Abstract
Background
The prognosis of patients with linitis plastica (LP) gastric cancer is reported to be poor. The purpose of our retrospective study was to characterize the clinicopathologic features and survival outcomes of patients with LP, using a univocal definition.
Methods
We defined LP as gastric cancer that involves more than 1/3 of the gastric wall macroscopically. We reviewed a prospectively maintained institutional database of gastric cancer patients and summarized and compared clinicopathologic factors of patients with and without LP who had undergone gastrectomy. Patients were matched 1:1 using propensity score matching, and their overall survival (OS) rates and durations were compared. Multivariable Cox regression analyses were conducted, using gastrectomy as a time-varying covariate.
Results
We identified 740 patients with radiographically non-metastatic gastric cancer, 157 (21.2%) of whom had LP. Most patients with LP had advanced-stage disease (75.8% had stage IV disease, mainly due to peritoneal involvement). Patients with LP had significantly shorter OS durations than did those without LP in the entire cohort (median OS, 14.0 vs. 33.5 months; p value < 0.001) and in the surgical cohort (median OS after gastrectomy, 21.8 vs. 91.0 months; p < 0.001), as well as in the propensity-matched surgical cohort. In the LP cohort, chemotherapy (hazard ratio [HR] = 0.594; p = 0.076), chemoradiation therapy (HR = 0.346; p = 0.001), and gastrectomy (HR = 0.425; p = 0.003) were associated with a longer OS.
Conclusions
LP is a phenotype of gastric cancer that often presents at an advanced stage, with a high rate of peritoneal involvement. The survival durations of patients with LP were poor in our study, even in the surgical cohort. The use of preoperative chemotherapy, chemoradiation therapy, and gastrectomy appeared to be important in carefully selected patients with localized LP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer death worldwide.1 Linitis plastica (LP) is a distinct phenotype of gastric cancer. Macroscopically, it is characterized as a thickened stomach, with prominent diffusion of the tumor into the submucosal and muscular layers; microscopically, it is often associated with signet ring cell features and diffuse and scirrhous (referring to the histologic characteristic of abundant stromal cells) histologic types.2,3,4,5,6,7,8,9,10 The term “scirrhous gastric cancer,” which is commonly defined as a Borrmann type 4 or large (≥ 8 cm in diameter) type 3 gastric cancer, is often, but inconsistently, used interchangeably with LP gastric cancer to describe this phenotype of gastric cancer in Eastern Asian countries.9, 11, 12
LP gastric cancer has been consistently reported to have a poor prognosis; patients with LP often present with advanced-stage disease, and their median overall survival (OS) duration ranges from 6 to 14 months.3,4,5, 8, 13,14,15,16,17,18 These patients have a high non-curative resection rate4, 5, 13, 14, 16, 18 and high rates of locoregional and peritoneal recurrence.16 As a consequence, some authors have proposed that patients with LP should not be considered for gastrectomy,3, 8, 17 while others have reported that gastrectomy may have a survival benefit.4, 5, 13, 16 It remains unknown whether the LP phenotype is independently associated with a shorter survival duration and whether patients benefit from surgical resection. In addition, there is no clear definition of LP, which makes inter-institutional collaborations and cross-study comparisons difficult or unreliable3,4,5, 8, 13,14,15,16,17,18; therefore, it is important that the definition be standardized.
The objectives of this retrospective study were (1) to propose a clear definition of LP and identify the proportion of gastric cancer patients with LP who had been treated in our surgical oncology practice, (2) to determine the effect of the LP phenotype on OS after controlling for other clinicopathologic factors, and (3) to determine the effects of gastrectomy on OS in patients with LP. We achieved these objectives by performing high-quality statistical analyses, such as propensity score matching, and using a time-varying covariate, to evaluate data from our institutional database.
Methods
Patients
We retrospectively reviewed the records of 1517 patients with gastroesophageal or gastric cancer who had been evaluated in the Department of Surgical Oncology at The University of Texas MD Anderson Cancer Center (Houston, TX) between August 1994 and October 2016. Patients’ records had been collected in an institutional database.
As LP has been commonly defined by its endoscopic findings, imaging, and appearance during surgical procedures,6, 16, 19 we used these three diagnostic modalities as part of our inclusion criteria. We included patients with gastric adenocarcinoma, including Siewert type 3 esophagogastric cancer, who had undergone (1) preoperative endoscopy or endoscopic ultrasonography (EUS), (2) a CT scan or PET/CT scan, and (3) staging laparoscopy. Patients with obvious stage IV disease do not undergo staging laparoscopy; therefore, as this study was designed to evaluate radiographically localized gastric cancer, these patients were not included. Patients with no diagnosis of adenocarcinoma and patients with synchronous tumors that would affect prognosis, a history of gastrectomy performed elsewhere, or remnant gastric cancer were excluded. Patients with incomplete or missing records were also excluded. Patients were classified using the 7th edition of the American Joint Committee on Cancer staging system.20 The study was approved by the MD Anderson Institutional Review Board.
Definition of LP
LP was defined as thickening of the gastric wall, with a lack of distensibility and stiffening that involved more than 1/3 of the gastric surface of at least some part circumferentially.10 These features must have been confirmed using at least two of three staging methods. All patient cases in the database were reviewed by two independent physicians (B.B. and A.A.) to determine whether they had LP using this definition; conflict between the two physicians was resolved by an additional reviewer (N.I.).
Data collection
Information collected from medical records included age, sex, gastroesophageal junction involvement, the presence of signet ring cells, tumor grade, preoperative clinical stage (by endoscopy/EUS, CT, and laparoscopy), LP, upfront therapy type, surgical procedure (if performed), additional organ resection, results of staging laparoscopy with lavage cytology (grossly positive peritoneal carcinomatosis, cytology-only positive, or negative), pathologic stage, lymphadenectomy extent, lymph node ratio, margin status, postoperative major morbidity (defined as a grade 3 or 4 complication by the Clavien-Dindo classification, occurring within 90 days of surgery), mortality (defined as death occurring within 90 days of surgery), and OS.
Preoperative Therapy
At MD Anderson, patients’ treatment strategies are decided at a multidisciplinary conference. Non-early resectable gastric cancer (≥ cT2 or cN positive) is generally treated with either preoperative chemotherapy alone or preoperative induction chemotherapy followed by chemoradiation therapy, while early gastric cancer (cT1) is recommended for upfront resection. The standard regimen of preoperative chemoradiation therapy is 45 Gy radiation administered concurrently with 5-fluorouracil (5-FU), commonly administered after induction chemotherapy with a 5-FU–based regimen. Preoperative chemotherapy regimens during the study period included epirubicin/oxaliplatin/capecitabine, 5-FU/cisplatin/paclitaxel, 5-FU/oxaliplatin/paclitaxel, and epirubicin/cisplatin/5-FU.21,22,23,24
Staging Laparoscopy and Surgical Procedures
Staging laparoscopy was performed before the initiation of a neoadjuvant regimen and was used to complete staging, evaluate the presence of peritoneal carcinomatosis or other metastatic lesions, and evaluate peritoneal lavage cytologic findings.25 The standard surgical procedure at MD Anderson is subtotal or total gastrectomy with D2 lymph node dissection.24, 26,27,28
Statistical Analysis
Clinicopathologic characteristics were summarized and compared between patients with and without LP using Pearson’s chi-squared test or Fisher’s exact test, as appropriate, for categorical variables and Student’s t test for continuous variables. OS duration was calculated from the date of diagnosis to the date of death or was censored at last follow-up. Survival curves were estimated with the Kaplan-Meier method and compared with the log-rank test. Univariate and multivariable Cox proportional hazards models were used to determine the associations between clinicopathologic factors and OS. Gastrectomy was treated as a time-varying covariate to minimize overestimation of the benefit of gastrectomy on survival.29, 30 The multivariable analysis included variables that were significant at the 0.1 level in the univariate analysis. Backward model selection was implemented with a significance level of 0.05 to build the final model.
To create comparable cohorts of patients, we matched surgical LP patients 1:1 with surgical non-LP patients using the propensity score-matching method (optimal matching).31, 32 Matching variables included age, sex, presence of signet ring cells, preoperative therapy type, gastrectomy type, nodal dissection type, concomitant organ resection, postoperative complications, pStage, pathologic P status, node ratio, and R status. After propensity score matching, the balances in the clinicopathologic characteristics between patients with and without LP were assessed using McNemar’s test for categorical variables and a paired t test for continuous variables.
The statistical analysis was conducted using SPSS v.23 for Windows XP software (SPSS, Chicago, IL, USA); SAS 9.3, the SPSS PSMatching extension tool developed by Thoemmes33 (The SAS Institute, Cary, NC); and Stata 13.1 (Stata Corp., College Station, TX). All statistical tests were two-sided with a significance level of 0.05.
Results
Seven hundred forty patients met the inclusion and exclusion criteria and were included in this study; 157 (21.2%) had a diagnosis of LP (Fig. 1). Patients with LP were younger and more often female than were those without LP. Patients with LP predominantly had signet ring cell (77.7%) and poorly differentiated histologic types (91.1%) (Table 1). We also found that 75.8% had clinical stage IV disease, mostly determined by the presence of gross peritoneal carcinomatosis (56.7%) or positive cytologic results (18.5%). Gastrectomy was performed in only 31 (19.7%) patients (Table 1).
Of the 740 patients in our study, 348 (47.0%) underwent gastrectomy. Of these, 174 (50.0%) underwent a total gastrectomy and 167 (48.0%) underwent a subtotal gastrectomy (Table 2). Patients with LP more often had positive cytologic findings, peritoneal carcinomatosis, and an advanced pathologic disease stage. They underwent total gastrectomy more often and had a higher R1 resection rate than did those without LP (Table 2).
OS
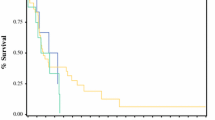
The median follow-up duration among survivors was 33.4 months after diagnosis in all patients. The median OS duration after diagnosis was 25.0 months. The 1-, 3-, and 5-year OS rates were 59%, 12%, and 5%, respectively, in the LP group and 80%, 48%, and 36% in the non-LP group. Patients with LP had a significantly shorter median OS duration than did those without LP (14.0 vs. 33.5 months; p < 0.001) (Fig. 2a).
We performed a further OS analysis of the 335 patients who underwent total or subtotal gastrectomy without 30-day postoperative mortality. The 1-, 3-, and 5-year OS rates after gastrectomy were 69%, 27%, and 18%, respectively, in the LP group and 89%, 67%, and 56% in the non-LP group. Similarly, patients with LP had a significantly shorter median OS duration after gastrectomy than did those without LP (21.8 vs. 91.0 months; p < 0.001) (Fig. 2b). After propensity score matching, LP patients had a remarkably shorter OS duration than did non-LP patients, although it did not reach statistical significance (stratified log-rank test, p = 0.0593; stratified Cox regression model, p = 0.0694; HR = 2.600; 95% CI = 0.927–7.293) (Fig. 2c).
In the LP cohort, the median OS duration after diagnosis was 29.0 months in patients who underwent gastrectomy and 12.5 months in patients who did not undergo gastrectomy (p < 0.001). The median OS duration was significantly longer in patients who achieved R0 resection (37.2 months) than in those with R1 resection (16.1 months; p = 0.01). The multivariable Cox regression models, adjusted by preoperative clinical stage, demonstrated that the use of systemic therapy (chemotherapy: HR = 0.594; p = 0.076 or chemoradiation therapy: HR = 0.346; p = 0.001) and gastrectomy (HR = 0.425; p = 0.003) were associated with improved OS (Table 3).
Discussion
On the basis of our preoperative diagnostic criteria, 21.2% of the gastric cancer patients in this study had LP gastric cancer. More of these patients presented with advanced-stage disease than did those with non-LP cancer, with a stronger propensity towards peritoneal dissemination; they had extremely poor OS durations. LP was independently associated with a poor OS on the basis of the results of propensity score-matched survival analyses of the surgical cohorts. Systemic therapy (chemotherapy or chemoradiation therapy) and gastrectomy were associated with a longer OS duration in LP patients by multivariable analysis, using gastrectomy as a time-varying covariate; upfront systemic therapy, followed by gastrectomy, seemed to be a promising approach in carefully selected patients with localized LP gastric cancer.
In accordance with our findings, most previous studies uniformly reported that LP patients had extremely poor survival, with median survival durations in surgical patients of 5–17 months.4, 8, 10, 13, 15,16,17,18 However, a significant obstacle in the interpretation of the results of previous studies was the use of heterogeneous and often unclear definitions of LP. Some studies included poorly differentiated histologic features or signet ring cell type as part of the definition.14,15,16,17 However, a microscopic or pathologic definition of LP is difficult to consistently apply because of the difficulty in obtaining representative tissue samples by biopsy, differences in the histologic criteria of pathologic classifications across countries, and the effects of preoperative therapy on the final pathologic results; in addition, most patients with LP do not undergo gastrectomy because of stage IV disease.10 In Eastern Asian countries, scirrhous gastric cancer has been consistently defined as Borrmann type 4 or large (≥ 8 cm in diameter) type 3 gastric cancer.9, 11, 12 However, patients with Borrmann type 4 tumors localized in less than two-thirds of the stomach are reported to have similar survival as patients with other non-scirrhous gastric cancers,8 which indicates that definitions based exclusively on the Borrmann classification underrepresent the patients with LP gastric cancer who are seen in western countries. After careful consideration of previous studies, we used a definition that we believe most accurately represents LP gastric cancer and is consistently applicable on the basis of three common staging methods.
Our results prompt several considerations. As patients with LP have a high risk of peritoneal involvement (75.2% among patients with radiographically non-metastatic disease in this series), diagnostic laparoscopy with lavage cytology should always be performed as part of the staging process. These patients may also benefit from repeat staging laparoscopy after preoperative therapy before surgical resection: our previous analysis showed that approximately 35% of patients with negative pre-treatment laparoscopy results had peritoneal disease on second examination.34
The optimum treatment strategy for LP gastric cancer is unknown, but our results support the use of preoperative therapy followed by gastrectomy in select patients. A previous study showed that LP patients experienced poor responses to systemic therapy,2 likely because of the disease’s scirrhous stromal component,10 which may protect cancer cells from the host’s immune response and from conventional chemotherapeutic agents.35,36,37 However, we consider preoperative chemotherapy a reliable strategy for testing the tumor’s biologic behavior and propose the selective use of gastrectomy in patients who do not experience progression during preoperative therapy. Preoperative chemoradiation therapy would also be helpful to improve the R0 resection rate.
The major limitation of this study, as well as previous studies of this topic, was the difficulty in defining LP. Some Eastern Asian studies have defined it as a large Borrmann type 3 or any Borrmann type 4 gastric cancer; our multidisciplinary team believe that this definition underrepresents the significance of the LP gastric cancer we often encounter in our practice in the west. Moreover, most recent studies have not provided a clear definition of LP. In addition, the infiltrative morphological characteristics of diffuse gastric cancer often lack clear demarcation of the tumor edge; therefore, it can be difficult to determine whether the tumor meets specific criteria. Although the ideal criteria for defining LP remain unknown, we feel that our study has provided a clear proposed definition that may help clarify the outcomes of LP.
Although selection bias in the use of preoperative therapy and gastrectomy was the major limitation of this retrospective study, multivariable analyses using gastrectomy as a time-varying covariate minimized its lead-time bias. Moreover, the long median OS duration of LP patients who underwent gastrectomy (37.2 months after R0 gastrectomy and 16.1 months after R1 gastrectomy)—compared with the 12.5 months in patients who have not undergone resection and to the findings of previous reports—supports the use of preoperative therapy and gastrectomy in carefully selected patients.
Conclusions
In conclusion, LP is a phenotype of gastric cancer that often presents at an advanced stage, with a high rate of peritoneal involvement. Our proposed definition of LP is feasible, which may help standardize the terminology. The use of staging laparoscopy is important for classifying LP, as well as for ruling out peritoneal carcinomatosis. The prognosis of LP patients is poor; however, the use of preoperative therapy, followed by gastrectomy in select patients, appears to be a reasonable treatment strategy for patients with localized LP gastric cancer.
Future studies should focus on defining the optimum preoperative therapy regimen and thus further improving the OS duration of patients with LP gastric cancer.
References
Ferlay J, Soerjomataram I, Ervik M. GLOBOCAN, cancer incidence and mortality worldwide: IARC cancer base no. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. 2012.
Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250(6):878-887.
Jafferbhoy S, Shiwani H, Rustum Q. Managing gastric linitis plastica: keep the scalpel sheathed. Sultan Qaboos Univ Med J. 2013;13(3):451-453.
Blackham AU, Swords DS, Levine EA, et al. Is Linitis Plastica a Contraindication for Surgical Resection: A Multi-Institution Study of the U.S. Gastric Cancer Collaborative. Annals of surgical oncology. 2016;23(4):1203-1211.
Schauer M, Peiper M, Theisen J, Knoefel W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. European journal of medical research. 2011;16(1):29-33.
Mastoraki A, Papanikolaou IS, Sakorafas G, Safioleas M. Facing the challenge of managing linitis plastica--review of the literature. Hepato-gastroenterology. 2009;56(96):1773-1778.
Otsuji E, Kuriu Y, Okamoto K, et al. Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. American journal of surgery. 2004;188(3):327-332.
Endo K, Sakurai M, Kusumoto E, et al. Biological significance of localized Type IV scirrhous gastric cancer. Oncol Lett. 2012;3(1):94-99.
Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017;20(1):1-19.
Agnes A, Estrella JS, Badgwell B. The significance of a nineteenth century definition in the era of genomics: linitis plastica. World journal of surgical oncology. 2017;15(1):123.
Iwasaki Y, Sasako M, Yamamoto S, et al. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). Journal of surgical oncology. 2013;107(7):741-745.
Hartgrink HH. Improving outcome for scirrhous gastric cancer. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2009;12(1):3-5.
Kodera Y, Ito S, Mochizuki Y, et al. The number of metastatic lymph nodes is a significant risk factor for bone metastasis and poor outcome after surgery for linitis plastica-type gastric carcinoma. World journal of surgery. 2008;32(9):2015-2020.
Kodera Y, Nakanishi H, Ito S, et al. Detection of disseminated cancer cells in linitis plastica-type gastric carcinoma. Japanese journal of clinical oncology. 2004;34(9):525-531.
Hamy A, Letessier E, Bizouarn P, et al. Study of survival and prognostic factors in patients undergoing resection for gastric linitis plastica: a review of 86 cases. International surgery. 1999;84(4):337-343.
Pedrazzani C, Marrelli D, Pacelli F, et al. Gastric linitis plastica: which role for surgical resection? Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2012;15(1):56-60.
Aranha GV, Georgen R. Gastric linitis plastica is not a surgical disease. Surgery. 1989;106(4):758-762; discussion 762-753.
Thompson RJ, Ranaghan L, Kennedy R, Clements W, Carey PD, Kennedy JA. Survival following operative management of gastric linitis plastica compared with non-operative management. Annals of the Royal College of Surgeons of England. 2016:1-5.
Burgain C, Germain A, Bastien C, et al. Computed tomography features of gastrointestinal linitis plastica: spectrum of findings in early and delayed phase imaging. Abdom Radiol (NY). 2016;41(7):1370-1377.
Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. Stomach. AJCC Cancer Staging Book 7th ed Berlin: Springer. 2010:145-152.
Badgwell B, Ajani J, Blum M, et al. Postoperative Morbidity and Mortality Rates are Not Increased for Patients with Gastric and Gastroesophageal Cancer Who Undergo Preoperative Chemoradiation Therapy. Annals of surgical oncology. 2016;23(1):156-162.
Badgwell B, Blum M, Estrella J, et al. Predictors of Survival in Patients with Resectable Gastric Cancer Treated with Preoperative Chemoradiation Therapy and Gastrectomy. Journal of the American College of Surgeons. 2015;221(1):83-90.
Badgwell B, Blum M, Elimova E, et al. Frequency of Resection After Preoperative Chemotherapy or Chemoradiotherapy for Gastric Adenocarcinoma. Annals of surgical oncology. 2016;23(6):1948-1955.
Ikoma N, Blum M, Estrella JS, et al. Left Gastric Artery Lymph Nodes Should Be Included in D1 Lymph Node Dissection in Gastric Cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2017.
Ikoma N, Blum M, Chiang YJ, et al. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Annals of surgical oncology. 2016;23(13):4332-4337.
Ikoma N, Blum M, Chiang YJ, et al. Survival rates in T1 and T2 gastric cancer: A Western report. Journal of surgical oncology. 2016;114(5):602-606.
Ikoma N, Blum M, Estrella JS, et al. Evaluation of the American Joint Committee on Cancer 8th edition staging system for gastric cancer patients after preoperative therapy. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017.
Ikoma N, Chen HC, Wang X, et al. Patterns of Initial Recurrence in Gastric Adenocarcinoma in the Era of Preoperative Therapy. Annals of surgical oncology. 2017;24(9):2679-2687.
John P. Klein MLM. Survival Analysis: Techniques for Censored and Truncated Data (Statistics for Biology and Health). Springer; 2005.
Tsujitani M, Tanaka Y. Analysis of heart transplant survival data using generalized additive models. Comput Math Methods Med. 2013;2013:609857.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55.
Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Statistics in medicine. 2014;33(7):1242-1258.
Thoemmes F. Propensity score matching in SPSS. arXiv preprint arXiv:12016385. 2012.
Thiels CA, Ikoma N, Fournier K, et al. Repeat staging laparoscopy for gastric cancer after preoperative therapy. Journal of surgical oncology. 2018;118(1):61-67.
Terai S, Fushida S, Tsukada T, et al. Bone marrow derived “fibrocytes” contribute to tumor proliferation and fibrosis in gastric cancer. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2015;18(2):306-313.
Naito Y, Sakamoto N, Oue N, et al. MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer. Cancer science. 2014;105(2):228-235.
Dvorak HF. Tumors: wounds that do not heal-redux. Cancer immunology research. 2015;3(1):1-11.
Acknowledgments
We thank the Department of Scientific Publications at MD Anderson for editorial assistance.
Funding
This study was supported in part by the National Institutes of Health under award number P30 CA016672 and by the Clinical Trials Support Resource.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ikoma, N., Agnes, A., Chen, HC. et al. Linitis Plastica: a Distinct Type of Gastric Cancer. J Gastrointest Surg 24, 1018–1025 (2020). https://doi.org/10.1007/s11605-019-04422-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04422-7