Abstract
Background
In China, hepatitis B virus (HBV) is an important causative factor of hepatocellular carcinoma (HCC). The contribution and interaction of fibrosis-4 (FIB-4) score and total tumor volume (TTV) in association with HCC recurrence is unknown. A reliable point score based on the FIB-4 score, TTV, and differentiation grade was established to predict the postoperative recurrence of HBV-related HCC patients who underwent hepatic resection (HR).
Methods
Three hundred thirty-eight HBV-related HCC patients from three institutions treated by HR were enrolled in this retrospective study. Prognostic factors were also evaluated by univariate and multivariate analysis using Cox’s proportional hazards model in the training cohort. The DFT score was established by a Cox regression model and validated in the internal cohort and the external cohorts from the other two institutions.
Results
The DFT score differentiated four groups of HBV-related HCC patients (0, 1–2, 3, 4–5 points) with distinct prognosis (median recurrence-free survival (RFS), 72.7 vs. 53.0 vs. 23.2 vs. 5.7 months; P < 0.05). Its predictive accuracy as determined by the area under the receiver operating characteristic curve (AUC) at 1, 3, and 5 years (AUCs 0.7319, 0.7031, and 0.6972) was greater than the other three staging systems for HCC. These findings were supported by the validation cohorts.
Conclusions
The DFT model is a reliable and objective model to predict the RFS of HBV-related HCC patients after HR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a common malignant tumor and ranks as the third leading cause of cancer-related death worldwide. Currently, hepatic resection (HR) was recommended as a first-line treatment for HCC patients with good liver functional reserve. However, there is a high incidence of recurrence and metastasis after HR.1, 2 Therefore, it is necessary to make some individualizing decisions about their treatment and individualizing surveillance after HR. Until now, the Barcelona Clinic Liver Cancer (BCLC) classification staging system has been endorsed as the best staging system and treatment algorithm for HCC by the European Association for the Study of Liver Disease (EASL) and the American Association for the Study of Liver Disease (AALSD).3, 4 BCLC staging system establishes a link between staging and estimating prognosis. However, the risk scoring models based on tumor characteristics and peritumoral fibrosis for predicting HCC recurrence are still little.
In China, more than 90% of HCC is related to hepatitis B.5 Ample evidence indicated that various inflammatory activities induced by fibrosis or cirrhosis could promote the recurrence of HCC after HR.6, 7 Liver biopsy has been the gold standard for evaluation of liver fibrosis. However, it is not routinely performed in clinical practice due to some limitations of this procedure. Recently, “non-invasive” scoring systems based on laboratory tests have been developed and could be an alternative method for assessing the extent of hepatic fibrosis. Fibrosis-4 (FIB-4) is an index score calculated from age, platelet count, alanine aminotransferase (ALT), and aspartate aminotransferase (AST).8 A number of studies have described that FIB-4 could assess the severity of liver fibrosis and predict the relapse of HCC after HR. More interestingly, Maeda et al. showed that the pretreatment FIB-4 index was associated with recurrence and the 5-year recurrence rate after hepatectomy was 69.6% in HCC patients with a FIB-4 index > 3.25.9 A recent study also indicated that a novel nomogram comprising the liver functions (combination of the albumin–bilirubin and FIB-4), peritumoral inflammatory score, AFP, tumor number, tumor size, and microvascular invasion could accurately predict the recurrence-free survival (RFS) of an individual with HCC after curative resection.10 On the other hand, some studies reported that total tumor volume (TTV) is a more accurate indicator of tumor burden as opposed to the current standard of number and size of tumor nodules of HCC.11, 12 Huo TI et al. showed that the AFP/TTV ratio might be useful in selecting super-high-risk patients for tumor recurrence.13 Of note, these studies suggested that FIB-4 score and TTV in the different scoring systems played the different roles in predicting HCC recurrence after curative hepatectomy. The contribution and interaction of FIB-4 score and TTV in association with HCC recurrence is not clear.
In this multicenter retrospective study, we constructed a reliable scoring system comprising the FIB-4 score, TTV, and differentiation grade to predict the postoperative RFS of HBV-related HCC patients who underwent HR. The aim was to validate its efficacy in predicting the RFS of HBV-related HCC patients after HR.
Methods
Clinical Samples and Follow-up
In this study, 255 HBV-related HCC patients who underwent curative hepatectomy were selected from the third affiliated hospital of Sun Yat-sen University (ZSSY) between December 2006 and February 2014. These patients were divided into a training cohort (n = 162, from August 2008 to November 2013) and an internal validation cohort (n = 93, from December 2006 and February 2014). Eighty-three HBV-related HCC patients as the external validation cohorts were enrolled at two independent centers as follows: 51 patients from The Affiliated Hospital/Clinical Medical College of Chengdu University (CDFY) between January 2005 and October 2016 and 32 patients from the Huashan Hospital of Fudan University (FDHS) between September 2015 and July 2017. All HBV-related HCC patients were underwent R0 resection and not received pre-operative treatment (ie. liver transplant, transarterial chemoembolization, or radiofrequency ablation). The hepatic resection procedure was performed as described previously.14
The data were censored on December 31, 2016, in the ZSSY cohort and CDFY cohort and on May 1, 2018, in the FDHS cohort. The follow-up examinations were conducted, including physical examination, serum alpha-fetoprotein (AFP), ultrasonography, chest X-ray, and abdominal computed tomography (CT) and/or magnetic resonance imaging (MRI) every 3 months in the first 2 years and 6 months thereafter. RFS was defined as the time from the day of operation to the date of recurrence, metastasis, or last follow-up.
Study Design
The study flowchart was shown in Fig. 1. In our study, clinicopathologic characteristics (i.e., gender, age, albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alpha-fetoprotein (AFP), tumor size, microvascular invasion, capsular invasion) of enrolled patients were prospectively collected. Two hundred fifty-five HBV-related HCC patients from ZSSY were divided randomly into the training (n = 162) and internal validation (n = 93) cohorts. Univariate and multivariate Cox regression analysis were performed to establish the DFT risk score in the training cohort. To validate the finding, we also performed internal and external validation in 176 HBV-related HCC patients from three independent centers. In view of the short-term follow-up in the external validation 2 (FDHS), we only assessed the performance of the DFT scoring system in predicting 1-year recurrence for these patients.
The retrospective study was approved by the ethics committee of each study center and written informed consent was obtained from each participant before HR.
Calculation of Score Values
TTV (cm3) was calculated as the sum of all tumor nodule volumes, and each tumor nodule volume is calculated as 4/3 × 3.14 × (maximum radius of the tumor nodule)3 as previously described.13 The patients were divided into two groups: TTV < 115 cm3 and TTV ≥ 115 cm3. The FIB-4 score was calculated as the following formula exactly as originally described10: age × AST/platelet count (× 103/μL) × ALT1/2. The FIB-4 categories were defined as follows: FIB-4 < 3.25 and FIB-4 ≥ 3.25.
Statistical Analysis
All demographic and clinicopathological data had been prospectively collected in computer databases before this retrospective analysis. Continuous variables were expressed as mean ± standard deviation. The RFS was determined by the Kaplan–Meier analysis. Univariate and multivariate analyses were performed with the Cox proportional hazard regression model to generate hazard ratios (HRs) and 95% confidence intervals (CIs). The regression coefficients (B-values) of the Cox regression model were multiplied by a factor of 3 and divided by 2, and rounded to the nearest unit to obtain simple point numbers to facilitate the calculation of the DFT score. To further validate the discriminative ability of the DFT scoring system, the area under the receiver operating characteristic curve (ROC) of the DFT scoring system was compared with that of commonly used staging systems. All statistical analyses were performed with the IBM SPSS 19.0 statistical package (SPSS, Armonk, USA). All reported P values were the result of two-sided tests and P < 0.05 was considered statistically significant.
Results
Patient Characteristics
The baseline characteristics of the ZSSY cohort were presented in Table 1. In the training cohort, 60.5% of HCC patients after HR (98 of 162) developed recurrence, and 21.0% of the patients (34 of 162) died. The 1-, 3-, and 5-year RFS rates were 35.8%, 53.1%, and 58.0%, respectively. In the internal validation cohort, the 1-, 3-, and 5-year RFS rates were 32.3%, 51.6%, and 61.3%, respectively.
Building a Risk Score Model
In the training cohort, the median RFS was 29.2 months. Next, we selected some variables that might affect RFS of HCC patients (i.e., pre-operative AFP level, tumor size, differentiation grade, microvascular invasion, clinically significant portal hypertension (PHT) (Table 2), neutrophil-to-lymphocyte ratio (NLR), FIB-4, and TTV) to perform univariate and multivariate Cox regression analysis. Univariate analysis revealed that pre-operative AFP level, tumor size, differentiation grade, microvascular invasion, NLR, FIB-4, and TTV were independent prognostic factors of RFS (Table 3). However, multivariate analysis demonstrated that only differentiation grade, FIB-4, and TTV were all significantly associated with RFS (Table 3).
To better investigate the performance of these risk factors in predicting recurrence, the DFT risk score model was built, with the regression coefficients weighted by the Cox model in the training cohort. In this study, the calculated regression coefficients (B-values) were multiplied by a factor of 3 and divided by 2, and rounded to facilitate the DFT score (Table 4). The DFT risk score was calculated as follows: DFT score = D (III–IV = 2; I–II = 0) + F(≥ 3.25 = 1; < 3.25 = 0) + T(≥ 115 = 2; < 115 = 0).
DFT Score Predicts RFS in the Training and Validation Cohorts
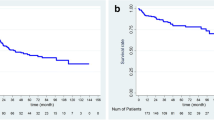
According to the DFT score, 162 HBV-related HCC patients in the training cohort were divided into six subgroups. Then, we further assessed the performance of the DFT score in predicting RFS for these patients. As was shown in Fig. 2a, there was no significant difference between DFT = 1 group and DFT = 2 group in RFS (52.4 months vs. 49.3 months, P = 1.693). Furthermore, the media RFS of DFT = 4 group and DFT = 5 group were 5.0 months and 7.1 months, respectively.
Kaplan–Meier estimated RFS curves by DFT score. a Prognostic significance of the single-point scores for RFS in the training cohort. HBV-related HCC patients in the training cohort were divided into four groups (0 point, 1–2 points, 3 points, 4–5 points) based on favorable median RFS in the Kaplan–Meier curves. b–d Kaplan–Meier estimated RFS curves by DFT score. The prognostic significance of the four DFT score groups (0 point, 1–2 points, 3 points, 4–5 points) for RFS in the training (b), the internal validation cohort (c), and the external validation cohort 1 (d)
Based on these results, 162 HBV-related HCC patients were classified into A stage (DFT = 0), B stage (DFT = 1 and DFT = 2), C stage (DFT = 3), and D stage (DFT = 4 and DFT = 5) (Table 5). In the training cohort, the median RFS of the HBV-related HCC patients with A (n = 66), B (n = 80), C (n = 6), and D (n = 10) stage was 72.7 months (95% CI, 61.9–83.5 months), 53.0 months (95% CI, 42.2–63.7 months), 23.2 months (95% CI, 1.8–44.6 months), and 5.7 months (95% CI, 3.8–7.5 months), respectively (Fig. 2b). Consistently, the novel DFT scoring system also had good performance in RFS prediction, with four significantly different prognostic subgroups in the internal validation and the external validation 1 (Fig. 2c, d).
The Predictive Accuracy of the DFT Scoring System in the Training and Validation Cohorts
To better evaluate the predictive value of the DFT scoring system in predicting RFS, we compared the accuracy of DFT scoring system with that of the current common used BCLC, HongKong Liver Cancer (HKLC), and American Joint Committee on Cancer (AJCC, TNM 8th) staging systems. In the training cohort, the areas under ROC curve (AUCs) of the DFT scoring system at 1, 3, and 5 years were 0.7319, 0.7031, and 0.6972, respectively, and were greater than those of the other three commonly used staging systems for HBV-related HCC (Figs. 3a and 4a, b). In the internal validation cohort, the AUCs of the DFT scoring system at 1, 3, and 5 years were 0.6429, 0.6203, and 0.6345, respectively, and were greater than those of the other three commonly used staging systems for HBV-related HCC (Figs. 3b and 4c, d). In the external validation cohort 1, the AUCs of the DFT scoring system at 1, 3, and 5 years were 0.6667, 0.6026, and 0.6345, respectively, and were greater than those of the other three commonly used staging systems for HBV-related HCC (Figs. 3c and 4e, f). In the external validation cohort 2, the AUCs of the DFT scoring system, BCLC, HKLC, and TNM at 1 year were 0.6345, 0.5000, 0.5263, and 0.6250, respectively (Figs. 3d and 4g, h). Altogether, our study indicated that the DFT scoring system had a better predictive value than the other three staging systems in predicting RFS.
DFT Score Predicts RFS in HBV-Related HCC Patients with BCLC B/C Stage
Here, we further evaluated the predictive value of the DFT scoring system in predicting RFS of HBV-related HCC patients with BCLC B/C stage. Because of the sample size of HCC patients with BCLC B/C stage in the training and validation cohorts, we combined the training cohort and internal validation cohort as the ZSSY cohort and the two relatively small sample data sets (CDFY and FDHS) as an external validation cohort.
When HCC patients with BCLC B/C stage were stratified by the DFT scoring system in the ZSSY cohort, the median RFS of the patients with A (n = 30), B (n = 54), C (n = 7), and D (n = 15) stage was 53.3 months (95% CI, 36.5–70.2 months), 29.6 months (95% CI, 20.3–39.1 months), 10.7 months (95% CI, 1.47–21.8 months), and 4.8 months (95% CI, 3.2–6.4 months), respectively (Fig. 5a). Furthermore, the AUCs of the DFT scoring system at 1, 3, and 5 years were 0.7150, 0.6857, and 0.7148, respectively, and were greater than those of the other two commonly used staging systems for BCLC B/C stage HCC (Fig. 5b–d).
Kaplan–Meier estimated RFS curves by DFT score in HBV-related HCC patients with BCLC B/C stage in the ZSSY cohort (a). The AUC of DFT score system and the other two clinical staging systems (TNM and HKLC staging system) in predicting RFS of HBV-related HCC patients with BCLC B/C stage in the ZSSY cohort at 1 year, 3 years, and 5 years (b–d)
In external validation cohort, HBV-related HCC patients with BCLC B/C stage were stratified by the DFT scoring system (Fig. 6a). The AUCs of the DFT scoring system at 1, 3, and 5 years were 0.7619, 0.7388, and 0.6949, respectively, and were greater than those of the other two commonly used staging systems for BCLC B/C stage HCC (Fig. 6b–d).
Kaplan–Meier estimated RFS curves by DFT score in HBV-related HCC patients with BCLC B/C stage in the external validation cohort (a). The AUC of DFT scoring system and the other two clinical staging systems (TNM and HKLC staging system) in predicting RFS of HBV-related HCC patients with BCLC B/C stage in the external validation cohort at 1 year, 3 years, and 5 years (b–d)
Discussion
To date, only the BCLC and HKLC systems provided stage-appropriate treatment modalities. According to the guidelines of EASL and AASLD, recommended treatments for BCLC B/C stage patients include transarterial chemoembolization (TACE), sorafenib, and radioembolization, but not HR. However, more and more studies from China indicated that HR could benefit many patients with intermediate or advanced HCC or with HCC associated with portal hypertension.5, 15, 16 Herein, we constructed a novel scoring system (DFT) comprising the liver functions (FIB-4), tumor burden (TTV), and differentiation grade, and this scoring system allowed for a more accurate prognostic prediction for RFS of an individual with HBV-related HCC after HR.
It is widely accepted that tumor burden and underlying liver function are associated with the prognosis of HCC patients. In China, the majority of HCC patients was infected by HBV and developed HCC from chronic hepatitis B (CHB) and CHB-induced liver cirrhosis. Previous studies have showed that the inflammatory activity could promote HCC recurrence by promoting the proliferation of premalignant cells6, 7 and aggravating liver damage. The FIB-4 was associated with peritumoral inflammatory activities in HCC and could predict the severity of fibrosis. On the other hand, some studies demonstrated that TTV, a more accurate marker of tumor burden, played a crucial role in predicting tumor recurrence.13, 17,18,19,20,21,22,23 In our DFT scoring system, TTV and FIB-4 partly supports the seed and soil theory of metastasis and recurrence. The DFT model, based on the tumor biology and the inflammatory status of noncancerous microenvironment, may contribute to a significantly increased predictive accuracy due to that the recurrence of HBV-related HCC depends on the interaction between cancer cells and the inflammatory noncancerous microenvironment. More interestingly, a recent study reported that a nomogram, incorporating PIS and combined ALBI and FIB-4, was associated with recurrence for HCC following curative hepatectomy.10 The authors also focused on the associations between inflammatory status and the recurrence of HCC patients after HR. The combined ALBI and FIB-4 might provide an entirely objective tool for assessing the progression, recurrence, and liver function of patients with HCC.
Although BCLC, HKLC, and AJCC (TNM 8th) staging systems showed the abilities to stratify HCC patients after HR into different risk categories, the ROC analysis supported that our DFT scoring system was superior to the BCLC, HKLC, and AJCC (TNM 8th) staging systems. These suggested that the DFT scoring system was a reliable staging system and had a better predictive value for RFS of HBV-related HCC patients compared to the representative HCC systems. Previous studies indicated that the FIB-4 index that reflects the degree of fibrosis of the remnant liver seems to be stronger when predicting long-term outcomes.24 In line with these results, we demonstrated that the difference in survival rates between the four groups became apparent approximately 1 year after hepatectomy.
In the present study, we first validated that HBV-related HCC patients who underwent HR were successfully classified into four stages according to the DFT score. These finding suggested that it is feasible to prolong the survival of HCC patients by reducing the DFT score. As for the methods of downstaging, TACE, hepatic arterial infusion (HAI), and antiviral treatment could be recommended to appropriate patients with potential high DFT score. In addition, our studies may help to identify the subgroup of HBV-related HCC patients who could benefit most from bridge treatment while awaiting liver transplantation. More significantly, pre-operative tumor biopsy is recommended in the DFT scoring system. The information of molecular and histological characterization from tumor tissue may provide prognostic data that are useful in the selection of therapy. Now, we face a time when oncology is moving towards personalized/precision medicine. In the context of histological HCC sub-classes, each with distinct molecular patterns, histological characterization, and prognostic impacts, the need for liver biopsy in HCC management may become a necessity.
There are several limitations in the present study. First, HCC patients had a background with HBV infection. Therefore, the DFT model may not be suitable for non-HBV-related HCC patients. Second, in the West, HR is not recommended as the first-line treatment for HCC patients with BCLC B/C stage. So, it limited the application of the DFT scoring system in the West. Third, our study was a retrospectively multicenter study, limited by the retrospective nature of the analysis. To increase reliability of our study, we confirmed our results in internal cohort and external cohorts.
Conclusion
In conclusion, the DFT model is a reliable and objective model to predict the RFS of HBV-related HCC patients after HR. HBV-related HCC patients with low score of DFT, especially in BCLC B/C stage patients, could benefit from aggressive hepatectomy. In the future, a large-scale prospective study is needed to perform and then verify its extensive applicability.
References
Makuuchi M, Takayama T, Kubota K, Kimura W, Midorikawa Y, Miyagawa S, Kawasaki S. Hepatic resection for hepatocellular carcinoma—Japanese experience. Hepatogastroenterology. 1998; 45(Suppl 3): 1267–1274.
Poon RT, Fan ST. Evaluation of the new AJCC/UICC staging system for hepatocellular carcinoma after hepatic resection in Chinese patients. Surg Oncol Clin North Am. 2003; 12(1): 35–50.
Bruix J, Sherman M, Practice Guidelines Committee American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42(5): 1208–1236.
European Association For The Study Of The Liver and European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56(4): 908–943.
Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014; 260(2): 329–340.
Sherman M: Recurrence of hepatocellular carcinoma. N Engl J Med. 2008; 359(19): 2045–2047.
Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014; 63(5): 844–855.
Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, Janssen HL, Lampertico P, Lau D, Bornstein JD, Schall RE, Dinh P, Yee LJ, Martins EB, Lim SG, Loomba R, Petersen J, Buti M, Marcellin P. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016; 64(4): 773–780.
Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery. 2015; 157(4): 699–707.
Liao R, Li DW, Du CY, Li M. Combined Preoperative ALBI and FIB-4 Is Associated with Recurrence of Hepatocellular Carcinoma After Curative Hepatectomy. J Gastrointest Surg. 2018; 22 (10):1679–1687.
Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, Chiou YY, Chiang JH, Lee PC, Huo TI, Lee SD. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010; 53(1): 108–117.
Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009; 49(3): 832–838.
Lee YH, Hsu CY, Huang YH, Su CW, Lin HC, Hsia CY, Huo TI. α-fetoprotein-to-total tumor volume ratio predicts post-operative tumor recurrence in hepatocellular carcinoma. J Gastrointest Surg. 2013; 17(4): 730–738.
Wei Q, Tian H, Luo HX, Zhang YC, Deng YN, Yao J, Li H, Chen GH, Yang Y. Better prognosis of hepatic resection combined with antiviral therapy for HBV-related hepatocellular carcinoma with BCLC Stage B/C. Asian J Surg. 2017; 40(6): 453–462.
Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009; 249(1): 118–23.
He W, Zeng Q, Zheng Y, Chen M, Shen J, Qiu J, Chen M, Zou R, Liao Y, Li Q, Wu X, Li B, Yuan Y. The role of clinically significant portal hypertension in hepatic resection for hepatocellular carcinoma patients: a propensity score matching analysis. BMC Cancer. 2015;15: 263.
Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003; 38(2): 200–207.
Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009; 51(5): 890–897.
Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007; 141(3): 330–339.
Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ, Wei SH, Curley SA, Laurent A, Poon RT, Belghiti J, Nagorney DM, Aloia TA. International Cooperative Study Group on Hepatocellular Carcinoma. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013; 17(1): 66–77.
Cucchetti A, Piscaglia F, Caturelli E, Benvegnù L, Vivarelli M, Ercolani G, Cescon M, Ravaioli M, Grazi GL, Bolond L, Pinna AD. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009; 16(2): 413–422.
Truty MJ, Vauthey JN. Surgical resection of high-risk hepatocellular carcinoma: patient selection, preoperative considerations, and operative technique. Ann Surg Oncol. 2010; 17(5): 1219–1225.
Hosaka T, Ikeda K, Kobayashi M, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase Y, Kumada H. Predictive factors of advanced recurrence after curative resection of small hepatocellular carcinoma. Liver Int. 2009; 29(5): 736–742.
Okamura Y, Ashida R, Yamamoto Y, Ito T, Sugiura T, Uesaka K. FIB-4 Index is a Predictor of Background Liver Fibrosis and Long-Term Outcomes After Curative Resection of Hepatocellular Carcinoma. Ann Surg Oncol. 2016; 23(Suppl 4): 467–474.
Financial Support
This work was supported by the grants from the Project funded by China Postdoctoral Science Foundation (2017M611459), National Natural Science Foundation of China (No. 81370575, 81570593), Sci-tech Research Development Program of Guangzhou city (No. 1581000156, 201400000001-3), and Sun Yat-sen University Clinical Research 5010 Program (2014006).
Author information
Authors and Affiliations
Contributions
Conception and design: Wei Qin, Guihua Chen, Yang Yang
Acquisition of data: Li Wang, Beiyuan Hu, Shusheng Leng, Huanxian Luo
Analysis and interpretation of data: Huan Tian, Jia Yao, Chao Wu
Writing, review, and/or revision of the manuscript: Wei Qin, Xiaolong Chen, Yang Yang
Corresponding authors
Ethics declarations
This study was approved by the Ethics Committee of The Third Affiliated Hospital of Sun Yat-Sen University, Huashan Hospital, Fudan University, The Affiliated Hospital/Clinical Medical College of Chengdu University, and conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from all the patients at the time of admission.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Qin, W., Wang, L., Hu, B. et al. A Novel Score Predicts HBV-Related Hepatocellular Carcinoma Recurrence After Hepatectomy: a Retrospective Multicenter Study. J Gastrointest Surg 23, 922–932 (2019). https://doi.org/10.1007/s11605-018-4037-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-4037-x