Abstract
Purpose
To analyze the features of gastrointestinal linitis plastica obtained by computed tomography (CT).
Materials and methods
We conducted a single-center, retrospective analysis of 45 cases of gastrointestinal tract linitis plastica collected over a 10-year period. “Linitis plastica” was defined based on histological characteristics. Primary and secondary linitis plastica were included. Two readers independently assessed the radiological findings (i.e., number of lesions, mass, wall thickening, and enhancement).
Results
The patient cohort comprised 23 men and 22 women with an average age of 63.2 years. The main presenting signs and symptoms were impaired general health and ascites (22/45 patients, 48.8%). The stomach was the affected organ in 68.3% of the cases, while the rectum was affected in 11.7% of the cases. Primary linitis was found in 73.3% of the cases, and solitary lesions were found in 77.8% of the cases. The most common CT finding was wall thickening (91.7%) with a complete disappearance of folds and enhancement of the entire wall at 2 min. Four lesions (6.6%) were described as masses, and only one (1.7%) was described as a wall atrophy.
Conclusion
Linitis plastica can affect the entire digestive system. Its potentially secondary nature necessitates a systematic search for a primary tumor. An appropriate CT protocol is required to detect the specific radiological features of this fibrous cancer. CT can help confirm the diagnosis of linitis plastica, rule out differential diagnoses, and indicate the need for deep biopsies where possible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Linitis is a histological form of cancer that primarily affects the stomach and was first described by Brinton in 1859 [1] and Borrmann in 1926 [2]. Linitis plastica lesions are adenocarcinomas whose cell populations are more than 50% “independent signet ring” cells. The lesions have a characteristic “frozen” aspect, infiltrating the bowel wall up to the lamina propria [3]. The prognosis is poor compared to other types of cancer; therefore, early diagnosis is critical [4, 5].
Gastroscopy is often used as an initial examination to establish a diagnosis, determine tumor extent, and provide histological proof of the disease. However, gastroscopy findings are often falsely negative, as only the mucosal wall is analyzed, and superficial biopsies are negative in this setting [6, 7].
Linitis can affect any part of the digestive tract and may be primary or secondary [8]. Although there are several published studies about gastric linitis and case reports of extra-gastric forms, we are aware of no epidemiological studies of this type of cancer within a particular population. In addition, the radiological findings of gastrointestinal linitis are poorly documented. “Linitis plastica” is a term that is typically used on the endoscopic and macroscopic plane; therefore, we aimed to correlate the histological definition of this condition (namely, “scirrhous carcinoma with more than 50% of signet ring cells”) into the realm of imaging. The aim of this study was to analyze the computed tomography (CT) features of gastrointestinal linitis plastica, determine the frequency of involvement of different sites, and assess its primary or secondary nature.
Materials and methods
Study population
This single-center, retrospective study analyzed all cases of gastrointestinal linitis observed over the 10-year period spanning from January 2003 to September 2014 at Nancy Regional University Hospital in France. The institutional review board approved the study and did not require additional informed consent for reviewing the patients’ medical records and images. A search of digital radiological reports using the terms “gastric linitis plastica” or “linitis plastica” identified 80 eligible patients. Following this, a search of digital pathological reports using the terms “linitis plastica” and “scirrhous carcinoma with more than 50% of signet ring cells” confirmed 65 of the cases.
Of the 65 cases, 45 patients with both firm pathological diagnosis and available pre-treatment imaging studies were enrolled (eight of the originally identified patients had only post-treatment imaging available, two of the originally identified patients underwent chemotherapy, and ten of the originally identified patients had no access to imaging) (Fig. 1). General data, such as patient's age and sex, mode of discovery, and history of malignancy, were also analyzed.
Collection of imaging data
Imaging data were retrieved from our PACS (Picture Archiving and Communication System). All patients underwent thoraco-abdominopelvic or abdominopelvic CT using various devices (Scan Lightspeed, General Electric, 16- to 64-array). The CT protocol included the injection of an iodinated contrast agent and the collection of images during the arterial phase and the delayed portal post-equilibrium phase. The arterial phase images were acquired 35 s after the injection of 80–120 mL of non-ionic contrast material (XENETIC 350 or IMERON 350, depending on the body weight) at a rate of 3 mL/s, and the delayed phase images were acquired 2 min after injection. Portal phase images were acquired 65 s after injection, but we did not include this phase in our analysis. Reconstructed images of 3- or 5-mm slice thickness were used for assessment.
Reading of CT scans
Two radiologists independently read the CT images according to Appendix. This included one junior radiologist (CB, a 3rd year radiology resident) and one senior radiologist (VL, a radiologist with over 20 years of experience, including 15 years in abdominal imaging).
Qualitative data collection
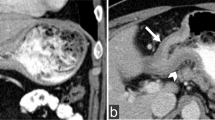
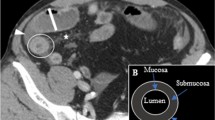
The following radiological features were analyzed (Fig. 2) (Appendix):
-
Lesion characteristics, such as the presence of mass syndrome, gastrointestinal wall thickening (>5 mm for the stomach [9]; >3 mm for the small intestine, colon and anus [10]), and wall atrophy (<1 mm).
-
Wall thickening, which was categorized as either segmental (<0 cm in length) or diffuse (affecting the entire organ). Fold disappearance, involvement beyond the serous layer, and the presence of stenosis were also recorded.
-
Lesion enhancement kinetics, which were recorded as no enhancement, enhancement during the early arterial phase of the mucosal-submucosal complex (corresponding to the two innermost layers of the gastrointestinal wall), and/or enhancement during the delayed phase of the entire lesion (fibrous type).
-
Linitis was considered secondary if a patient had a history of cancer and primary in all other cases. When multiple synchronous lesions were detected, gastric site lesions were considered to be primary, and the others were considered to be metastatic.
Quantitative data collection
The following quantitative CT data were collected (Fig. 3):
-
The two maximum cross-sectional diameters (in mm) when there was a mass.
-
The maximum thickness (in mm) in cases with wall thickening.
-
The maximum length (in mm) of segmental thickening.
Enhancement was quantified using density region of interest (ROIs) (in Hounsfield units (HU)) of 50 mm2 located in the region of wall thickening on acquisitions without contrast injection when they were available. The following assessments were made during the delayed portal post-equilibrium phase: density (HU) without contrast injection, density (HU) in the delayed portal post-equilibrium phase, and density variation (ΔHU) between the two acquisitions.
Results
Clinical data
The study population consisted of 23 men and 22 women (51.1%/48.9%), with an average age of 63.2 years (range 31–86). The presenting signs and symptoms were poor general health and/or ascites (n = 22), abdominal pain (n = 7), ulcer or dysphagia (n = 4), jaundice (n = 3), or an obstructive syndrome (n = 2). Seven patients presented with anuria, anemia, or hematemesis, while the diagnosis was incidental in four cases (Fig. 4).
CT findings
Epidemiological data
A total of 60 lesions were detected in 45 patients (1.3 lesions/patient). These lesions were found in the stomach (n = 41, 68.3%), colon (n = 6, 10%), rectum (n = 7, 11.7%), sigmoid (n = 2, 3.3%), small intestine (n = 2, 3.3%), duodenum, and anus (one case each, 1.7%). No esophageal lesions were found. The majority (73.3%) of the lesions were primary, while 26.7% (n = 16) were secondary (from gastric linitis, n = 7; breast cancer, n = 5; bladder urothelioma, n = 2; non-Hodgkin’s lymphoma, n = 1; and hepatocellular carcinoma, n = 1). The secondary tumors were metachronous with the primary tumors in 43.7% of cases (n = 7).
Solitary lesions were found in 77.8% (n = 35/45) of the patients. The maximum number of lesion sites per patient was 3 (Fig. 5).
Imaging features
We observed wall thickening in 55 cases (91.7% of lesions), mass syndrome in four cases (6.6%) and wall atrophy in one case (1.7%) (Table 1). In the four cases with mass syndrome, the average maximum mass diameter was 61.5 mm (range 23–114), and no enhancement of the mass was evident at any phase. Wall atrophy affected the entire stomach and was only enhanced in the delayed phase.
Diffuse wall thickening was present in 46.7% of cases (n = 28/60) and involved the stomach, sigmoid, rectum, or anus. Segmental lesions were present in 45% of cases (n = 27/60) and involved the stomach, small intestine, or colon. The average lesion length was 64.4 mm (range 43–97), and the average lesion thickness was 15.1 mm (range 9–28.5).
Wall thickening affecting the entire stomach was present in 51.3% of cases (n = 20/39). Segmental involvement was generally antropyloric (84.2%, n = 16/19). Only one stenotic lesion was found in association with segmental wall thickening. No involvement beyond the serosa was observed.
When considering enhancement kinetics (Table 2), the following observations were made:
-
A total of 90.6% (n = 48/53) of the lesions assessed in the arterial phase showed early enhancement of the mucosal-submucosal complex.
-
A total of 93.2% (n = 55/59) of the lesions assessed in the delayed phase showed homogenous enhancement of the entire lesion, including all 55 cases with wall thickening.
-
The average variation in contrast enhancement between the pre-injection phase and the delayed phase was 70.8 HU (Table 3).
Table 3 Densities before contrast injection and at the delayed portal post-equilibrium phase (2 min) and the difference between them
Reader agreement
Satisfactory inter-reader agreement was found for the type of wall thickening (Kappa = 0.66), early enhancement (Kappa = 0.71), and delayed enhancement (Kappa = 0.87).
Discussion
Linitis plastica of the digestive tract is a particular form of gastrointestinal malignancy [2]. Histologically, it consists of tumor infiltration of the entire gut wall, fibrous stroma, and no distinct mass or obvious ulceration [3]. It is often discovered late and associated with peritoneal carcinomatosis [5, 11]. This diagnostic delay is due to the atypical nature of the associated clinical signs and symptoms [12] and to negative biopsy results on initial endoscopy (30% of cases of gastric linitis) [13]. Repeated deep biopsies are needed to reveal the presence of tumor cells.
Treatment of linitis plastica of the digestive tract is generally limited to surgery, which may or may not be curative, and/or palliative chemotherapy. The overall survival rates range from 0% to 21.8% at 5 years [11, 14], with a median survival time of 8–17 months [15].
CT is central to the positive diagnosis of gastric linitis, as it provides an earlier diagnosis than endoscopy (74.9% vs 44.1%) by revealing segmental or diffuse thickening of the stomach wall with contrast uptake [16]. Despite the importance of CT in this setting, the specific CT features associated with this malignancy have not previously been analyzed.
Gastrointestinal linitis affects similar numbers of men and women (51.1% and 48.9%, sex ratio = 1.04). The published results vary widely from one study to another, probably because of population differences and the varying forms of linitis [11, 17].
The average age at diagnosis was 63.2 years (range 31–86 years) in our study, in agreement with that reported by Otsuji et al. (57.9 years) [17]. In contrast, Kong et al. [18] reported that gastric linitis primarily occurred before age 45. It should be noted that our study focused on a Caucasian population and included all sites of involvement.
One unique feature of linitis plastica is that it may be primary or secondary. Secondary linitis was found in 26.7% of our cases. This proportion corresponds well with the 20% reported by Nguyen et al. [8]. Various histological types of cancer can give rise to secondary linitis, but breast and urothelial carcinomas appear to be the most common primary tumors [19]. Secondary linitis was synchronous in 56.3% of the cases in our study. Multiple sites of involvement (a maximum of three per patient in our series) must therefore be systematically identified.
All sections of the digestive tract can be involved [20], although we observed no cases of esophageal linitis. The two most common sites in our study were the stomach (68.3%) and rectum (11.7%) (Table 1).
Linitis plastica exhibits specific and similar CT features regardless of its location within the digestive tract. Wall thickening was observed in 91.7% of our cases. This thickening had a regular, concentric, and symmetrical appearance and reduced the caliber of the intestinal lumen. In our study, the average thickness of the involved walls was 15 mm, which was concordant with a previous report by Ha et al. [21]. This type of thickening rarely causes stenosis (with stenosis noted in only one case in our study).
Linitis plastica lesions can be diffuse and affect entire organ, which was the case in the sigmoid, rectum, and anus in our study. Alternatively, the lesions may be segmental, particularly in the small intestine and colon (the average lesion length was 64.4 mm in these two locations). Only the stomach showed both diffuse and segmental lesions [mainly of the pylorus and antrum (84.2%)].
Enhancement after contrast injection is particularly specific in this setting, as enhancement of the entire lesion was observed during the delayed phase in 93.2% of our cases, rising to 100% in the cases with wall thickening because the various wall layers and folds were no longer discernible. This delayed enhancement (at 2 min) concerned all wall layers and corresponded to tumor infiltration. This defining feature reflects the abundant stroma associated with linitis (Fig. 6). Infiltration by fibrous stroma also explains the initial lack of distension described in esophagogastroduodenal transit studies [6, 22]. This lack of distension reflects the loss of wall flexibility due to fibrous infiltration. However, this feature is difficult to assess by CT, as dynamic analyses cannot be performed. A loss of compliance is nonetheless a sign of diffuse gastric involvement.
Segmental gastric linitis. A contrast-enhanced CT scan (A) showing concentric cardiac wall thickening (arrows). White, regular thickening was macroscopically evident (B). Photomicrographs [×2.5 (C) and ×200 (D)] showing a diffuse, poorly differentiated adenocarcinoma (C) with signet ring cell morphology (D) affecting the entire wall
The above CT features are evocative of linitis plastica and thus could rule out the most probable differential diagnoses. In particular, gastric involvement may suggest lymphoma. However, in the case of lymphoma, wall thickening is irregular and nodular [23] and some degree of gastric distensibility persists. In addition, there is no tumor enhancement due to the absence of stromal fibrosis [24]. Adenocarcinoma takes the form of a relatively ulcerated mass with heterogeneous contrast enhancement [25, 26], while endocrine and connective tissue tumors are more necrotic and show early enhancement. Metastases of the small intestine may be secondary to breast cancer, lung cancer, or melanoma [27], but the lesions are shorter, the thickening is less uniform, and the contrast uptake is more irregular [28].
Gastrointestinal wall thickening can also be observed in inflammatory and infectious diseases. However, in these settings, hypodense submucosal edema is observed after contrast injection, the different wall layers remain distinct [10, 28, 29], and there is no homogeneous wall enhancement on delayed images. Likewise, there is no loss of wall compliance or folds.
The main difficulty in diagnosing linitis plastica is distinguishing it from chronic inflammatory bowel disease (IBD), as fibrous components are present in both conditions. This distinction is particularly challenging, as malignant transformation is a common complication of IBD, and linitis is one of the forms this transformation can take [30]. Only biopsy or surgery to excise a specimen for histological examination can ensure the absence of underlying neoplasia.
The key limitation of our study is its retrospective, single-center design, which limited patient recruitment (only 46 patients). Our study focused only on a Caucasian population. The dynamic enhancement of the thickened wall during the portal phase is unknown due to our analysis. Furthermore, “linitis plastica” is a term that is typically used only on the endoscopic and macroscopic plane. We aimed to correlate the histological definition of this condition with its imaging features, which explains why we found four unexpected masses and an atrophied wall in our series. A prospective study including an analysis of wall enhancement in all cases with gastrointestinal wall involvement would provide additional valuable information.
In conclusion, linitis plastica can affect any segment of the digestive tract. It may be primary or secondary, with breast and urothelial carcinomas being the most common primary lesions in cases of secondary linitis plastica. Multiple lesion sites are rare and must be systematically identified. In cases with wall thickening without a mass syndrome, an appropriate CT protocol must be used, with both acquisitions prior to contrast injection and in the delayed phase (2 min after injection).
In summary, the CT features suggestive of gastrointestinal linitis include regular, concentric wall thickening and delayed (2 min), regular, homogeneous enhancement associated with losses of wall compliance and folds. The detection of these specific features should indicate the need for deep endoscopic biopsy where possible.
References
Brinton W (1859) The diseases of the stomach with an introduction on its anatomy and physiology. London: J. Churchill, p 310
Borrmann R (1926) Geschwülste des Magens und Duodenums. In: Borchardt H, Borrmann R, Christeller E, Dietrich A, Fischer W, Von Gierke E, et al. (eds) Verdauungsschlauch. Berlin: Springer, pp 812–1054
Aaltonen LA, Hamilton SR (eds) (2000) Pathology and genetics of tumours of the digestive system, vol. 38. Lyon: IARC Press
An JY, Kang TH, Choi MG, et al. (2008) Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J Gastrointest Surg 12:1364–1369
Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C (2009) Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 250:878–887
Park MS, Ha HK, Choi BS, et al. (2004) Scirrhous gastric carcinoma: endoscopy versus upper gastrointestinal radiography. Radiology 231:421–426
Kanter MA, Isaacson NH, Knoll SM, Nochomovitz LE (1986) The diagnostic challenge of metastatic linitis plastica. Two cases and a consideration of the problem. Am Surgeon 52:510–513
Nguyen MD, Plasil B, Wen P, Frankel WL (2006) Mucin profiles in signet-ring cell carcinoma. Arch Pathol Lab Med 130:799–804
Insko EK, Levine MS, Birnbaum BA, Jacobs JE (2003) Benign and malignant lesions of the stomach: evaluation of CT criteria for differentiation. Radiology 228:166–171
Macari M, Balthazar EJ (2001) CT of bowel wall thickening: significance and pitfalls of interpretation. Am J Roentgenol 176:1105–1116
Kim DY, Kim HR, Kim YJ, Kim S (2002) Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg 72:739–742
Li C, Kim S, Lai JF, et al. (2007) Advanced gastric carcinoma with signet ring cell histology. Oncology 72:64–68
Levine MS, Kong V, Rubesin SE, Laufer I, Herlinger H (1990) Scirrhous carcinoma of the stomach: radiologic and endoscopic diagnosis. Radiology 175:151–154
Koufuji K, Aoyagi K, Yano S, et al. (2005) Peritoneal dissemination of scirrhous type 4 gastric cancers. Cancer Chemother 32:1384–1388
Schauer M, Peiper M, Theisen J, Knoefel W (2011) Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur J Med Res 16:29–33
Kim JI, Kim YH, Lee KH, et al. (2013) Type-specific diagnosis and evaluation of longitudinal tumor extent of Borrmann type IV gastric cancer: CT versus gastroscopy. Korean J Radiol 14:597–606
Otsuji E, Kuriu Y, Okamoto K, et al. (2004) Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg 188:327–332
Kong X, Wang JL, Chen HM, Fang JY (2012) Comparison of the clinicopathological characteristics of young and elderly patients with gastric carcinoma: a meta analysis. J Surg Oncol 106:346–352
Beyrouti MI, Beyrouti R, Amar MB, et al. (2007) Linite plastique gastrique. Presse Med 36:1782–1786
Dixon CF, Stevens G (1936) Carcinoma of linitis plastica type involving the intestine. Ann Surg 103:263–272
Ha HK, Jee KR, Yu E, et al. (2000) CT features of metastatic linitis plastica to the rectum in patients with peritoneal carcinomatosis. Am J Roentgenol 174:463–466
Rubesin SE, Levine MS, Laufer I (2008) Double-contrast upper gastrointestinal radiography: a pattern approach for diseases of the stomach. Radiology 246:33–48
Guermazi A, Brice P, de Kerviler EE, et al. (2001) Extranodal Hodgkin disease: spectrum of disease. Radiographics 21:161–179
Régent D, Laurent V, Antunes L, et al. (2002) Fibrous tissue(s): a key for lesion characterization in digestive diseases. J Radiol 83:292–312
Ba-Ssalamah A, Prokop M, Uffmann M, et al. (2003) Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics 23:625–644
Horton KM, Fishman EK (2003) Current role of CT in imaging of the stomach. Radiographics 23:75–87
Pickhardt PJ, Kim DH, Menias CO, et al. (2007) Evaluation of submucosal lesions of the large intestine: part 1. Neoplasms. Radiographics 27:1681–1692
Macari M, Megibow AJ, Balthazar EJ (2007) A pattern approach to the abnormal small bowel: observations at MDCT and CT enterography. Am J Roentgenol 188:1344–1355
Fernandes T, Oliveira MI, Castro R, et al. (2014) Bowel wall thickening at CT: simplifying the diagnosis. Insights Imaging 5:195–208
Hristova L, Soyer P, Hoeffel C, et al. (2013) Colorectal cancer in inflammatory bowel diseases: CT features with pathological correlation. Abdom Imaging 38:421–435
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
The Institutional review board approved the study and did not require additional informed consent for reviewing the patients’ medical records and images.
Appendix: The form used for CT interpretation
Appendix: The form used for CT interpretation
Qualitative analysis
Presence of individual features (Y/N):
A. Positive diagnosis: lesion features
-
a.
Mass syndrome,
-
b.
Gut wall thickening (stomach, >5 mm; small intestine, colon, or anus, >3 mm):
-
i.
Segmental (<100-mm-long) or diffuse,
-
ii.
Circumferential,
-
iii.
Stenotic,
-
iv.
Beyond the serous, and
-
v.
Disappearance of folds; and
-
i.
-
c.
Wall atrophy (<1 mm).
B. Qualitative analysis of lesion enhancement:
-
a.
No enhancement,
-
b.
Early arterial enhancement (A) of the mucosal-submucosal complex (two innermost layers of the gastrointestinal wall), and
-
c.
Delayed enhancement (T) of the whole lesion suggesting “fibrous” type tissue.
Quantitative analysis
Where possible
-
A.
Masses: two maximal orthogonal dimensions in the axial plane (mm);
-
B.
Wall thickening: maximum thickness (mm);
-
C.
Segmental thickening: maximum length (in mm);
-
D.
Density before injection (HU); and
-
E.
Density in the delayed portal, post-equilibrium phase (HU).
Rights and permissions
About this article
Cite this article
Burgain, C., Germain, A., Bastien, C. et al. Computed tomography features of gastrointestinal linitis plastica: spectrum of findings in early and delayed phase imaging. Abdom Radiol 41, 1370–1377 (2016). https://doi.org/10.1007/s00261-016-0652-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0652-8