminated PS. Hyperthermic intraperitoneal chemotherapy combined with cytoreductive surgery for HCC patient with PS was reported feasible to prolong survival in well-selected cases40,41. The effectiveness of sorafenib is not well-documented in HCC with PS. Among eight patients with PS who were not amenable to surgical resection in this study, two patients were treated with sorafenib, and both of them showed progressive disease. Various systemic agents of multi-kinase (lenvatinib, regorafenib, and cabozantinib) and immune checkpoint inhibitors (nivolumab and pembrolizumab) were recently granted approval for advanced HCC with promising outcomes42. This evolving landscape of systemic therapy for HCC could benefit recurrence in patients with ruptured HCC with PS.
Conclusion
In conclusion, surgical resection for ruptured HCC showed worse outcome than unruptured HCC. Spontaneous rupture of HCC itself had a negative effect on patient survival and disease recurrence. In patients with ruptured HCC, the risk of PS also increased. However, hepatectomy as a mainstay of assertive management combined with TACE in these patients could prolong survival outcome. Even after recurrence, aggressive treatment still stands for improved survival outcome after recurrence.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- AJCC/UICC:
-
American Joint Committee on Cancer/Union for International Cancer Control
- LCSGJ:
-
Liver Cancer Study Group of Japan
- HBV:
-
Hepatitis B virus
- PS:
-
Peritoneal seeding
- AFP:
-
Alpha fetoprotein
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- TACE:
-
Transcatheter arterial chemoembolization
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumors
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
References
Aoki T, Kokudo N, Matsuyama Y, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O, Makuuchi M, Liver Cancer Study Group of Japan. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg 2014;259:532–542.
Cheung TT, Poon RT, Chok KS, Chan AC, Tsang SH, Dai WC, Yau TC, Chan SC, Fan ST, Lo CM. Management of spontaneously ruptured hepatocellular carcinomas in the radiofrequency ablation era. PLoS One 2014;9:e94453.
Yang T, Sun YF, Zhang J, Lau WY, Lai EC, Lu JH, Shen F, Wu MC. Partial hepatectomy for ruptured hepatocellular carcinoma. Br J Surg 2013;100:1071–1079.
Kwak MS, Lee JH, Yoon JH, Yu SJ, Cho EJ, Jang ES, Kim YJ, Lee HS. Risk factors, clinical features, and prognosis of the hepatocellular carcinoma with peritoneal metastasis. Dig Dis Sci 2012;57:813–819.
Miyamoto M, Sudo T, Kuyama T. Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases. Am J Gastroenterol 1991;86:67–71.
Hiraoka A, Kawamura T, Aibiki T, Okudaira T, Toshimori A, Yamago H, Nakahara H, Suga Y, Azemoto N, Miyata H, Miyamoto Y, Ninomiya T, Murakami T, Ishimaru Y, Kawasaki H, Hirooka M, Abe M, Matsuura B, Hiasa Y, Michitaka K. Prognosis and therapy for ruptured hepatocellular carcinoma: problems with staging and treatment strategy. Eur J Radiol 2015;84:366–371.
Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg 2006;141:191–198.
Yoshida H, Mamada Y, Taniai N, Uchida E. Spontaneous ruptured hepatocellular carcinoma. Hepatol Res 2016;46:13–21.
Schwarz L, Bubenheim M, Zemour J, Herrero A, Muscari F, Ayav A, Riboud R, Ducerf C, Regimbeau JM, Tranchart H, Lermite E, Petrovai G, Suhol A, Doussot A, Capussotti L, Tuech JJ, Le Treut YP, FRENCH association. Bleeding recurrence and mortality following interventional management of spontaneous HCC rupture: results of a multicenter European study. World J Surg 2018;42:225–232.
Zhu Q, Li J, Yan JJ, Huang L, Wu MC, Yan YQ. Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol 2012;18:7302–7307.
Zhu Q, Qiao GL, Xu C, Guo DL, Tang J, Duan R, Li Y. Partial hepatectomy for spontaneous tumor rupture in patients with hepatocellular carcinoma: a retrospective cohort study. Cancer Manag Res 2017;9:525–537.
Miyoshi A, Kitahara K, Kohya N, Noshiro H, Miyazahi K. Outcomes of patients with spontaneous rupture of hepatocellular carcinoma. Hepatogastroenterology 2011;58:99–102.
Chan AC, Dai JW, Chok KS, Cheung TT, Lo CM. Prognostic influence of spontaneous tumor rupture on hepatocellular carcinoma after interval hepatectomy. Surgery 2016;159:409–417.
Liu CL, Fan ST, Lo CM, Tso WK, Poon RT, Lam CM, Wong J. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol 2001;19:3725–3732.
Joliat GR, Labgaa I, Uldry E, Demartines N, Halkic N. Recurrence rate and overall survival of operated ruptured hepatocellular carcinomas. Eur J Gastroenterol Hepatol 2018;30:792–796.
Sada H, Ohira M, Kobayashi T, Tashiro H, Chayama K, Ohdan H. An analysis of surgical treatment for the spontaneous rupture of hepatocellular carcinoma. Dig Surg 2016;33:43–50.
Tan FL, Tan YM, Chung AY, Cheow PC, Chow PK, Ooi LL. Factors affecting early mortality in spontaneous rupture of hepatocellular carcinoma. ANZ J Surg 2006;76:448–452.
Tanaka S, Kaibori M, Ueno M, Wada H, Hirokawa F, Nakai T, Iida H, Eguchi H, Hayashi M, Kubo S. Surgical outcomes for the ruptured hepatocellular carcinoma: multicenter analysis with a case-controlled study. J Gastrointest Surg 2016;20:2021–2034.
Uchiyama H, Minagawa R, Itoh S, Kajiyama K, Harimoto N, Ikegami T, Yoshizumi T, Shirabe K, Takenaka K, Maehara Y. Favorable outcomes of hepatectomy for ruptured hepatocellular carcinoma: Retrospective analysis of primary R0-hepatectomized patients. Anticancer Res 2016;36:379–385.
Lee HS, Choi GH, Kang DR, Han KH, Ahn SH, Kim DY, Park JY, Kim SU, Choi JS. Impact of spontaneous hepatocellular carcinoma rupture on recurrence pattern and long-term surgical outcomes after partial hepatectomy. World J Surg 2014;38:2070–2078.
Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. 6th Japanese ed. Tokyo: Kanehara & Co, 2015.
Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the liver cancer study group of Japan. Dig Dis 2015;33:765–770.
Amin MB, Edge SB, Greene FL, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, American Joint Committee on Cancer, eds. AJCC cancer staging manual. 8th ed. New York: Springer, 2017.
Pombo F, Arrojo L, Perez-Fontan J. Haemoperitoneum secondary to spontaneous rupture of hepatocellular carcinoma: CT diagnosis. Clin Radiol 1991;43:321–322.
Singhal M, Sinha U, Kalra N, Duseja A, Khandelwal N. Enucleation sign: a computed tomographic appearance of ruptured hepatocellular carcinoma. J Clin Exp Hepatol 2016;6:335–336.
Kim HC, Yang DM, Jin W, Park SJ. The various manifestations of ruptured hepatocellular carcinoma: CT imaging findings. Abdom Imaging 2008;33:633–642.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52–60.
Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ, Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698–711.
Buczkowski AK, Kim PT, Ho SG, Schaeffer DF, Lee SI, Owen DA, Weiss AH, Chung SW, Scudamore CH. Multidisciplinary management of ruptured hepatocellular carcinoma. J Gastrointest Surg 2006;10:379–386.
The Korean Liver Cancer Association, National Cancer Center. Korea practice guidelines for management of hepatocellular carcinoma. 2018 [cited 2019 17 April]; Available from: http://livercancer.or.kr/study/guidelines.php.
Chen WK, Chang YT, Chung YT, Yang HR. Outcomes of emergency treatment in ruptured hepatocellular carcinoma in the ED. Am J Emerg Med 2005;23:730–736.
Zhang XF, Wei T, Liu XM, Lv Y. Spontaneous tumor rupture and surgical prognosis of patients with hepatocellular carcinoma. Scand J Gastroenterol 2012;47:968–974.
Marini P, Vilgrain V, Belghiti J. Management of spontaneous rupture of liver tumours. Dig Surg 2002;19:109–113.
Yoshida H, Mamada Y, Taniai N, Mizuguchi Y, Kakinuma D, Ishikawa Y, Kanda T, Matsumoto S, Bando K, Akimaru K, Tajiri T. Long-term results of elective hepatectomy for the treatment of ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2008;15:178–182.
Kow AW, Kwon CH, Song S, Shin M, Kim JM, Joh JW. Risk factors of peritoneal recurrence and outcome of resected peritoneal recurrence after liver resection in hepatocellular carcinoma: review of 1222 cases of hepatectomy in a tertiary institution. Ann Surg Oncol 2012;19:2246–2255.
Yeh CN, Chen MF. Resection of peritoneal implantation of hepatocellular carcinoma after hepatic resection: risk factors and prognostic analysis. World J Surg 2004;28:382–386.
Ryu JK, Lee SB, Kim KH, Yoh KT. Surgical treatment in a patient with multiple implanted intraperitoneal metastases after resection of ruptured large hepatocellular carcinoma. Hepatogastroenterology 2004;51:239–242.
Yoshida H, Onda M, Tajiri T, Akimaru K, Takasaki H, Mamada Y, Taniai N, Nakamura Y, Kawano Y, Takahashi T. Successful surgical treatment of peritoneal dissemination of hepatocellular carcinoma. Hepatogastroenterology 2002;49:1663–1665.
Hung MC, Wu HS, Lee YT, Hsu CH, Chou DA, Huang MH. Intraperitoneal metastasis of hepatocellular carcinoma after spontaneous rupture: a case report. World J Gastroenterol 2008;14:3927–3931.
Tabrizian P, Franssen B, Jibara G, Sweeney R, Sarpel U, Schwartz M, Labow D. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with peritoneal hepatocellular carcinoma. J Surg Oncol 2014;110:786–790.
Mehta S, Schwarz L, Spiliotis J, Hsieh MC, Akaishi EH, Goere D, Sugarbaker PH, Baratti D, Quenet F, Bartlett DL, Villeneuve L, Kepenekian V, Psogi, Big-Renape Working Groups. Is there an oncological interest in the combination of CRS/HIPEC for peritoneal carcinomatosis of HCC? Results of a multicenter international study. Eur J Surg Oncol 2018;44:1786–1792.
Mody K, Abou-Alfa GK. Systemic therapy for advanced hepatocellular carcinoma in an evolving landscape. Curr Treat Options Oncol 2019;20:3.
Funding
The present work was supported by a fund from the National Research Foundation of Korea (NRF-2015K1A4A3046807).
Author information
Authors and Affiliations
Contributions
Our manuscript has 13 authors, all of whom contributed significantly to this study. Jae Hyun Kwon, Gi-Won Song, and Sung-Gyu Lee made substantial contributions to study conception and design. Shin Hwang, Ki-Hun Kim, Chul-Soo Ahn, Deok-Bog Moon, Tae-Yong Ha, Dong-Hwan Jung, Gil-Chun Park, Young-In Yoon, Ju Hyun Shim, and Kyong Won Kim participated in data acquisition and analysis. Jae Hyun Kwon and Gi-Won Song participated in the drafting of the article and critical revisions to ensure appropriate communication of important intellectual content.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kwon, J.H., Song, GW., Hwang, S. et al. Surgical Outcomes of Spontaneously Ruptured Hepatocellular Carcinoma. J Gastrointest Surg 25, 941–953 (2021). https://doi.org/10.1007/s11605-020-04555-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04555-0