Abstract
Background or Purpose
To compare the cost-performance between planned short-course radiation and upfront concurrent chemoradiation on metastatic rectal cancer.
Methods
A total of 75 patients with metastatic rectal cancer who underwent planned short-course radiation or upfront concurrent chemoradiation were enrolled. The Kaplan-Meier method was used to compute the survival rates. The χ2 test was used to compare baseline characteristics. The Cox proportional hazards model was applied to determine the prognostic influence of clinicopathological factors.
Results
The planned short-course radiation is superior to upfront concurrent chemoradiation in overall survival for the patients with metastatic rectal cancer (34.8 vs. 20.2 months, P = 0.010). The planned short-course radiation was an independent prognostic factor (P = 0.009, HR (95% CI) = 0.319(0.135–0.752)). The efficacy of radiation on downstaging was similar between planned short-course radiation and upfront concurrent chemoradiation. The total cost of concurrent chemoradiation is 4.52-fold more expensive than that of short-course radiation (340,142 vs. 75,106 NT dollars, respectively).
Conclusions
Based on the impressive cost-performance of planned short-course radiation compared with upfront concurrent chemoradiation (better OS, modest downstaging and lower cost), planned short-course radiation should be the preferred radiation approach for managing metastatic rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the most common malignancy worldwide. A quarter of the cases are rectal cancer (RC). Unfortunately, approximately half of these patients diagnosed with RC eventually progress to metastatic RC (mRC).1 Despite advances in multimodal treatment, the median overall survival (OS) of patients with mRC is approximately 30 months.2,3,4,5,6,7,8,9 However, 10–15% of mRC patients can survive the cancer after metastasectomy and aggressive bio-chemotherapy.7,8,9,10,11
Radiation therapy (RT) is the standard treatment in stage II–III RC with low-lying or local advanced lesions. However, the optimal radiation therapy for treating mRC remains uncertain. Most recommendations are based on extrapolation from stage II/III RC and small retrospective reports in patients with mRC.8,12,13,14,15,16,17,18,19,20,21,22,23 According to the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) 2018 guidelines, RT is recommended for patients with mRC. This is consistent with our previous report that upfront concurrent chemoradiation (CCRT) provided a survival benefit only in those patients with stage IV rectal cancer who underwent subsequent metastasectomy.8 However, there were two types of RT: long-course CCRT therapy and short-course radiation (scRT) therapy. This fact raises a dilemma in selecting the radiation therapy for mRC and the strategy (timing) of incorporating RT into mRC management.
Currently, there is no randomized clinical trial to answer this question. In the case of stage II–III RC, the advantages of CCRT included decreased risk of pelvic failure following surgery and higher rates of pathological response. The disadvantages of CCRT are longer duration of hospital stay and increased intolerance of systemic bio-chemotherapy.24 In contrast, scRT is another emerging choice for treating mRC. Its advantages include uncompromised progression-free survival (PFS) and OS, lower acute radiation-associated toxicity, and higher tolerance of systemic bio-chemotherapy.16 In addition, the timing of scRT remains unclear. It has been reported that a combination of scRT, chemotherapy, and bio-chemotherapy is another strategy for RC treatment.15,17,22,23,25 To our knowledge, there has been no study to compare planned scRT (scRT was restricted to patients who responded to bio-chemotherapy) with upfront CCRT (CCRT was arranged immediately after the diagnosis) in mRC.
Before 2012, if radiation was arranged in treating metastatic rectal cancer (mRC), upfront CCRT was the only choice in our hospital. In our previous study,8 upfront CCRT might only provide the survival benefit in patient with curative intent and scRT provided the equal biologically equivalent dose (BED) to CCRT.26 Thus, our multidisciplinary team (MDT) promoted an alternative choice: planned scRT in treating mRC. Our MDT wished that our patients received the right radiation which lead to survival benefit and escaped from any unnecessary adverse events of radiation (no radiation if not fitted). Thus, during 2012 to 2017, either upfront CCRT or planned scRT were used in treating mRC and the decision of planned scRT or upfront CCRT depended on physicians’ individual decision. This was a specific, but not mandatory, protocol in our hospital.
Herein, we conducted a retrospective study to compare the performance of upfront CCRT with planned scRT in mRC treatment (a different strategy for the incorporation of radiation in treating mRC). We aimed to show that planned scRT is superior to upfront CCRT for treating mRC with respect to cost and performance.
Materials and Methods
Study Population and Data Collection
This study retrospectively reviewed patients’ medical records (data was stored in health information systems) with initially clinical mRC who underwent “upfront CCRT” or “planned scRT” at the Taipei Veterans General Hospital between January 2008 and January 2017. RC was defined as a cancerous lesion located within 10 cm from the anal verge.27 Tumors were staged according to the American Joint Committee on Cancer staging system 6th edition. The general characteristics, clinicopathological staging, response, and surveillance were obtained from a computer database containing information on these patients. Follow-up was continued until July 2017 or the time of death. The patients were followed up every 3–6 months in the first 2 years, every 6–9 months in the next 5 years, and annually thereafter. Surveillance included physical examination, recto-digital examination, measurement of the level of carcinoembryonic antigen (CEA; ng/mL), measurement of the level of carbohydrate antigen 19–9 (CA19–9; U/mL), chest radiography, and abdominal CT scans. Resectability of primary tumors or metastatic lesions were evaluated by a multidisciplinary team. Upfront chemotherapy was according to physician choices and included 5-fluorouracil (5-FU)/leucovorin/oxaliplatin (FOLFOX4/FOLFOX6), 5-FU/leucovorin/irinotecan (FOLFIRI), and 5-FU/leucovorin (HDFL/DeGramont), plus cetuximab or bevacizumab. Cetuximab was only used in patients with wild-type K/N-RAS genes. Tumor regression grade was defined as follows: grade 0: pathologically complete remission; grade 1: tumor remission > 90%; grade 2: tumor remission > 50%, and grade 3 tumor remission < 50%. RECIST criteria was used to determine response.
The Radiation Schemes for Planned scRT and Upfront CCRT
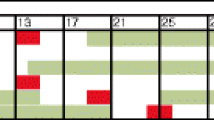
The radiation scheme is shown in Fig. 1. In the upfront CCRT group, the determination of treatment made was not based on resectability. Grossly, some of our physicians individually determine the upfront CCRT by size of the tumor, T4 and N1–2. On the contrary, potential resectability is our criterion in selecting patients into planned scRT strategy.
Arm 1: In the upfront CCRT group, we used the “sandwich” approach as follows: upfront chemotherapy ×1–2 cycles followed by CCRT (the same chemotherapy with approximately 70–100% of the dosage individually and the radiation doses to the pelvis were 45–50 Gy in 25–28 fractions). Then, a multidisciplinary team decided whether surgery was performed or not. After that, we continued the same bio-chemotherapy.28
Arm 2: In the planned scRT group, the approach was different from the CCRT group. In patients with unresectable mRC, induction bio-chemotherapy was arranged in 4–12 cycles, and scRT (5 fractions of radiotherapy, 5 Gy per fraction, administered each day for 5 days, with a total dose of 25 Gy) was arranged in patients who responded to complete remission (CR)/partial remission (PR)/stable disease (SD). After that, we continued the same bio-chemotherapy. This strategy was designed to protect our patients from unnecessary radiation exposure and radiation-related adverse events.
Statistical Analyses
The Kaplan-Meier method was used to compute the survival rates. OS was defined as the time from the mRC diagnosis to death from the cancer. We compared categorical variables between the upfront CCRT and the planned scRT groups using the χ2 test. The Cox proportional hazards model was applied for univariate and multivariate analyses to determine the prognostic influence of clinicopathological factors on the survival endpoints. We defined P values < 0.05 as statically significant. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS 18.0 for Windows; SPSS Inc., Chicago, IL).
Results
Baseline Clinical Characteristics
A total of 75 patients diagnosed as mRC at Taipei Veterans General Hospital were enrolled. The comparison of the baseline demographics of patients with mRC patients who underwent planned scRT or upfront CCRT is shown in Table 1. Forty-four (58.7%) patients received upfront CCRT and 31 (41.3%) patients experienced planned scRT. Grossly, the distribution of the patients who underwent upfront CCRT or those receiving planned scRT did not differ significantly except that the patients in the CCRT group had significantly shorter durations between diagnosis and radiation (mean 22 days) than those in the scRT group (mean 83 days) (P = 0.046), had different metastasis sites (P = 0.034), and had different periradiation chemotherapy regimens (P < 0.001). The median (interquartile range) cycles of chemotherapy in the CCRT group and the scRT group were 1 (0) and 4 (7) respectively. This difference came from our study protocol (Fig. 1). The approach to the planned scRT group, unlike the upfront CCRT group, was arranged in patients who responded to CR/PR/SD or patients with initially resectable mRC. The completion of scRT and CCRT was 100% and 86.4% (38/44), respectively. The denominator of the planned scRT group in our study was around 36 patients. The failure rate of neoadjuvant chemotherapy in the planned scRT group was around 13.8%.
Planned scRT Is Superior to Upfront CCRT for Treating mRC
The prognostic factors for OS in patients with mRC are shown in Table 2. In univariate Cox regression analysis, only this type of radiation was a significantly good prognostic marker (P = 0.012, HR (95% CI) = 0.434 (0.226–0.832)). Moreover, based on additional insights from the multivariate Cox regression analysis, we found that both the type of radiation and metastasectomy were good prognostic factors (P = 0.023, HR(95% CI) = 0.420 (0.198–0.889)); furthermore, the clinical N status was a poor prognostic factor (P = 0.045, HR(95% CI) = 2.092 (1.015–4.310)). Interestingly, both colectomy and the duration between diagnosis and radiation were not significant prognostic factors.
The estimated median OS (not reached) was 34.8 months with planned scRT compared with 20.2 months with upfront CCRT (Fig. 2a), and there was a significant difference between the arms (P = 0.010). The clinical N status was a poor prognostic factor (P = 0.027) (Fig. 2b). The N0, N1, N2 median OSs were 45.0 months (estimated; not reached), 40.5 months, and 26.1 months, respectively. The median OS was 40.5 months with metastasectomy compared with 27.5 months without metastasectomy (Fig. 2c). There was a significant difference between arms (P = 0.063). Whether a colectomy was performed was not a significant prognostic factor (P = 0.143) (Fig. 2d).
The Efficacy on Downstaging and Tumor Regression Grade of Planned scRT Is Not Inferior to Upfront CCRT
At the end of the analysis, 58 patients had received colectomies after radiation (Table 3). On the surface of Table 3, compared with upfront CCRT, planned scRT had relatively low efficacy on downstaging (57.7% vs. 62.5%) and tumor regression rate (TRG) (TRG 0–1 vs TRG 2–3: 44.0% vs. 52.9%); however, the difference is not statistically significant. Recently, scRT with delayed surgery has become an alternative choice for treating mRC. The comparison of the efficacies of planned scRT or upfront CCRT in patients with stage IV rectal cancer who underwent colectomy with a duration between radiation and colectomy of > 60 days is shown in Table 4. Even though the P values were still not significant, the downstage rate rose from 57.7 to 70.0%; however, the TRG did not rise.
The Delayed Surgery Strategy Increases the T Down-Stage Rate in Patients with Planned scRT
The comparison of the efficacies of planned scRT in patients with mRC who underwent colectomy and the duration between radiation and colectomy ≤ 60 or > 60 days is shown in Table 5. The values of the downstage rate, T downstage rate, and N downstage rate increased, but the TRG did not increase. Only the T downstage increased significantly (P = 0.034).
The Cost of CCRT Is 4.52-Fold More Expensive than scRT in Taiwan Reimbursement
The cost-performance of scRT was far higher than CCRT under Taiwan reimbursement. The schedule and the cost of each radiation procedure are listed in Table 6. The cost of scRT is 55,456 NT dollars and CCRT is 210,452 NT dollars. The cost of CCRT is 2.8-fold higher than that of scRT for outpatients. With additional hospital stays, the hospital fee for CCRT is 6.6-fold higher than that for scRT. Based on the above costs, the total cost of an entire course of CCRT is 4.52-fold higher than that of scRT (340,142 NT dollars vs. 75,106 NT dollars). With respect to public health policy, planned scRT offered a more impressive cost-performance ratio, and it should be the only preferred radiation approach for managing mRC.
Discussion
In the empire of radiation therapy for managing mRC, OS is King, the adverse effects are Queen, and the strategies for incorporating radiation into systemic bio-chemotherapy are the princes and princesses of the realm. In other words, the primary end point of radiation in mRC treatment is increased OS, the secondary end-point is fewer adverse effects, and the third end-point is identifying the strategy offering the highest cost-performance for patients; these are the cornerstones of incorporating radiation into mRC management.29
Data to guide the strategy regarding the incorporation of radiation into mRC management are scarce. Most recommendations came from small studies and experts’ discussion.8,12,13,14,15,16,17,19,20,21,28,30,31 Based on this literature8,12,13,14,15,16,17,19,20,21,28,30,31 and the NCCN and ESMO 2018 guidelines, either scRT or CCRT are advised for managing mRC. However, there is no consensus about which one is the best choice in this area. With the growing evidence based on small studies, the preference for scRT is increasing.
Our report offers evidence that the planned use of scRT is the preferred strategy for treating mRC compared with upfront CCRT. Our results showed that planned scRT was superior to upfront CCRT in improving the OS mRC patients (34.8 vs. 20.2 months, P = 0.010). In addition, we also showed that the efficacy of radiation in downstaging was similar between planned scRT and upfront CCRT. The key element of our strategy for planned scRT was that it was performed in patients who responded to bio-chemotherapy.
Our results are supported by literature,18,19,20,21,22,23 as in previous studies the median OS range was from 25.0 to 45.6 months. If the treatment goal for the enrolled patient is to cure, the OS was better (45.6 months),20 and if the goal is palliation, the median OS declines (25.0 months).19 In the convertible or resectable patients, we found an OS of 34.8 months, which is similar to the findings of Kim et al. (33.6 months).18 If we took metastasectomy into consideration in our study, the median OS of our patients after radiation and metastasectomy was 40.5 months, comparable to 45.6 months.
Moreover, there was another independent prognostic marker in the multivariate Cox regression analysis, i.e., metastasectomy. This possibility was suggested in our previous report8 and Emma’s report.21 They showed the similar finding that definitive management of metastases was associated with improved OS [hazard ratio (HR) 0.03, 95% confidence interval (CI) 0.01–0.33]; P = 0.003, and ≤ 2 months of neoadjuvant chemotherapy was associated with decreased OS (HR 11.7, 95% CI 2.11–106; P = 0.004). Interestingly, colectomy is not an independent prognostic factor in either of these studies.
In our report, we could not assess the different occurrence of adverse effects between planned scRT and upfront CCRT. However, the comparison of adverse effects are extensively reviewed in the literature, especially in stage II–III RC. The most striking difference between scRT and CCRT is a lower risk of early adverse effects,32 and notably, there is no difference in the long-term outcomes between the two groups.13,33 In our clinical experience, the adverse effects are manageable.
With regard to the cost, the comparison between scRT and CCRT is listed in Table 6. From the viewpoint of reimbursement and the attempt to end the so-called postcode lottery of healthcare, the most important issue is the cost-performance ratio. The total cost of an entire course of CCRT is 4.52-fold higher than that of scRT (340,142 NT dollars vs. 75,106 NT dollars, respectively). With respect to public health policy, planned scRT offered the most impressive cost-performance ratio (less cost and better OS), and therefore, it should be the only preferred radiation therapy for managing mRC.
There were some limitations in our study. First, our report is based on a small patient number, and we must arrange a large study to confirm the finding. Second, our study is a retrospective analysis, and the planned scRT strategy has only been used since 2012 (short length of KM cure in Fig.2a), and some survival benefit might come from new agents for mCRC treatment. Nevertheless, our estimated OS of 34.8 months exceed 30 months. Generally speaking, this means that our strategy is on the way to providing increased survival for our patients. Third, some selection bias existed. It came from the fact that planned scRT is not used in patients with disease progression after the start of bio-chemotherapy. This is our strategy to protect our patients from unnecessary radiation exposure and from radiation-related adverse effects, such as fistula. Finally, we did not report the comparison of adverse effects, because the data are not reported in detail in our datasheet. In the future, we need more prospective trials to compare planned scRT with upfront CCRT in stage IV rectal cancer.
Conclusion
The goal of our study is not to compare the efficacy of different radiation schemas but to determine the right strategy for the incorporation of radiation in treating mRC (The right patients). Based on the impressive benefits of planned scRT compared with upfront CCRT, i.e., better OS, modest downstaging, and lower cost, we concluded that planned scRT, rather than upfront CCRT, is the preferred radiation strategy for managing mRC.
References
Naishadham D, Lansdorp-Vogelaar I, Siegel R et al. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev 2011; 20: 1296–1302
Cunningham D, Humblet Y, Siena S et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–345
Falcone A, Ricci S, Brunetti I et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007; 25: 1670–1676
Montagnani F, Chiriatti A, Turrisi G et al. A systematic review of FOLFOXIRI chemotherapy for the first-line treatment of metastatic colorectal cancer: improved efficacy at the cost of increased toxicity. Colorectal Dis 2011; 13: 846–852
Saltz LB, Clarke S, Diaz-Rubio E et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008; 26: 2013–2019
Souglakos J, Androulakis N, Syrigos K et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br J Cancer 2006; 94: 798–805
Teng HW, Huang YC, Lin JK et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. Journal of surgical oncology 2012; 106: 123–129
Lin JK, Lee LK, Chen WS et al. Concurrent chemoradiotherapy followed by metastasectomy converts to survival benefit in stage IV rectum cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2012; 16: 1888–1896
Hsu YN, Lin JK, Chen WS et al. A new classification scheme for recurrent or metastatic colon cancer after liver metastasectomy. Journal of the Chinese Medical Association : JCMA 2011; 74: 493–499
Van Cutsem E, Nordlinger B, Adam R et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. European journal of cancer 2006; 42: 2212–2221
Choti MA, Sitzmann JV, Tiburi MF et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002; 235: 759–766
Bosset JF, Collette L, Calais G et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: 1114–1123
Bujko K, Nowacki MP, Nasierowska-Guttmejer A et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–1223
Gerard JP, Conroy T, Bonnetain F et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006; 24: 4620–4625
Radu C, Berglund A, Pahlman L et al. Short-course preoperative radiotherapy with delayed surgery in rectal cancer - a retrospective study. Radiother Oncol 2008; 87: 343–349
Ceelen W, Fierens K, Van Nieuwenhove Y et al. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: a systematic review and meta-analysis. International journal of cancer Journal international du cancer 2009; 124: 2966–2972
Shin SJ, Yoon HI, Kim NK et al. Upfront systemic chemotherapy and preoperative short-course radiotherapy with delayed surgery for locally advanced rectal cancer with distant metastases. Radiat Oncol 2011; 6: 99
Kim KH, Shin SJ, Cho MS et al. A phase II study of preoperative mFOLFOX6 with short-course radiotherapy in patients with locally advanced rectal cancer and liver-only metastasis. Radiother Oncol 2016; 118: 369–374
Picardi V, Deodato F, Guido A et al. Palliative Short-Course Radiation Therapy in Rectal Cancer: A Phase 2 Study. Int J Radiat Oncol Biol Phys 2016; 95: 1184–1190
Bisschop C, van Dijk TH, Beukema JC et al. Short-Course Radiotherapy Followed by Neoadjuvant Bevacizumab, Capecitabine, and Oxaliplatin and Subsequent Radical Treatment in Primary Stage IV Rectal Cancer: Long-Term Results of a Phase II Study. Ann Surg Oncol 2017; 24: 2632–2638
Holliday EB, Hunt A, You YN et al. Short course radiation as a component of definitive multidisciplinary treatment for select patients with metastatic rectal adenocarcinoma. J Gastrointest Oncol 2017; 8: 990–997
Goodman KA, Milgrom SA, Herman JM et al. ACR Appropriateness Criteria(R) rectal cancer: metastatic disease at presentation. Oncology (Williston Park) 2014; 28: 867–871, 876, 878
Lutz MP, Zalcberg JR, Glynne-Jones R et al. Second St. Gallen European Organisation for Research and Treatment of Cancer Gastrointestinal Cancer Conference: consensus recommendations on controversial issues in the primary treatment of rectal cancer. European journal of cancer 2016; 63: 11–24
Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740
Fernandez-Martos C, Pericay C, Aparicio J et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 2010; 28: 859–865
Viani GA, Stefano EJ, Soares FV et al. Evaluation of biologic effective dose and schedule of fractionation for preoperative radiotherapy for rectal cancer: meta-analyses and meta-regression. Int J Radiat Oncol Biol Phys 2011; 80: 985–991
Kapiteijn E, Marijnen CA, Nagtegaal ID et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345: 638–646
Madoff RD. Chemoradiotherapy for rectal cancer--when, why, and how? N Engl J Med 2004; 351: 1790–1792
Cady B. Basic principles in surgical oncology. Arch Surg 1997; 132: 338–346
Bartlett DL, Berlin J, Lauwers GY et al. Chemotherapy and regional therapy of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006; 13: 1284–1292
Minsky BD. Counterpoint: long-course chemoradiation is preferable in the neoadjuvant treatment of rectal cancer. Semin Radiat Oncol 2011; 21: 228–233
Bujko K, Bujko M. Point: short-course radiation therapy is preferable in the neoadjuvant treatment of rectal cancer. Semin Radiat Oncol 2011; 21: 220–227
Ngan SY, Burmeister B, Fisher RJ et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012; 30: 3827–3833
Acknowledgments
We thank our colleague Chang Miawerl who provided some work that assisted the research.
Author Declaration
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Funding
This study was funded by grants from the Taiwan Clinical Oncology Research Foundation and from the Ministry of Science and Technology, Taiwan (MOST 105-2314-B-075-051; MOST 106-2314-B-075-062), and the Taipei Veterans General Hospital (V105C-132, V106C-166, V107C-120).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors. This retrospective study was conducted based on population-based data from the Taipei Veterans General Hospital, Taipei, Taiwan, and under the guidelines of the Helsinki declaration, approved by the Human Subjects Protection Offices (IRB) at the Taipei Veterans General Hospital (VGHIRB number: 2015-07-003AC). Because all identifying patient information was removed prior to analysis in this study, informed consent was not obtained.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teng, HW., Lin, JK., Lin, TC. et al. Planned Short-Course Radiation (scRT) is Superior to Upfront Concurrent Chemoradiation (CCRT) in Treating Metastatic Rectal Cancer. J Gastrointest Surg 24, 1092–1100 (2020). https://doi.org/10.1007/s11605-019-04256-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04256-3